Multidimensional Transcriptomics Reveals the Key Genes and Pathways Regulating the Acidity of Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Sampling Method
2.3. Extraction of Acid Components and Determination
2.4. RNA Extraction and Library Construction
2.5. Transcriptomic Analysis
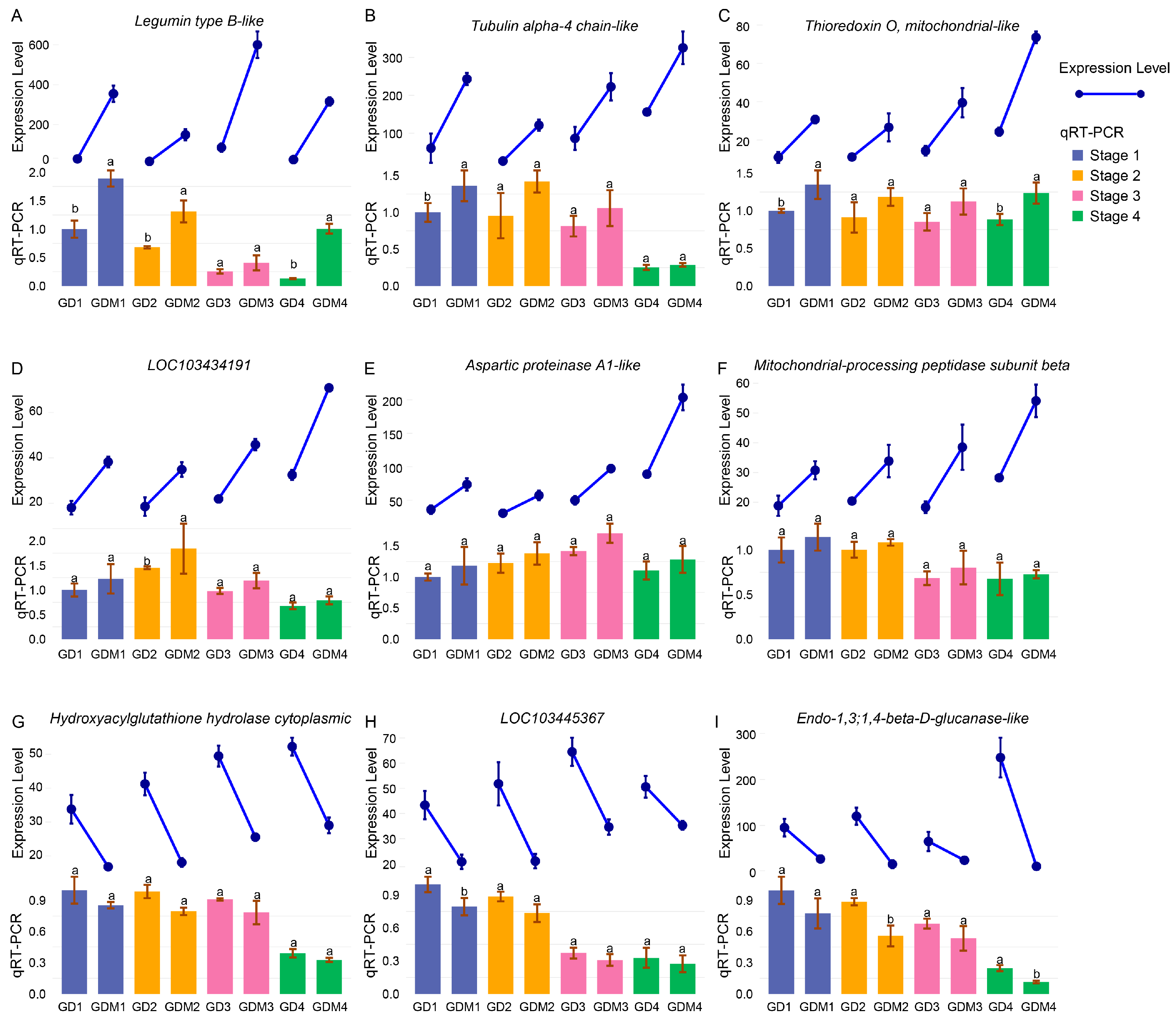
2.6. qRT-PCR Verification
3. Results
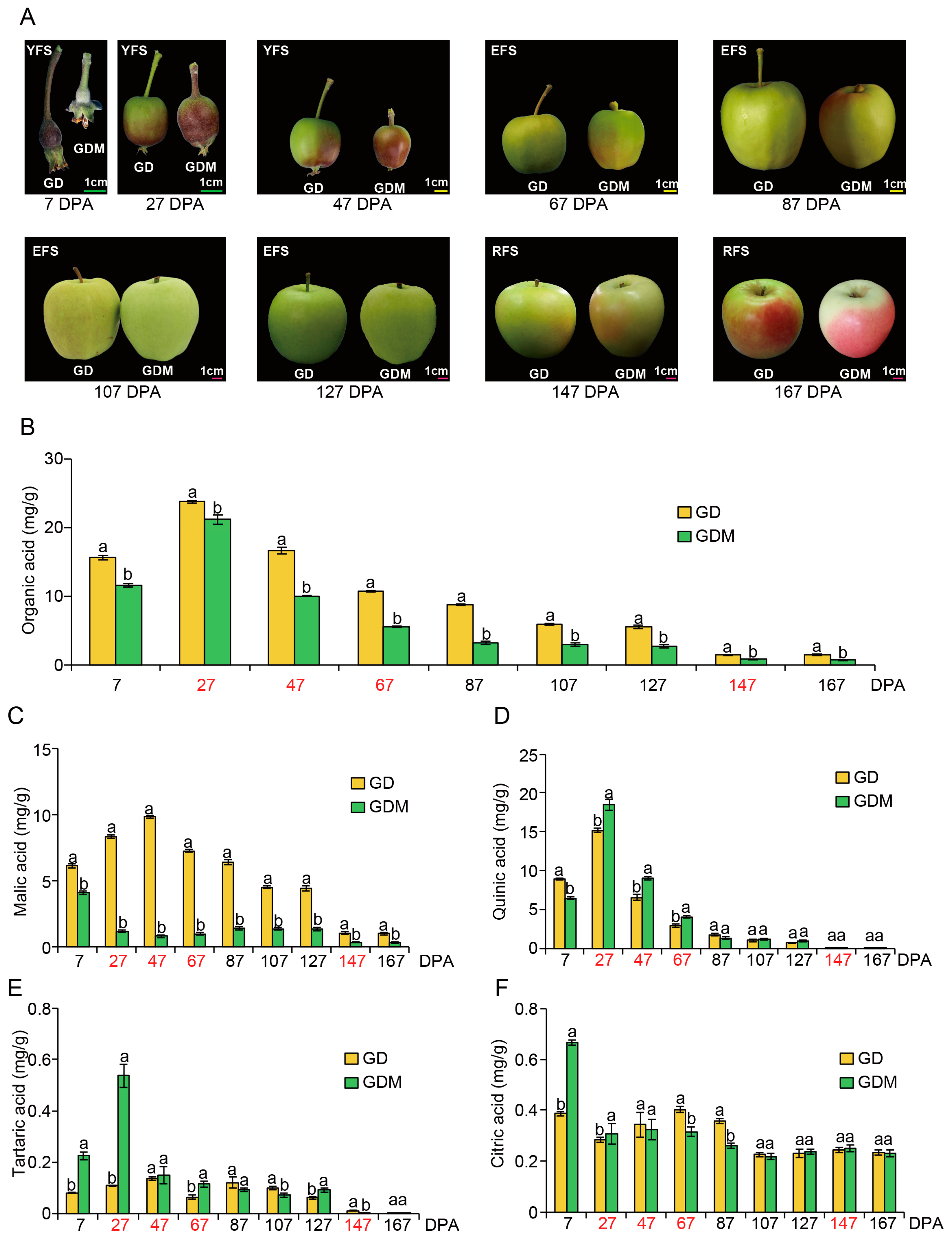
3.1. Organic Acid Profiles of GD and GDM Apples During Development
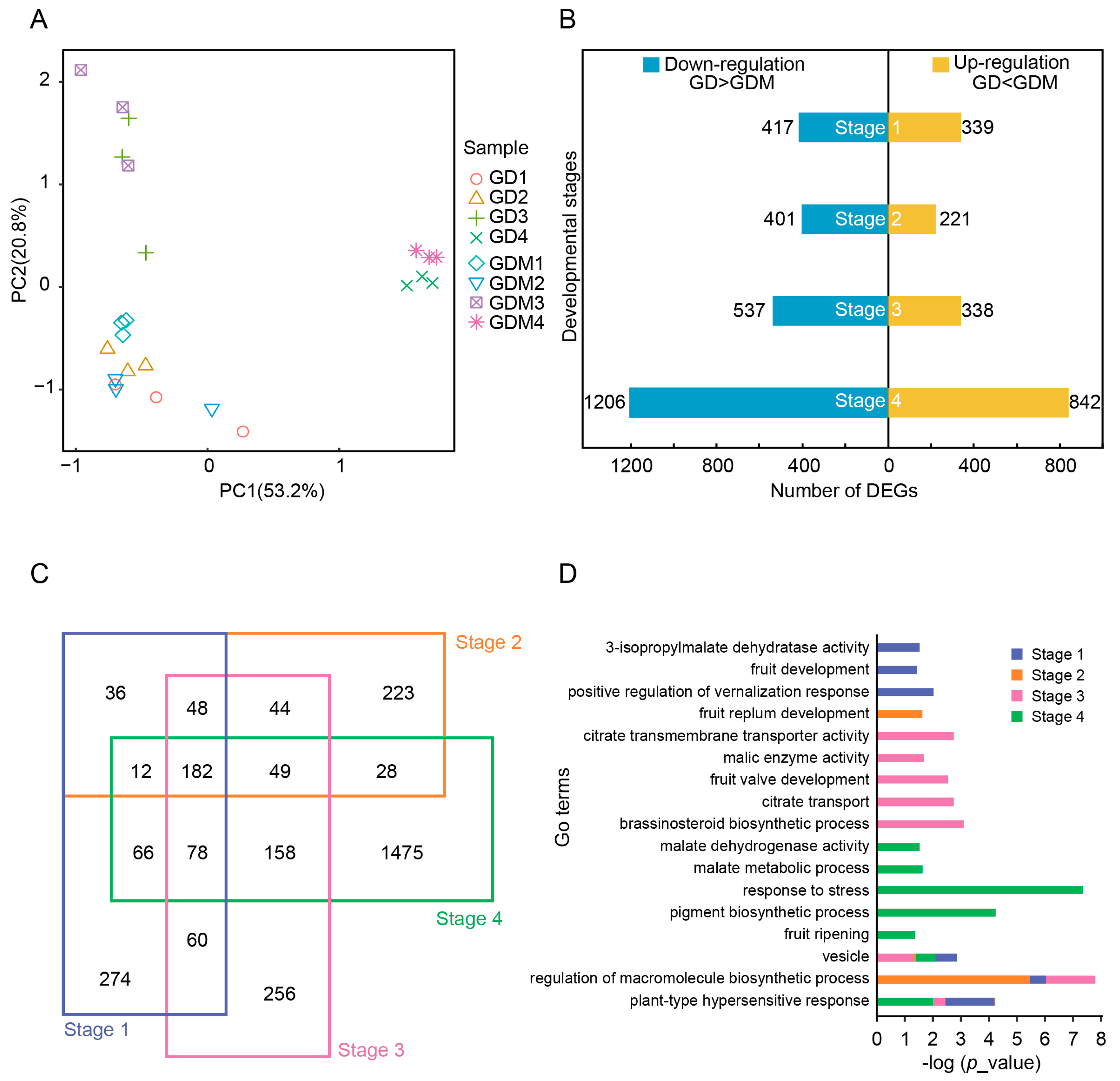
3.2. Analysis of Differentially Expressed Genes (DEGs) Between GD and GDM Apples
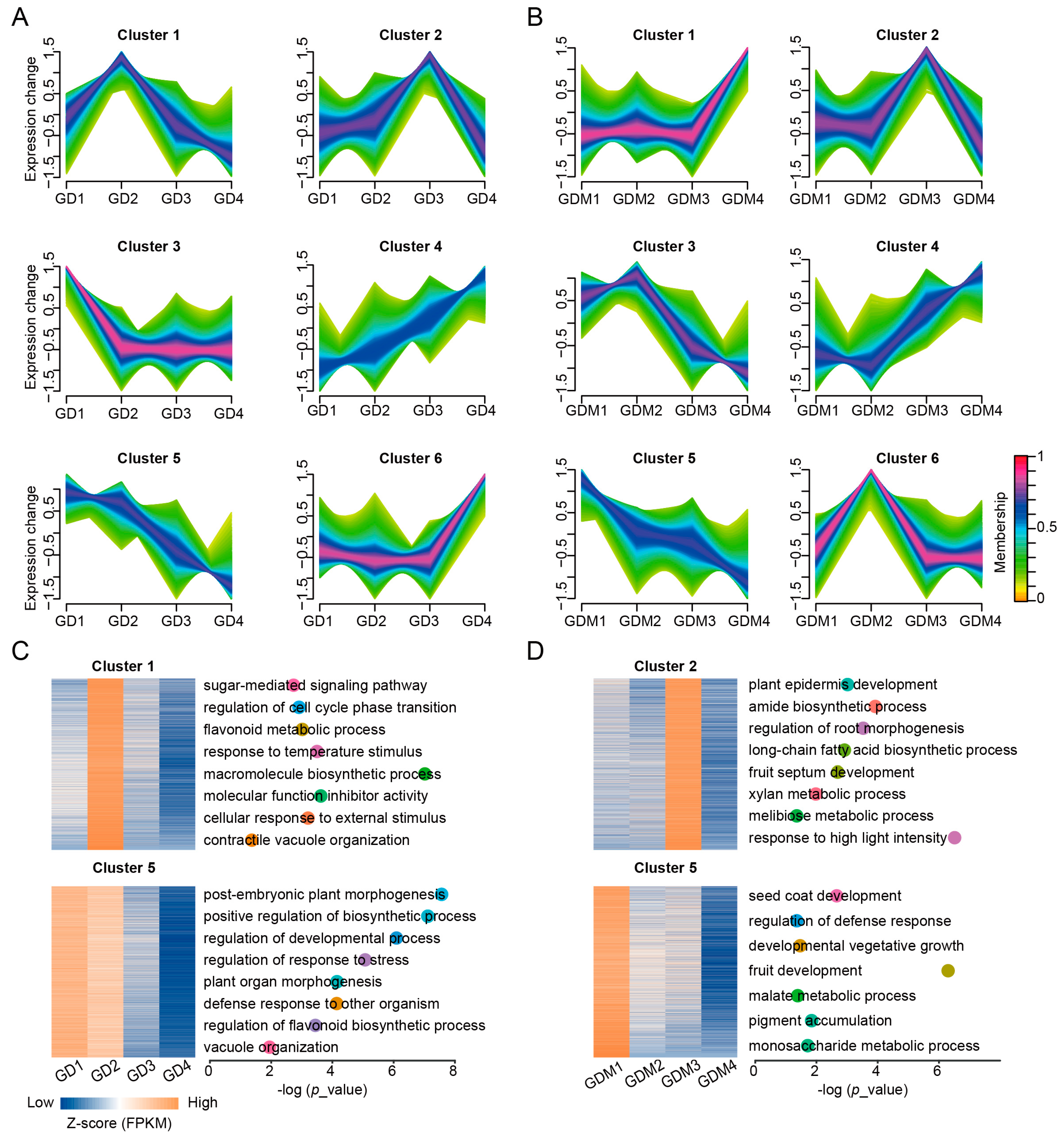
3.3. Dynamic Changes in Gene Expression During Fruit Ripening
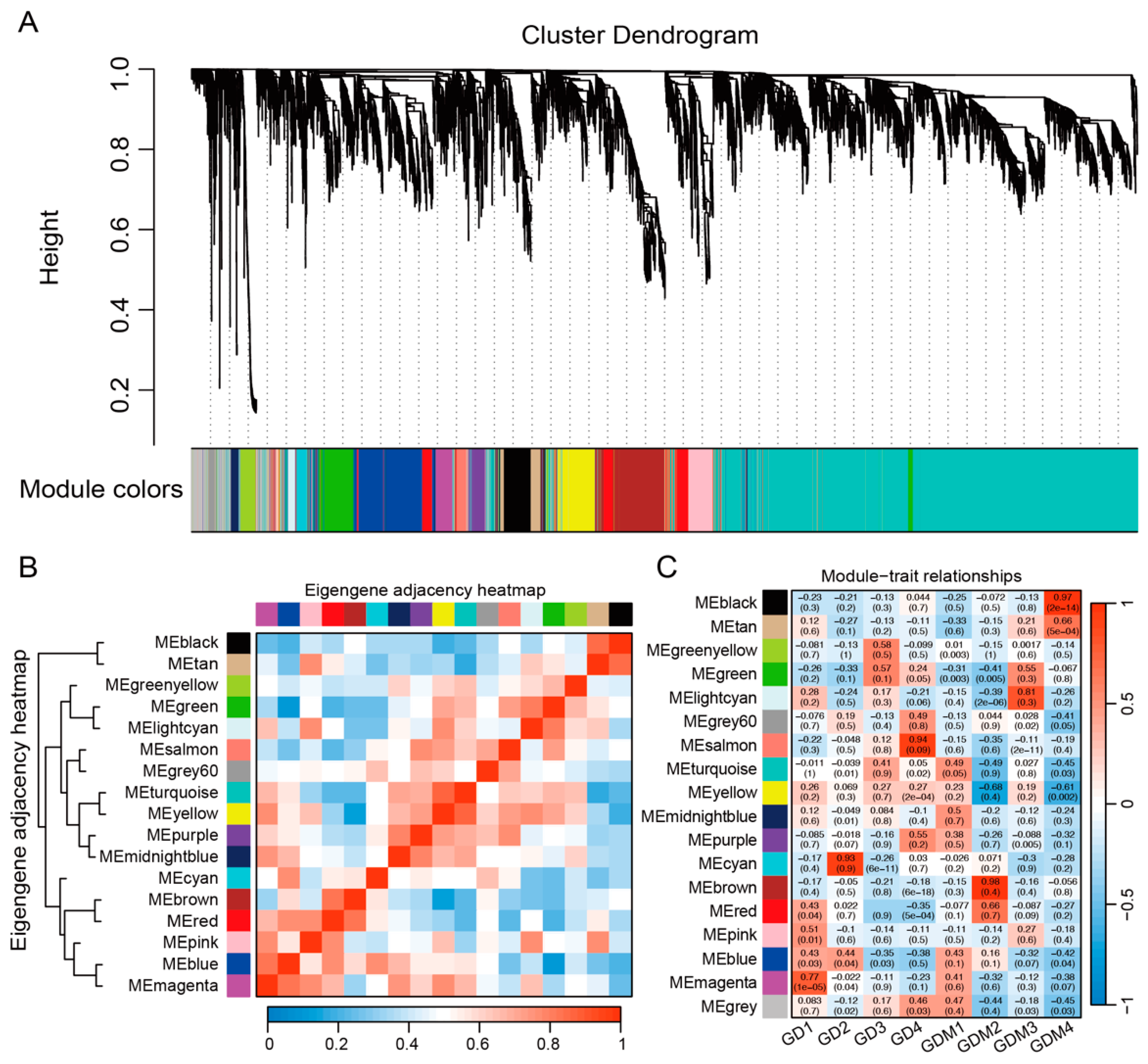
3.4. Co-Expression Network Analysis
3.5. Patterns of Gene Regulation of Acidity in Apple Fruit
3.6. Functional Validation of Critical Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene Name | Primer Forward | Primer Reverse |
|---|---|---|
| Actin | AATGGAACTGGAATGGTCAAGGC | TGCCAGATCTTCTCCATGTCATCCCA |
| LOC103409144 | ACAAGCTGGGTTAAGTGCGA | CAGGCCTGCAAAAATAATACCA |
| LOC114823285 | GAACTTCTGTCGAAACAGCCG | AGCAGCATCTGCCATTCCTT |
| LOC103421679 | GTGTCCTCAGACTCCCCAAG | TTTGCGCACTTTTTGTTGCAG |
| LOC103434191 | CTGCAACAAAAAGTGCGCAAA | CGGTATGTATTGCGCGAAGC |
| LOC114827605 | ACTCGACCAGTGTTGCTGAG | ACGACACAATACAAGGGGGA |
| LOC103442405 | TGTTCACTGGCTGCTCCATT | CAGAAGGATGAGCGTTCGGT |
| LOC103445367 | CAACATGCTGCAATAAGGCCA | CATGCATGCGGTTCAGTACG |
| LOC103438582 | GCACGCATTCTATCGCCAAG | TCGACGGTCGGCATATGTTT |
| LOC103424034 | TCAAAACAGAAGGCAGGTGGT | ACGGTCTAGAACAAGATCGGG |
References
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hajihashemi, S. Specific Secondary Metabolites of Medicinal Plants and Their Role in Stress Adaptation. In Plant Secondary Metabolites and Abiotic Stress; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 425–479. [Google Scholar]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef]
- Albertini, M.-V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in Organic Acids and Sugars during Early Stages of Development of Acidic and Acidless Citrus Fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]
- Melgarejo, P.; Salazar, D.M.; Artés, F. Organic acids and sugars composition of harvested pomegranate fruits. Eur. Food Res. Technol. 2000, 211, 185–190. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents-a review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A review on nutritional features, chemical composition, traditional and medicinal value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Harris, S.A.; Robinson, J.P.; Juniper, B.E. Genetic clues to the origin of the apple. Trends Genet. 2002, 18, 426–430. [Google Scholar] [CrossRef]
- Mabrok, H.B.; Mohamed, D.A.; Sytar, O.; Smetanska, I. Biological evaluation of golden delicious apples exposure to UV lights in rats. Pak. J. Biol. Sci. 2019, 22, 564–573. [Google Scholar] [CrossRef]
- Li, W.; Chu, C.; Zhang, T.; Sun, H.; Wang, S.; Liu, Z.; Wang, Z.; Li, H.; Li, Y.; Zhang, X. Pan-genome analysis reveals the evolution and diversity of Malus. Nat. Genet. 2025. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, J.; Liu, X.; Wang, B.; Zhao, Y.; Liao, L.; Allan, A.C.; Sun, C.; Duan, Y.; Li, X. A metabolic perspective of selection for fruit quality related to apple domestication and improvement. Genome Biol. 2023, 24, 95. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Araújo, W.L.; Obata, T.; Fernie, A.R. Regulation of the mitochondrial tricarboxylic acid cycle. Curr. Opin. Plant Biol. 2013, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yuan, Y.; Gao, M.; Xing, L.; Li, C.; Li, M.; Ma, F. Genome-wide Identification, Classification, Molecular Evolution and Expression Analysis of Malate Dehydrogenases in Apple. Int. J. Mol. Sci. 2018, 19, 3312. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-j.; Yang, Z.-q.; Wei, T.-t.; Zhao, H.-l. Response of tomato sugar and acid metabolism and fruit quality under different high temperature and relative humidity conditions. Phyton-Int. J. Exp. Bot. 2022, 91, 2033–2054. [Google Scholar] [CrossRef]
- Moing, A.; Rothan, C.; Svanella, L.; Just, D.; Diakou, P.; Raymond, P.; Gaudillère, J.P.; Monet, R. Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development. Physiol. Plant. 2000, 108, 1–10. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Wang, C.-K.; Zhao, Y.-W.; Sun, C.-H.; Hu, D.-G. Mechanisms and regulation of organic acid accumulation in plant vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef]
- Shiratake, K.; Martinoia, E. Transporters in fruit vacuoles. Plant Biotechnol. 2007, 24, 127–133. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, C.; Lu, C.; Wang, Y.; Ge, C.; Huang, G.; Wang, H. The lemon genome and DNA methylome unveil epigenetic regulation of citric acid biosynthesis during fruit development. Hortic. Res. 2024, 11, uhae005. [Google Scholar] [CrossRef]
- Huang, Y.; He, J.; Xu, Y.; Zheng, W.; Wang, S.; Chen, P.; Zeng, B.; Yang, S.; Jiang, X.; Liu, Z. Pangenome analysis provides insight into the evolution of the orange subfamily and a key gene for citric acid accumulation in citrus fruits. Nat. Genet. 2023, 55, 1964–1975. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, Y.; Mao, S.; Wu, F.; Liu, Y.; Mao, X.; Adhikari, P.B.; Xu, Y.; Wang, L.; Zuo, H. The high-quality genome of pummelo provides insights into the tissue-specific regulation of citric acid and anthocyanin during domestication. Hortic. Res. 2022, 9, uhac175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, B.; Wang, C.; Chen, X.; Ruan, Y.-L.; Yuan, Y.; Ma, F.; Li, M. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5). Plant Physiol. 2022, 188, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, N.; Shao, Y.; Shen, T.; Li, W.; Ma, B.; Wei, X.; Ruan, Y.-L.; Ma, F.; Li, M. An insertion in the promoter of a malate dehydrogenase gene regulates malic acid content in apple fruit. Plant Physiol. 2024, 196, 432–445. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, W.; Liu, P.; Song, S.; Wang, L.; Yang, W.; Ren, H.; Wei, X.; Zhu, L.; Peng, J.; et al. Transcriptional factor MdESE3 controls fruit acidity by activating genes regulating malic acid content in apple. Plant Physiol. 2024, 196, 261–272. [Google Scholar] [CrossRef]
- Wang, Y. China’s apple production reigns as world’s No. 1. China Farmers’ Daily, 9 November 2024; p. 007. [Google Scholar]
- Nisperos-Carriedo, M.O.; Buslig, B.S.; Shaw, P.E. Simultaneous detection of dehydroascorbic, ascorbic, and some organic acids in fruits and vegetables by HPLC. J. Agric. Food Chem. 1992, 40, 1127–1130. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar]
- Zhang, L.-H.; Zhang, A.-N.; Xu, Y.; Zhu, L.-C.; Ma, B.-Q.; Li, M.-J. Accumulation and regulation of malate in fruit cells. Fruit Res. 2024, 4, e031. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 6, 1451–1469. [Google Scholar] [CrossRef]
- Guo, L.-X.; Shi, C.-Y.; Liu, X.; Ning, D.-Y.; Jing, L.-F.; Yang, H.; Liu, Y.-Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provide a novel insight for an orange mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Niu, X.-Q.; Zheng, X.-L.; Chen, X.; Zheng, G.-H.; Wu, J.-C. Comparative transcriptome analysis reveals key genes potentially related to organic acid and sugar accumulation in loquat. PLoS ONE 2021, 16, e0238873. [Google Scholar] [CrossRef]
- Volk, G.M.; Olmstead, J.W.; Finn, C.E.; Janick, J. The ASHS outstanding fruit cultivar award: A 25-year retrospective. HortScience 2013, 48, 4–12. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Jia, R.; Yang, N.; Jin, L.; Zhu, L.; Ma, B.; Yao, Y.-X.; Ma, F.; Li, M. Malate metabolism mediated by the cytoplasmic malate dehydrogenase gene MdcyMDH affects sucrose synthesis in apple fruit. Hortic. Res. 2022, 9, uhac194. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, H.; Zheng, L.; Zhang, L.; Peng, Y.; Ma, W.; Tian, R.; Yuan, Y.; Ma, F.; Li, M.; et al. Overexpression of apple Ma12, a mitochondrial pyrophosphatase pump gene, leads to malic acid accumulation and the upregulation of malate dehydrogenase in tomato and apple calli. Hortic. Res. 2022, 9, uhab053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Quan, P.; Liu, H.; Li, L.; Qi, S.; Zhang, M.; Zhang, B.; Li, H.; Zhao, Y.; Ma, B.; et al. Transcriptomic and Metabolic Analyses Provide New Insights into the Apple Fruit Quality Decline during Long-Term Cold Storage. J. Agric. Food Chem. 2020, 68, 4699–4716. [Google Scholar] [CrossRef]
- Schumacher, K. pH in the plant endomembrane system—An import and export business. Curr. Opin. Plant Biol. 2014, 22, 71–76. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, X.-Y.; Zhao, Y.-W.; Wang, C.-K.; Sun, Q.; Hu, D.-G. Optimization of apple fruit flavor by MdVHP1-2 via modulation of soluble sugar and organic acid accumulation. Plant Physiol. Biochem. 2024, 206, 108227. [Google Scholar] [CrossRef]
- Brune, A.; Müller, M.; Taiz, L.; Gonzalez, P.; Etxeberria, E. Vacuolar acidification in citrus fruit: Comparison between acid lime (Citrus aurantifolia) and sweet lime (Citrus limmetioides) juice cells. J. Am. Soc. Hortic. Sci. 2002, 127, 171–177. [Google Scholar] [CrossRef]
- Terrier, N.; Sauvage, F.-X.; Ageorges, A.; Romieu, C. Changes in acidity and in proton transport at the tonoplast of grape berries during development. Planta 2001, 213, 20–28. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shiratake, K.; Yamaki, S. Seasonal changes in the activities of vacuolar H+-pumps and their gene expression in the developing Japanese pear fruit. J. Jpn. Soc. Hortic. Sci. 2000, 69, 15–21. [Google Scholar] [CrossRef]
- Xu, K.; Wang, A.; Brown, S. Genetic characterization of the Ma locus with pH and titratable acidity in apple. Mol. Breed. 2012, 30, 899–912. [Google Scholar] [CrossRef]
- Ma, W.; Li, B.; Zheng, L.; Peng, Y.; Tian, R.; Yuan, Y.; Zhu, L.; Su, J.; Ma, F.; Li, M. Combined profiling of transcriptome and DNA methylome reveal genes involved in accumulation of soluble sugars and organic acid in apple fruits. Foods 2021, 10, 2198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yu, H.; Tao, L.; Xie, H. Multidimensional Transcriptomics Reveals the Key Genes and Pathways Regulating the Acidity of Apples. Curr. Issues Mol. Biol. 2025, 47, 341. https://doi.org/10.3390/cimb47050341
Yang W, Yu H, Tao L, Xie H. Multidimensional Transcriptomics Reveals the Key Genes and Pathways Regulating the Acidity of Apples. Current Issues in Molecular Biology. 2025; 47(5):341. https://doi.org/10.3390/cimb47050341
Chicago/Turabian StyleYang, Wenyuan, Hang Yu, Lian Tao, and Hongjiang Xie. 2025. "Multidimensional Transcriptomics Reveals the Key Genes and Pathways Regulating the Acidity of Apples" Current Issues in Molecular Biology 47, no. 5: 341. https://doi.org/10.3390/cimb47050341
APA StyleYang, W., Yu, H., Tao, L., & Xie, H. (2025). Multidimensional Transcriptomics Reveals the Key Genes and Pathways Regulating the Acidity of Apples. Current Issues in Molecular Biology, 47(5), 341. https://doi.org/10.3390/cimb47050341







