Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Method
2.3. Renal Histopathological Analysis
2.4. Hematological Analysis
2.5. Renal Biochemical Analysis
2.6. Quantification of TNF-α Concentrations in Plasma
2.7. Assessment of Lipid Peroxidation
2.8. Statistical Analysis
3. Results
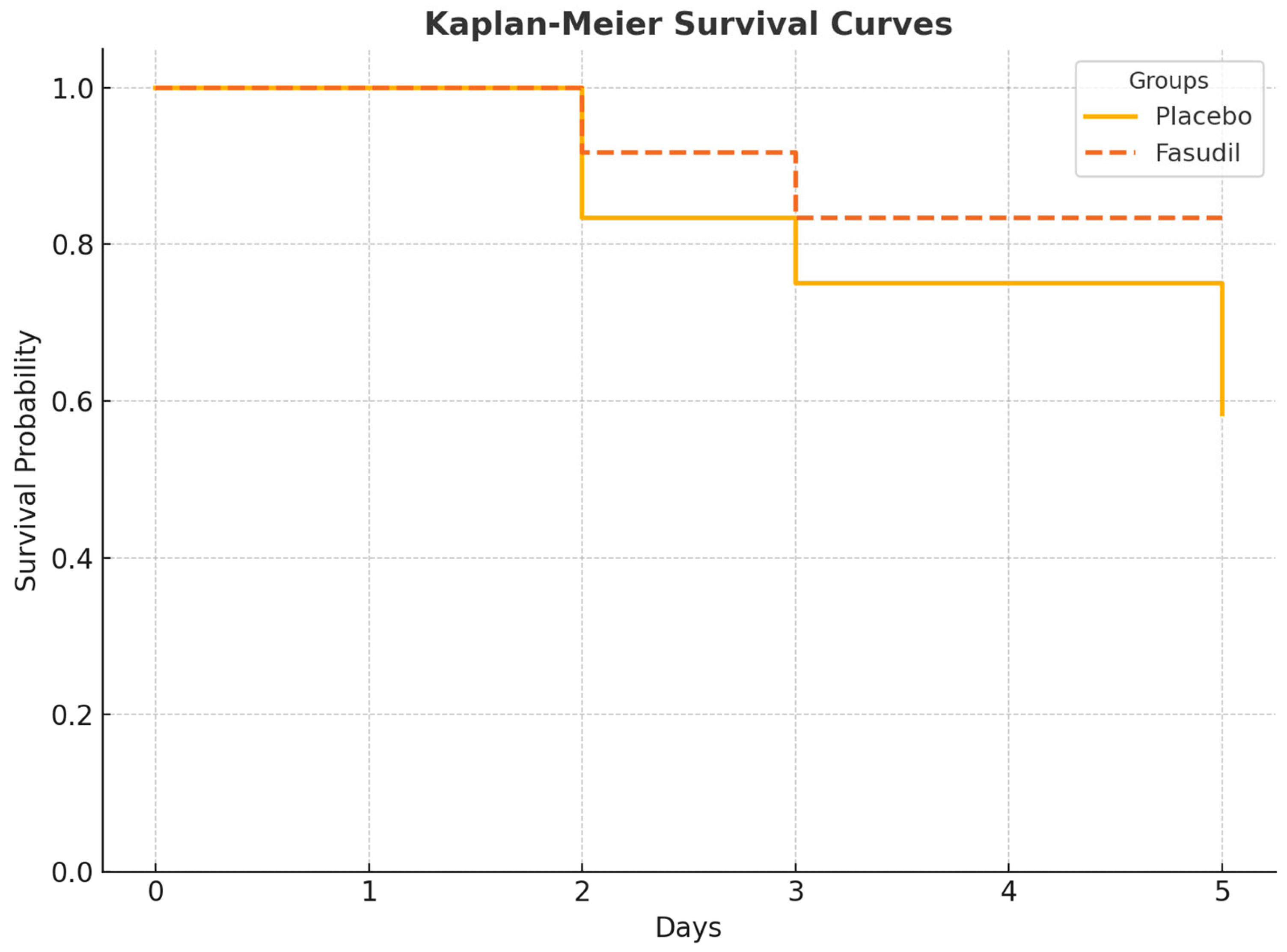
3.1. Mortality and Survival
3.2. Biochemical Parameters
3.3. Histopathological Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manrique-Caballero, C.L.; Del Rio-Pertuz, G.; Gómez, H. Sepsis-associated acute kidney injury. Crit. Care Clin. 2021, 37, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute Dialysis Quality Initiative workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology, needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Fukushima, K.; Fujita, M.; Nakanishi, M.; Hamori, M.; Nishimura, A.; Shibata, N.; Sugioka, N. Alterations in cisplatin pharmacokinetics and its acute/sub-chronic kidney injury over multiple cycles of cisplatin treatment in rats. Biol. Pharm. Bull. 2017, 40, 1948–1955. [Google Scholar] [CrossRef]
- Ferguson, M.A.; Vaidya, V.S.; Bonventre, J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2008, 245, 182–193. [Google Scholar] [CrossRef]
- Waikar, S.S.; Curhan, G.C.; Wald, R.; McCarthy, E.P.; Chertow, G.M. Declining mortality in patients with acute renal failure, 1988 to 2002. J. Am. Soc. Nephrol. 2006, 17, 1143–1150. [Google Scholar] [CrossRef]
- Wakino, S.; Kanda, T.; Hayashi, K. Rho/Rho kinase as a potential target for the treatment of renal disease. Drug News Perspect. 2005, 18, 639–643. [Google Scholar] [CrossRef]
- Homma, K.; Hayashi, K.; Wakino, S.; Tokuyama, H.; Kanda, T.; Tatematsu, S.; Hasegawa, K.; Fujishima, S.; Hori, S.; Saruta, T.; et al. Rho-kinase contributes to pressure-induced constriction of renal microvessels. Keio J. Med. 2014, 63, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, J.M.; Su, T.; Zhang, S.Y.; Lin, X.J. Fasudil, a rho-kinase inhibitor, exerts cardioprotective function in animal models of myocardial ischemia/reperfusion injury: A meta-analysis and review of preclinical evidence and possible mechanisms. Front. Pharmacol. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Deng, B.; Yang, X.; Zhu, Z.; Zhang, C. A rho-kinase inhibitor, fasudil, attenuates progressive glomerulosclerosis induced by daunorubicin in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 720–724. [Google Scholar] [CrossRef]
- Nishikimi, T.; Akimoto, K.; Wang, X.; Mori, Y.; Tadokoro, K.; Ishikawa, Y.; Shimokawa, H.; Ono, H.; Matsuoka, H. Fasudil, a rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J. Hypertens. 2004, 22, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Gao, Q.; Ni, L.; Wang, M.; Shen, F. Fasudil inhibits epithelial-myofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem. Pharm. Bull. 2013, 61, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Tu, Y.; Jia, R. The influence of fasudil on the epithelial-mesenchymal transdifferentiation of renal tubular epithelial cells from diabetic rats. Biomed. Pharmacother. 2010, 64, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, T.; Matsuoka, H. Molecular mechanisms and therapeutic strategies of chronic renal injury: Renoprotective effect of rho-kinase inhibitor in hypertensive glomerulosclerosis. J. Pharmacol. Sci. 2006, 100, 22–28. [Google Scholar] [CrossRef]
- Erbas, O.; Taskiran, D. Sepsis-induced changes in behavioral stereotypy in rats: Involvement of tumor necrosis factor-alpha, oxidative stress, and dopamine turnover. J. Surg. Res. 2014, 186, 262–268. [Google Scholar] [CrossRef]
- Başol, N.; Erbaş, O.; Çavuşoğlu, T.; Meral, A.; Ateş, U. Beneficial effects of agomelatine in experimental model of sepsis-related acute kidney injury. Ulus. Travma Acil. Cerrahi. Derg. 2016, 22, 121–126. [Google Scholar] [CrossRef]
- Ercan, G.; Bora, E.S.; Çınaroğlu, O.S.; Karaali, R.; Erbas, O. Hydroxychloroquine attenuates sepsis-induced acute respiratory distress syndrome in rats. Ulus. Travma Acil. Cerrahi. Derg. 2024, 30, 465–471. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Yang, Z.; Miao, D.; Zhang, D. Rho kinase inhibitor, fasudil, attenuates contrast-induced acute kidney injury. Basic Clin. Pharmacol. Toxicol. 2018, 122, 278–287. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Tan, Y.; Guo, J.; Chen, H.; Wu, P.-Y.; Wang, X.; Zhang, H. Assessment of fasudil on contrast-associated acute kidney injury using multiparametric renal MRI. Front. Pharmacol. 2022, 13, 905547. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Yan, Y.; Zhang, D. Alleviation of the doxorubicin-induced nephrotoxicity by fasudil in vivo and in vitro. J. Pharmacol. Sci. 2021, 145, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, S.; Liu, R.; Guo, M.; Wang, Q.; Chai, Z.; Xiao, B.; Ma, C. Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis. Neural Regen Res. 2024, 19, 671–679. [Google Scholar] [CrossRef]
- Hong, X.; Zhu, G.; Li, J.; Zhuang, B.; Chen, J.; Zhang, B. The protective effects of different doses of fasudil on hepatic ischemia/reperfusion injury in rats with cirrhosis. Chin. J. Gen. Surg. 2016, 31, 1038–1041. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, H.; Yu, J.; Liu, C.; Feng, L.; Li, J.; Xiao, B.; Ma, C. Inhibition of Fasudil on lipopolysaccharide-induced TNF-α and IL-1β expressions through TLR4 pathway in murine BV-2 cells in vitro. Chin. J. Cell. Mol. Immunol. 2014, 30, 11–14. [Google Scholar]
- Zhang, X.; Liu, T.; Tian, C.; Herbert, L. Application of fasudil in the treatment of contrast-ınduced acute renal insufficiency based on computerized tomography intelligent information monitoring (Preprint). JMIR Med. Inform. 2021. [Google Scholar] [CrossRef]
- Wan, J.; Liu, D.; Pan, S.; Zhou, S.; Liu, Z. NLRP3-mediated pyroptosis in diabetic nephropathy. Front. Pharmacol. 2022, 13, 998574. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Ali, F.E.M.; Kozman, M.R.; El-Ghafar, O.A.M. Umbelliferone attenuates gentamicin-induced renal toxicity by suppression of TLR-4/NF-κB-p65/NLRP-3 and JAK1/STAT-3 signaling pathways. Environ. Sci. Pollut. Res. 2021, 28, 11558–11571. [Google Scholar] [CrossRef]
- Satoh, M.; Tabuchi, T.; Itoh, T.; Nakamura, M. NLRP3 inflammasome activation in coronary artery disease: Results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin. Sci. 2014, 126, 233–241. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, P.; Yu, J.; Wang, J.; Yu, J.; Guo, M. Fasudil improves cognitive function in APP/PS1 transgenic mice by promoting mitophagy and inhibiting NLRP3 inflammasome activation. Chin. J. Cell. Mol. Immunol. 2024, 40, 696–703. [Google Scholar]
- Tian, F.; Huang, S.; Xu, W.; Xie, G.; Gan, Y.; Huang, F.; Fan, Y.; Bao, J. Fasudil compensates podocyte injury via CaMK4/Rho GTPases signal and actin cytoskeleton-dependent activation of YAP in MRL/lpr mice. Int. Immunopharmacol. 2023, 119, 110199. [Google Scholar] [CrossRef] [PubMed]
- Mang, J.Z.; Guo, A.H.; Li, D.D.; Yu, Y.; Zhou, C.H.; Liu, W.Y. The study of fasudil hydrochloride prevented acute kidney injury rat caused by sepsis. J. Biomed. Eng. Res. 2010, 29, 197–201. [Google Scholar]
- Xia, D.; Lai, X.; Wu, K.; Zhou, P.Y.; Li, L.; Guo, Z.-Y.; Xu, S. Metabolomics study of fasudil on cisplatin-induced kidney injury. Biosci. Rep. 2019, 39, BSR20192940. [Google Scholar] [CrossRef] [PubMed]


| Normal Control | CLP and Saline Group | CLP and 100 mg/kg Fasudil (Fas) Group | |
|---|---|---|---|
| Plasma MDA (nM) | 61.8 ± 10.1 | 144.2 ± 9.5 ** | 107.6 ± 12.5 # |
| Plasma TNF alfa (pg/mL) | 12.8 ± 3.3 | 185.1 ± 12.6 ** | 89.5 ± 9.7 ## |
| Plasma BUN (mg/dL) | 24.5 ± 1.3 | 48.4 ± 1.9 ** | 34.2 ± 2.3 # |
| Plasma Creatinin (mg/dL) | 0.29 ± 0.1 | 0.57 ± 0.08 * | 0.43 ± 0.2 # |
| Kidney STAT-3 (pg/mg protein) | 0.93 ± 0.12 | 2.86 ± 0.3 ** | 1.75 ± 0.28 ## |
| Kidney NLRP-3 (pg/g protein) | 49.4 ± 3.7 | 125.1 ± 5.4 ** | 98.2 ± 7.7 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, N.; Bora, E.S.; Çınaroğlu, O.S.; Erbaş, O. Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway. Curr. Issues Mol. Biol. 2025, 47, 340. https://doi.org/10.3390/cimb47050340
Şahin N, Bora ES, Çınaroğlu OS, Erbaş O. Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway. Current Issues in Molecular Biology. 2025; 47(5):340. https://doi.org/10.3390/cimb47050340
Chicago/Turabian StyleŞahin, Neslihan, Ejder Saylav Bora, Osman Sezer Çınaroğlu, and Oytun Erbaş. 2025. "Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway" Current Issues in Molecular Biology 47, no. 5: 340. https://doi.org/10.3390/cimb47050340
APA StyleŞahin, N., Bora, E. S., Çınaroğlu, O. S., & Erbaş, O. (2025). Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway. Current Issues in Molecular Biology, 47(5), 340. https://doi.org/10.3390/cimb47050340







