Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks
Abstract
1. Introduction
1.1. The Place of Galectin-3 in the Guidelines
1.2. Ideal Cardiovascular Health
2. Materials and Methods
3. Properties of Galectin
3.1. Structure and Biological Functions of Galectin-3
3.2. The Role in Inflammation and Fibrosis
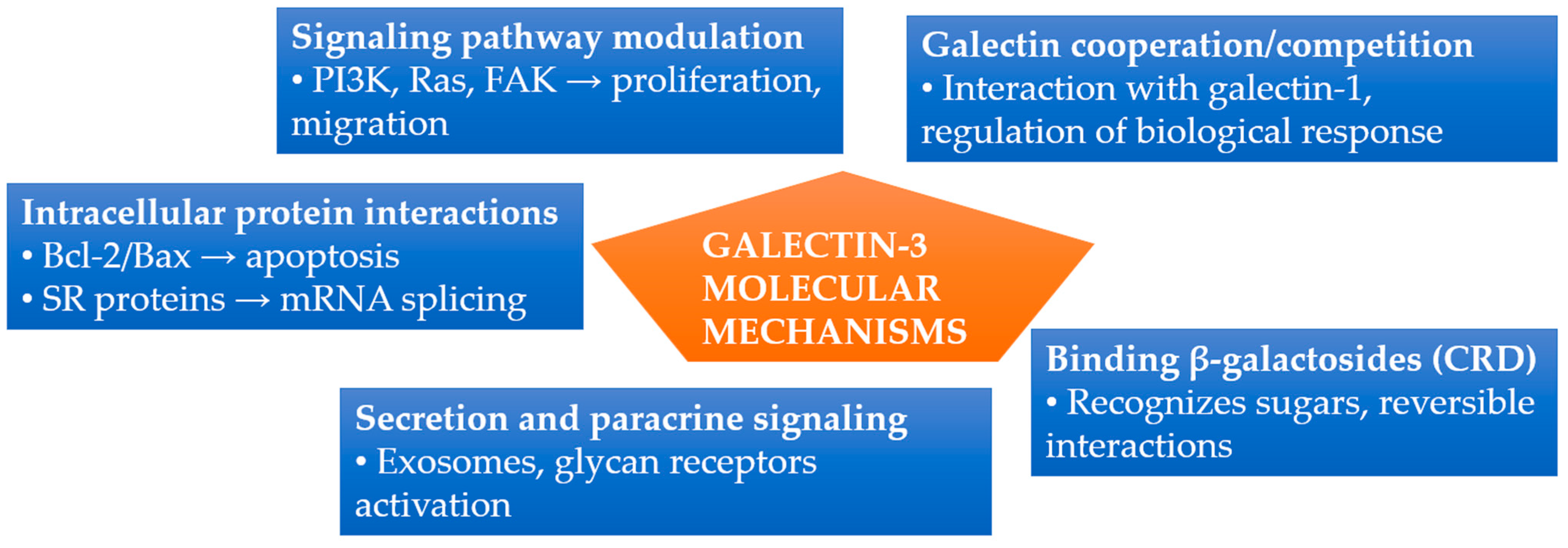
3.3. Molecular Mechanisms
3.4. Galectin-3 as a Biomarker in Cardiology
3.5. Galectin-3 Inhibitors
3.6. Galectin-3 and Myocardial Extracellular Volume
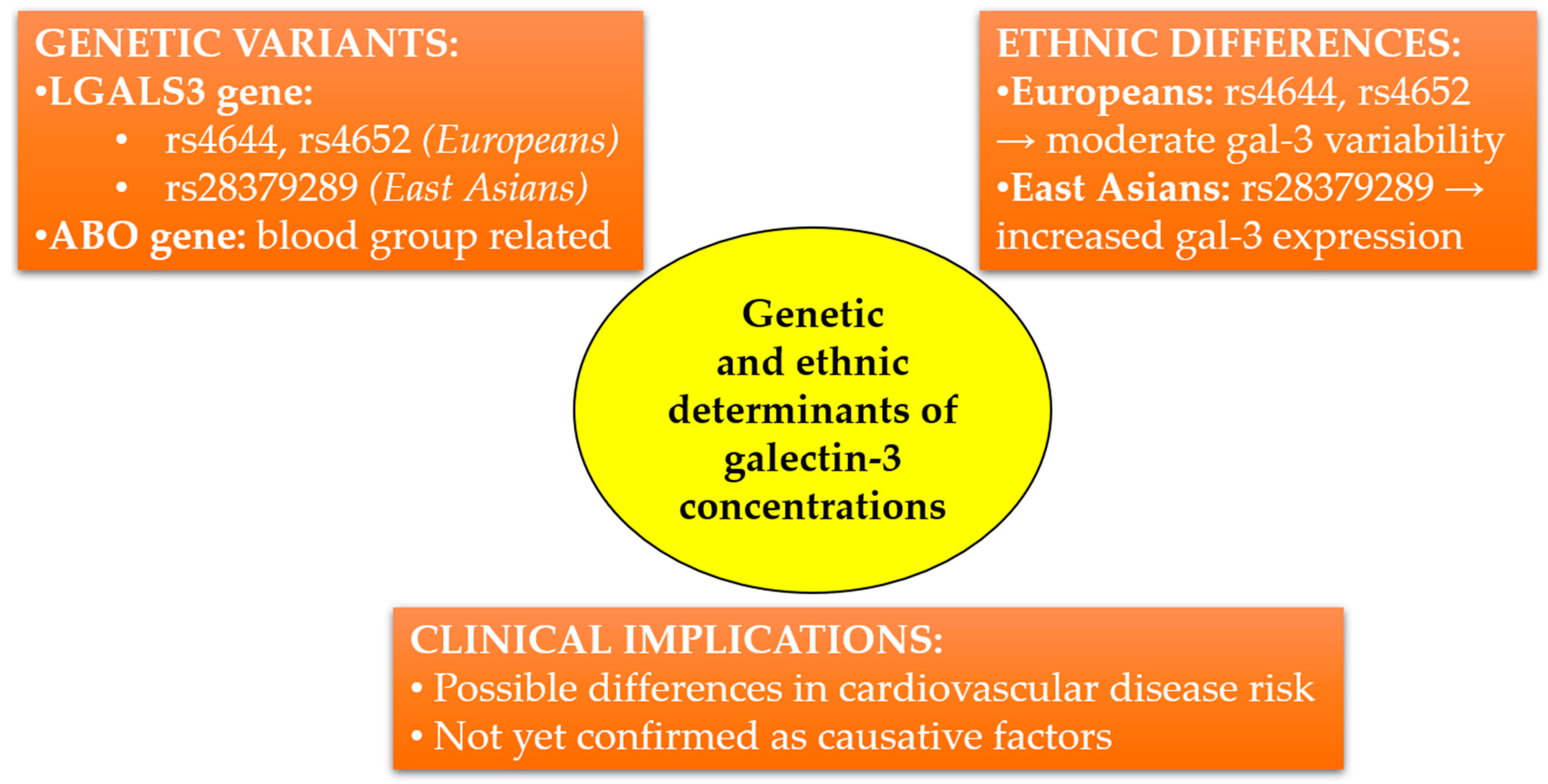
3.7. Genetic Determinants of Circulating Galectin-3 Concentrations and Ethnic Differences
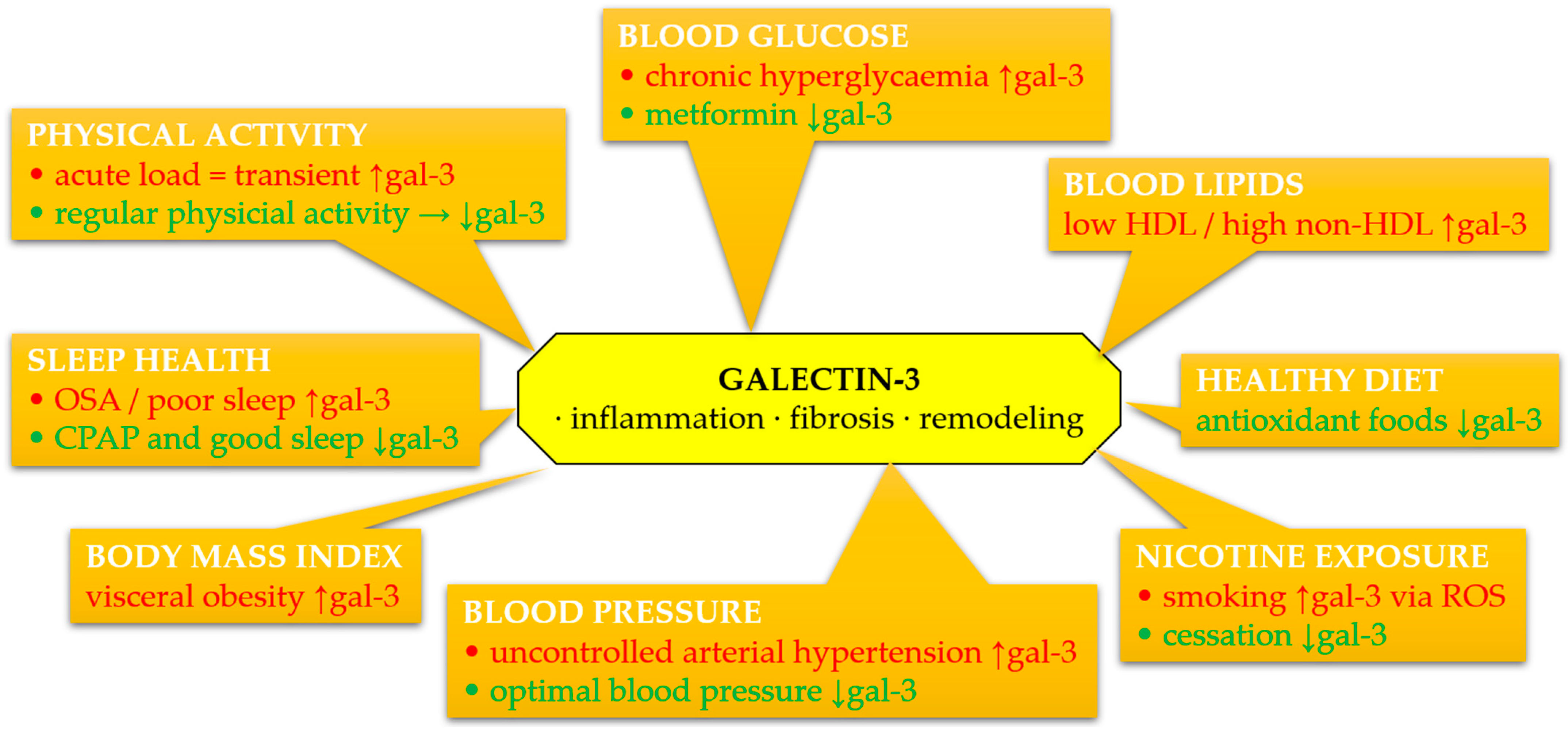
4. Association of Galectin-3 with Individual Components of LS7 and LE8
4.1. Tobacco Smoking
4.2. Diet
4.3. Physical Activity
4.4. BMI
4.5. Cholesterol
4.6. Blood Pressure
4.7. Glucose Concentration
4.8. Sleep Health
5. Limitations and Future Directions
- Prospective studies evaluating the predictive value of gal-3 in various risk populations;
- Interventional trials assessing the impact of lifestyle modifications on gal-3 concentration and related clinical outcomes;
- Validation of gal-3 as a marker of therapeutic response in CVDs, particularly in HF and atrial fibrillation;
- Assessment of its role as a marker of environmental and lifestyle effects in the context of LE8/LS7.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AECs | Airway epithelial cells |
| AHA | American Heart Association |
| AH | Arterial hypertension |
| AHI | Apnea-hypopnea index |
| ALEs | Advanced lipoxidation end-products |
| AMPK | AMP-activated protein kinase |
| AOR | Adjusted odds ratio |
| ARIC | Atherosclerosis Risk in Communities Study |
| BMI | Body mass index |
| BNP | B-type natriuretic peptide |
| BP | Blood pressure |
| CAD | Coronary artery disease |
| CD68 | Cluster of differentiation 68 |
| CHF | Chronic heart failure |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CPAP | Continuous positive airway pressure |
| CR | Cardiac rehabilitation |
| CSE | Cigarette smoke extract |
| CVD | Cardiovascular disease |
| CVH | Cardiovascular health |
| DAMP | Damage-associated molecular pattern |
| DCM | Diabetic cardiomyopathy |
| DEX | Dexpanthenol |
| ECHO | Echocardiographic |
| ECM | Extracellular matrix |
| ECV | Extracellular volume |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| EPCs | Endothelial progenitor cells |
| ERC | Elastin receptor complex |
| ESC | European Society of Cardiology |
| FAK kinases | Focal adhesion kinase kinases |
| FBG | Fasting blood glucose |
| FDA | Food and Drug Administration |
| FHS | Framingham Heart Study |
| Gal-3 | Galectin-3 |
| GSH-Px | Glutathione peroxidase |
| HbA1C | Hemoglobin A1c (glycated hemoglobin) |
| HCD | Hypercaloric diet |
| HF | Heart failure |
| HFD | High-fat diet |
| HFpEF | Heart failure with preserved ejection fraction |
| HFSA | Heart Failure Society of America |
| HIIT | High-intensity interval training |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| HR | Hazard ratio |
| hsCRP | High-sensitivity C-reactive protein |
| HUVECs | Human umbilical vein endothelial cells |
| HVS | Hepatic vein serum |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IF | Intermittent fasting |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LCN2 | Lipocalin-2 |
| LE8 | Life’s Essential 8 |
| LGALS3BP | Galectin-3 binding protein |
| LLPS | Liquid–liquid phase separation |
| LPC | Lysophosphatidylcholine |
| LPS | Lipopolysaccharide |
| LS7 | Life’s Simple 7 |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LVH | Left ventricular hypertrophy |
| MCP | Modified citrus pectin |
| MDA | Malondialdehyde |
| MIACT | Moderate-intensity aerobic continuous training |
| MRI | Magnetic resonance imaging |
| MUC1-C/EGFR | Mucin 1 C-terminal subunit/Epidermal Growth Factor Receptor |
| NAC | N-acetylcysteine |
| NADPH oxidase 2 | Nicotinamide adenine dinucleotide phosphate oxidase 2 |
| NASH | Nonalcoholic steatohepatitis |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NWGR | Asparagine-Tryptophan-Glycine-Arginine |
| NT-proBNP | N-terminal pro–B-type natriuretic peptide |
| OGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| OSA | Obstructive sleep apnea |
| oxLDL | Oxidized low-density lipoprotein |
| PI3K | Phosphoinositide 3-kinase |
| POEM | Prospective Investigation of Obesity, Energy and Metabolism |
| PREVEND | Prevention of Renal and Vascular End-stage Disease |
| PUFA | Polyunsaturated fatty acids |
| PVS | Portal vein serum |
| Ref. | References |
| ROS | Reactive oxygen species |
| RUNX-2 | Runt-related transcription factor 2 |
| RV | Right ventricular |
| RVEF | Right ventricular ejection fraction |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SAT | Subcutaneous adipose tissue |
| SBP | Systolic blood pressure |
| SGLT2i | Sodium-glucose co-transporter 2 inhibitors |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| SNP | Sodium nitroprusside |
| SPPB | Short physical performance battery |
| SR proteins | Serine/arginine-rich proteins |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STEMI | ST-elevation myocardial infarction |
| SVS | Systemic venous serum |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| TGF-β | Transforming growth factor beta. |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TOS | Total oxidative status |
| VAT | Visceral adipose tissue |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VCID | Vascular cognitive impairment and dementia |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| VO2 | Oxygen uptake |
| VSMCs | Vascular smooth muscle cells |
| WAT | White adipose tissue |
| WC | Waist circumference |
| WONDERFUL | Weekly ONe-Day WatER-only Fasting InterventionaL trial |
| WT | Wild type |
| YAP | Yes-associated protein |
| α9nAChR | α9 nicotinic acetylcholine receptor |
| ↑ | Increase |
| ↓ | Decrease |
References
- Charkiewicz, A.E. A Proposal for Research Involving New Biomarkers of Hypertension, Lifestyle, and Environmental Exposure. Curr. Issues Mol. Biol. 2025, 47, 206. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; De Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254. [Google Scholar] [CrossRef]
- Van Der Velde, A.R.; Meijers, W.C.; Van Den Heuvel, E.R.; Bakker, S.J.; Van Gilst, W.H.; Van Der Harst, P.; Hillege, H.; De Boer, R.A. Determinants of Temporal Changes in Galectin-3 Level in the General Population: Data of PREVEND. Int. J. Cardiol. 2016, 222, 385–390. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Suarez, G.; Meyerrose, G. Heart Failure and Galectin 3. Ann. Transl. Med. 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Cai, K.; Xu, C.; Zhan, Q.; Xu, X.; Xu, D.; Zeng, Q. Prognostic Value of Serum Galectin-3 in Chronic Heart Failure: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 783707. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Nuclear Transport of Galectin-3 and Its Therapeutic Implications. Semin. Cancer Biol. 2014, 27, 30–38. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Qiu, D.-C.; Chang, W.-H.; Yeh, Y.-Q.; Jeng, U.-S.; Liu, F.-T.; Huang, J. The Intrinsically Disordered N-Terminal Domain of Galectin-3 Dynamically Mediates Multisite Self-Association of the Protein through Fuzzy Interactions. J. Biol. Chem. 2017, 292, 17845–17856. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.; Bellotti, C.; Salehi, L.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Gaye, B.; Lloyd-Jones, D.M. Primordial Prevention of Cardiovascular Disease: Several Challenges Remain. Int. J. Cardiol. 2019, 274, 379–380. [Google Scholar] [CrossRef]
- Banach, M.; Burchardt, P.; Chlebus, K.; Dobrowolski, P.; Dudek, D.; Dyrbuś, K.; Gąsior, M.; Jankowski, P.; Jóźwiak, J.; Kłosiewicz-Latoszek, L.; et al. WYTYCZNE PTL/KLRwP/PTK/ PTDL/PTD/PTNT DIAGNOSTYKI I LECZENIA ZABURZEŃ LIPIDOWYCH W POLSCE 2021. Nadciśnienie Tętnicze Prakt. 2021, 7, 113–222. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. 2021 Guidelines on the Management of Patients with Diabetes. A Position of Diabetes Poland. Clin. Diabetol. 2021, 10, 1–113. [Google Scholar] [CrossRef]
- Patel, R.; Peesay, T.; Krishnan, V.; Wilcox, J.; Wilsbacher, L.; Khan, S.S. Prioritizing the Primary Prevention of Heart Failure: Measuring, Modifying and Monitoring Risk. Prog. Cardiovasc. Dis. 2024, 82, 2–14. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tian, P.; Li, L.; Qu, Q. Comparison of the Associations between Life’s Essential 8 and Life’s Simple 7 with Stroke: NHANES 1999–2018. J. Stroke Cerebrovasc. Dis. 2025, 34, 108238. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on P-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Kyriacou, D.N. The Enduring Evolution of the P Value. JAMA 2016, 315, 1113. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.; D’Agostino, R.B.; Gatsonis, C.; Hogan, J.W.; Hunter, D.J.; Normand, S.-L.T.; Drazen, J.M.; Hamel, M.B. New Guidelines for Statistical Reporting in the Journal. N. Engl. J. Med. 2019, 381, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Martuszewski, A.; Paluszkiewicz, P.; Poręba, R.; Gać, P. Galectin-3 in Cardiovascular Health: A Narrative Review Based on the Life’s Essential 8 and Life’s Simple 7 Frameworks. 2025. Available online: https://osf.io/xfwr5/resources (accessed on 3 May 2025). [CrossRef]
- Sato, S. Why Does Galectin-3 Have a Unique Intrinsically Disordered Region? Glycoforum 2023, 26, A8. [Google Scholar]
- Liu, F. Intracellular Functions of Galectins. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Lin, H.-M. Galectin-3 Mediates Genistein-Induced G2/M Arrest and Inhibits Apoptosis. Carcinogenesis 2000, 21, 1941–1945. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A Novel Antiapoptotic Molecule with a Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Zhang, T.; Hogan, V.; Raz, A. Galectin-3 Leads to Attenuation of Apoptosis through Bax Heterodimerization in Human Thyroid Carcinoma Cells. Oncotarget 2014, 5, 9992–10001. [Google Scholar] [CrossRef]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of Galectin-3 Modulates T-Cell Growth and Apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef]
- Fukumori, T.; Takenaka, Y.; Oka, N.; Yoshii, T.; Hogan, V.; Inohara, H.; Kanayama, H.; Kim, H.-R.C.; Raz, A. Endogenous Galectin-3 Determines the Routing of CD95 Apoptotic Signaling Pathways. Cancer Res. 2004, 64, 3376–3379. [Google Scholar] [CrossRef]
- Liu, L.; Sakai, T.; Sano, N.; Fukui, K. Nucling Mediates Apoptosis by Inhibiting Expression of Galectin-3 through Interference with Nuclear Factor kappaB Signalling. Biochem. J. 2004, 380, 31–41. [Google Scholar] [CrossRef]
- Yu, F.; Finley, R.L.; Raz, A.; Kim, H.-R.C. Galectin-3 Translocates to the Perinuclear Membranes and Inhibits Cytochrome c Release from the Mitochondria. J. Biol. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Fusco, O.; Tinari, N.; Natoli, C.; Liu, F.T.; Semeraro, M.L.; Malorni, W.; Iacobelli, S. Galectin-3 Overexpression Protects from Apoptosis by Improving Cell Adhesion Properties. Int. J. Cancer 2000, 85, 545–554. [Google Scholar] [CrossRef]
- Califice, S.; Castronovo, V.; Bracke, M.; Van Den Brûle, F. Dual Activities of Galectin-3 in Human Prostate Cancer: Tumor Suppression of Nuclear Galectin-3 vs Tumor Promotion of Cytoplasmic Galectin-3. Oncogene 2004, 23, 7527–7536. [Google Scholar] [CrossRef] [PubMed]
- Califice, S.; Castronovo, V.; Van Den Brûle, F. Galectin-3 and Cancer (Review). Int. J. Oncol. 2004, 25, 983–992. [Google Scholar]
- Moisa, A.; Fritz, P.; Eck, A.; Wehner, H.-D.; Mürdter, T.; Simon, W.; Gabius, H.-J. Growth/Adhesion-Regulatory Tissue Lectin Galectin-3: Stromal Presence but Not Cytoplasmic/Nuclear Expression in Tumor Cells as a Negative Prognostic Factor in Breast Cancer. Anticancer Res. 2007, 27, 2131–2139. [Google Scholar]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of Galectin-3 in the Nucleus and Cytoplasm. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 181–189. [Google Scholar] [CrossRef]
- Hughes, R. Secretion of the Galectin Family of Mammalian Carbohydrate-Binding Proteins. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1473, 172–185. [Google Scholar] [CrossRef]
- Jones, J.L.; Saraswati, S.; Block, A.S.; Lichti, C.F.; Mahadevan, M.; Diekman, A.B. Galectin-3 Is Associated with Prostasomes in Human Semen. Glycoconj. J. 2010, 27, 227–236. [Google Scholar] [CrossRef]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The Galectin Lattice at a Glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Markowska, A.I.; Liu, F.-T.; Panjwani, N. Galectin-3 Is an Important Mediator of VEGF- and bFGF-Mediated Angiogenic Response. J. Exp. Med. 2010, 207, 1981–1993. [Google Scholar] [CrossRef]
- Merlin, J.; Stechly, L.; De Beaucé, S.; Monté, D.; Leteurtre, E.; Van Seuningen, I.; Huet, G.; Pigny, P. Galectin-3 Regulates MUC1 and EGFR Cellular Distribution and EGFR Downstream Pathways in Pancreatic Cancer Cells. Oncogene 2011, 30, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Yik, J.J.; Mat Jusoh, H.; Norton, L.J.; Funnell, A.P.W.; Pearson, R.C.M.; Bell-Anderson, K.S.; Crossley, M.; Quinlan, K.G.R. Krüppel-like Factor 3 (KLF3/BKLF) Is Required for Widespread Repression of the Inflammatory Modulator Galectin-3 (Lgals3). J. Biol. Chem. 2016, 291, 16048–16058. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2021, 12, 46. [Google Scholar] [CrossRef]
- Kasai, K. The Keyhole of Galectin Is Made Loose, and the Inserted Key Easily Comes Out. Glycoforum 2020, 23, A17. [Google Scholar]
- Miyanishi, N.; Nishi, N.; Abe, H.; Kashio, Y.; Shinonaga, R.; Nakakita, S.; Sumiyoshi, W.; Yamauchi, A.; Nakamura, T.; Hirashima, M.; et al. Carbohydrate-Recognition Domains of Galectin-9 Are Involved in Intermolecular Interaction with Galectin-9 Itself and Other Members of the Galectin Family. Glycobiology 2007, 17, 423–432. [Google Scholar] [CrossRef]
- Tian, L.; Chen, K.; Han, Z. Correlation between Galectin-3 and Adverse Outcomes in Myocardial Infarction Patients: A Meta-Analysis. Cardiol. Res. Pract. 2020, 2020, 7614327. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming Growth Factor (TGF)-β Signaling in Cardiac Remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Nikolova, A.P.; Pancoast, J.R.; Lee, R.T. Heart Failure With Preserved Ejection Fraction: Molecular Pathways of the Aging Myocardium. Circ. Res. 2014, 115, 97–107. [Google Scholar] [CrossRef]
- Málek, F. Impact of galectin 3 as myofibrosis marker in clinical cardiology. Vnitr. Lek. 2014, 60, 327–330. [Google Scholar]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and Cancer Stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Saccon, F.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Role of Galectin-3 in Autoimmune and Non-Autoimmune Nephropathies. Autoimmun. Rev. 2017, 16, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, X.; Ma, L.; Zhuang, Y.; Wei, Y.; Zhang, L.; Jin, S.; Liang, W.; Shen, X.; Li, C.; et al. Galectin-3 May Serve as a Marker for Poor Prognosis in Colorectal Cancer: A Meta-Analysis. Pathol. Res. Pract. 2019, 215, 152612. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Ilarregui, J.M. Conveying Glycan Information into T-cell Homeostatic Programs: A Challenging Role for Galectin-1 in Inflammatory and Tumor Microenvironments. Immunol. Rev. 2009, 230, 144–159. [Google Scholar] [CrossRef]
- Pruc, M.; Gaca, Z.; Swieczkowski, D.; Kubica, J.; Galwankar, S.; Salak, A.; Szarpak, L. A Systematic Review and Meta-Analysis of the Diagnostic Value of Galectin-3 in Acute Coronary Syndrome. J. Clin. Med. 2024, 13, 4504. [Google Scholar] [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and Risk of Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin. Lab. Anal. 2020, 34, e23104. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic. Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef]
- Arangalage, D.; Nguyen, V.; Robert, T.; Melissopoulou, M.; Mathieu, T.; Estellat, C.; Codogno, I.; Huart, V.; Duval, X.; Cimadevilla, C.; et al. 0574: Determinants and Prognostic Value of Galectin-3 in Patients with Aortic Valve Stenosis—The COFRASA-GENERAC Study. Arch. Cardiovasc. Dis. Suppl. 2016, 8, 59. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. JNCI J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Jiménez-González, S.; Delgado-Valero, B.; Islas, F.; Romero-Miranda, A.; Luaces, M.; Ramchandani, B.; Cuesta-Corral, M.; Montoro-Garrido, A.; Martínez-Martínez, E.; Cachofeiro, V. The Detrimental Role of Galectin-3 and Endoplasmic Reticulum Stress in the Cardiac Consequences of Myocardial Ischemia in the Context of Obesity. FASEB J. 2024, 38, e23818. [Google Scholar] [CrossRef]
- Kirk, J.A.; Frangogiannis, N.G. Galectin-3 in the Pathogenesis of Heart Failure: A Causative Mediator or Simply a Biomarker? Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H1256–H1258. [Google Scholar] [CrossRef] [PubMed]
- Kram, M. Galectin-3 Inhibition as a Potential Therapeutic Target in Non-Alcoholic Steatohepatitis Liver Fibrosis. World J. Hepatol. 2023, 15, 201–207. [Google Scholar] [CrossRef]
- Aslanis, V.; Slack, R.J.; MacKinnon, A.C.; McClinton, C.; Tantawi, S.; Gravelle, L.; Nilsson, U.J.; Leffler, H.; Brooks, A.; Khindri, S.K.; et al. Safety and Pharmacokinetics of GB1211, an Oral Galectin-3 Inhibitor: A Single- and Multiple-Dose First-in-Human Study in Healthy Participants. Cancer Chemother. Pharmacol. 2023, 91, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Perea, R.J.; Morales-Ruiz, M.; Ortiz-Perez, J.T.; Bosch, X.; Andreu, D.; Borras, R.; Acosta, J.; Penela, D.; Prat-González, S.; De Caralt, T.M.; et al. Utility of Galectin-3 in Predicting Post-Infarct Remodeling after Acute Myocardial Infarction Based on Extracellular Volume Fraction Mapping. Int. J. Cardiol. 2016, 223, 458–464. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Fanisio, F.; Birtolo, L.I.; Costi, B.; Netti, L.; Chimenti, C.; Lavalle, C.; Maestrini, V.; et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 7650. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dong, G.; Liu, J.; Shuang, X.; Liu, C.; Yang, C.; Qing, W.; Qiao, W. Clinical Implications of Plasma Galectin-3 in Heart Failure With Preserved Ejection Fraction: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 854501. [Google Scholar] [CrossRef]
- Radziwolek, L.; Radunski, U.K.; Koopmann, K.; Bohnen, S.; Zeller, T.; Lund, G.; Krull, A.D.; Hauschild, N.; Stehning, C.; Adam, G.; et al. Myocardial Injury and Fibrogenesis: Extracellular Volume Expansion Is Associated with Elevated Galectin-3 Levels in Patients with Myocarditis. J. Cardiovasc. Magn. Reson. 2014, 16, P290. [Google Scholar] [CrossRef]
- De Boer, R.A.; Verweij, N.; Van Veldhuisen, D.J.; Westra, H.-J.; Bakker, S.J.L.; Gansevoort, R.T.; Muller Kobold, A.C.; Van Gilst, W.H.; Franke, L.; Leach, I.M.; et al. A Genome-Wide Association Study of Circulating Galectin-3. PLoS ONE 2012, 7, e47385. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhu, J.; Liu, Y.; Zhuang, L.; Zhang, Z.; Zhong, D.; Zhang, W.; Lai, D. Exploring the Causal Effects of Circulating ST2 and Galectin-3 on Heart Failure Risk: A Mendelian Randomization Study. Front. Cardiovasc. Med. 2022, 9, 868749. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, M.; Zhu, Z.; Cui, C. Galectin-3 and Peripheral Artery Disease: A Mendelian Randomization Study. Front. Cardiovasc. Med. 2024, 10, 1279396. [Google Scholar] [CrossRef]
- Henry, A.; Gordillo-Marañón, M.; Finan, C.; Schmidt, A.F.; Ferreira, J.P.; Karra, R.; Sundström, J.; Lind, L.; Ärnlöv, J.; Zannad, F.; et al. Therapeutic Targets for Heart Failure Identified Using Proteomics and Mendelian Randomization. Circulation 2022, 145, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.M.; Norwitz, E.R.; Kim, J.H.; Jung, Y.M.; Park, C.-W.; Jun, J.K.; Lee, D.; Jin, Y.; Kim, S.; et al. Placental Expression Quantitative Trait Loci in an East Asian Population. Hum. Genet. Genom. Adv. 2024, 5, 100276. [Google Scholar] [CrossRef]
- De Boer, R.A.; Van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; Van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; Van Der Harst, P. The Fibrosis Marker Galectin-3 and Outcome in the General Population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.R.; Agraval, H.; Yadav, U.C.S. Cigarette Smoke Induces Epithelial-to-Mesenchymal Transition, Stemness, and Metastasis in Lung Adenocarcinoma Cells via Upregulated RUNX-2/Galectin-3 Pathway. Life Sci. 2023, 318, 121480. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Bandyopadhyaya, G.; Polumuri, S.K.; Chumsri, S.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Nicotine Promotes Apoptosis Resistance of Breast Cancer Cells and Enrichment of Side Population Cells with Cancer Stem Cell-like Properties via a Signaling Cascade Involving Galectin-3, A9 Nicotinic Acetylcholine Receptor and STAT3. Breast Cancer Res. Treat. 2014, 145, 5–22. [Google Scholar] [CrossRef]
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Med. Sci. Monit. 2017, 23, 4612–4618. [Google Scholar] [CrossRef]
- Pei, C.; Wang, X.; Lin, Y.; Fang, L.; Meng, S. Inhibition of Galectin-3 Alleviates Cigarette Smoke Extract-Induced Autophagy and Dysfunction in Endothelial Progenitor Cells. Oxidative Med. Cell. Longev. 2019, 2019, 7252943. [Google Scholar] [CrossRef]
- Zhang, L.; Gallup, M.; Zlock, L.; Chen, Y.T.F.; Finkbeiner, W.E.; McNamara, N.A. Pivotal Role of MUC1 Glycosylation by Cigarette Smoke in Modulating Disruption of Airway Adherens Junctions in Vitro. J. Pathol. 2014, 234, 60–73. [Google Scholar] [CrossRef]
- Milanic, M.; Hren, R.; Stergar, J.; Simoncic, U. WITHDRAWAL Monitoring of Caffeine Consumption Effect on Skin Blood Properties by Diffuse Reflectance Spectroscopy. Physiol. Res. 2025, 74, 161. [Google Scholar] [CrossRef]
- Aslan, M.; Gürel, E.; Üremis, N.; Üremis, M.; Taslidere, E. Anti-Inflammatory and Antioxidative Effects of Dexpanthenol on Nicotine-Induced Lung Injury in Rats. Toxicol. Environ. Health Sci. 2023, 15, 303–313. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Zaaba, N.; Elzaki, O.; Pathan, A.; Ali, B. Waterpipe Smoke Inhalation Induces Lung Injury and Aortic Endothelial Dysfunction in Mice. Physiol. Res. 2023, 72, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Z.; Wang, L.; Li, J.; Cheng, X.; Tang, Y.; Xing, M.; Yao, P. Chronic High-Fat Diet Induces Galectin-3 and TLR4 to Activate NLRP3 Inflammasome in NASH. J. Nutr. Biochem. 2023, 112, 109217. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Ricci, C.; Fantauzzi, C.B.; Scipioni, A.; Salvi, L.; Cordone, S.; Delucchi, F.; Serino, M.; Federici, M.; et al. Galectin-3 Ablation Protects Mice from Diet-Induced NASH: A Major Scavenging Role for Galectin-3 in Liver. J. Hepatol. 2011, 54, 975–983. [Google Scholar] [CrossRef]
- Marín-Royo, G.; Gallardo, I.; Martínez-Martínez, E.; Gutiérrez, B.; Jurado-López, R.; López-Andrés, N.; Gutiérrez-Tenorio, J.; Rial, E.; Bartolomé, M.V.; Nieto, M.L.; et al. Inhibition of Galectin-3 Ameliorates the Consequences of Cardiac Lipotoxicity in a Rat Model of Diet-Induced Obesity. Dis. Models Mech. 2018, 11, dmm032086. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.; Silva, A.F.; Rodrigues, P.G.; Correia, E.; Moura, C.; Eloy, C.; Roncon-Albuquerque, R.; Falcão-Pires, I.; Leite-Moreira, A.F. Early Cardiac Changes Induced by a Hypercaloric Western-Type Diet in “Subclinical” Obesity. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H655–H666. [Google Scholar] [CrossRef] [PubMed]
- Pejnovic, N.N.; Pantic, J.M.; Jovanovic, I.P.; Radosavljevic, G.D.; Milovanovic, M.Z.; Nikolic, I.G.; Zdravkovic, N.S.; Djukic, A.L.; Arsenijevic, N.N.; Lukic, M.L. Galectin-3 Deficiency Accelerates High-Fat Diet–Induced Obesity and Amplifies Inflammation in Adipose Tissue and Pancreatic Islets. Diabetes 2013, 62, 1932–1944. [Google Scholar] [CrossRef]
- Wensvoort, G. Human C-Peptide Is a Ligand of the Elastin-Receptor-Complex and Therewith Central to Human Vascular Remodelling and Disease in Metabolic Syndrome. Med. Hypotheses 2022, 168, 110964. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; May, H.T.; Le, V.T.; Galenko, O.; Drakos, S.G.; Bair, T.L.; Knowlton, K.U.; Muhlestein, J.B. Intermittent Fasting and Changes in Galectin-3: A Secondary Analysis of a Randomized Controlled Trial of Disease-Free Subjects. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1538–1548. [Google Scholar] [CrossRef]
- Lee, J.; An, H.S.; Shin, H.J.; Jang, H.M.; Im, C.O.; Jeong, Y.; Eum, K.; Yoon, S.; Lee, S.J.; Jeong, E.A.; et al. Intermittent Fasting Reduces Neuroinflammation and Cognitive Impairment in High-Fat Diet-Fed Mice by Downregulating Lipocalin-2 and Galectin-3. Nutrients 2024, 16, 159. [Google Scholar] [CrossRef]
- Kim, K.E.; Shin, H.J.; Ju, Y.; Jung, Y.; An, H.S.; Lee, S.J.; Jeong, E.A.; Lee, J.; Hwang, G.-S.; Roh, G.S. Intermittent Fasting Attenuates Metabolic-Dysfunction-Associated Steatohepatitis by Enhancing the Hepatic Autophagy–Lysosome Pathway. Nutrients 2023, 15, 4574. [Google Scholar] [CrossRef]
- Bichara, M.; Attmane-Elakeb, A.; Brown, D.; Essig, M.; Karim, Z.; Muffat-Joly, M.; Micheli, L.; Eude-Le Parco, I.; Cluzeaud, F.; Peuchmaur, M.; et al. Exploring the Role of Galectin 3 in Kidney Function: A Genetic Approach. Glycobiology 2006, 16, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kröpfl, J.M.; Beltrami, F.G.; Gruber, H.; Schmidt-Trucksäss, A.; Dieterle, T.; Spengler, C.M. Circulating Gal-3 and sST2 Are Associated with Acute Exercise-induced Sustained Endothelial Activation: Possible Relevance for Fibrosis Development? Exp. Physiol. 2023, 108, 1259–1267. [Google Scholar] [CrossRef]
- Kaleta-Duss, A.M.; Lewicka-Potocka, Z.; Dąbrowska-Kugacka, A.; Raczak, G.; Lewicka, E. Myocardial Injury and Overload among Amateur Marathoners as Indicated by Changes in Concentrations of Cardiovascular Biomarkers. Int. J. Environ. Res. Public Health 2020, 17, 6191. [Google Scholar] [CrossRef]
- Vassalle, C.; Masotti, S.; Lubrano, V.; Basta, G.; Prontera, C.; Di Cecco, P.; Del Turco, S.; Sabatino, L.; Pingitore, A. Traditional and New Candidate Cardiac Biomarkers Assessed before, Early, and Late after Half Marathon in Trained Subjects. Eur. J. Appl. Physiol. 2018, 118, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Kaux, J.-F.; Farre Segura, J.; Stojkovic, V.; Ancion, A.; Seidel, L.; Lancellotti, P.; Cavalier, E. Evolution of the Slopes of ST2 and Galectin-3 during Marathon and Ultratrail Running Compared to a Control Group. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 314–321. [Google Scholar] [CrossRef]
- Lewicka-Potocka, Z.; Kaleta-Duss, A.M.; Lewicka, E.; Kubik, M.; Faran, A.; Szymeczko, P.; Gałąska, R.; Raczak, G.; Dąbrowska-Kugacka, A. Post-Marathon Decline in Right Ventricular Radial Motion Component Among Amateur Sportsmen. Front. Physiol. 2022, 12, 811764. [Google Scholar] [CrossRef]
- Trigiani, L.J.; Lacalle-Aurioles, M.; Bourourou, M.; Li, L.; Greenhalgh, A.D.; Zarruk, J.G.; David, S.; Fehlings, M.G.; Hamel, E. Benefits of Physical Exercise on Cognition and Glial White Matter Pathology in a Mouse Model of Vascular Cognitive Impairment and Dementia. Glia 2020, 68, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Trigiani, L.J.; Royea, J.; Tong, X.; Hamel, E. Comparative Benefits of Simvastatin and Exercise in a Mouse Model of Vascular Cognitive Impairment and Dementia. FASEB J. 2019, 33, 13280–13293. [Google Scholar] [CrossRef]
- Avazpour, S.; Amini, A.; Shirvani, H.; Arabzadeh, E. Exercise Modulation in Inflammation and Metabolic Hormonal Disorders of COVID-19 to Decrease Risk Factors in Coronary Heart Disease. Horm. Mol. Biol. Clin. Investig. 2023, 44, 199–206. [Google Scholar] [CrossRef]
- Keyhani, D.; Tartibian, B.; Dabiri, A.; Teixeira, A.M.B. Effect of High-Intensity Interval Training Versus Moderate-Intensity Aerobic Continuous Training on Galectin-3 Gene Expression in Postmenopausal Women: A Randomized Controlled Trial. J. Aging Phys. Act. 2020, 28, 987–995. [Google Scholar] [CrossRef]
- Ahmad, F.; Karim, A.; Khan, J.; Qaisar, R. Plasma Galectin-3 and H-FABP Correlate with Poor Physical Performance in Patients with Congestive Heart Failure. Exp. Biol. Med. (Maywood) 2023, 248, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, V.B.; Nery, G.; Fernandes, L.L.; Santana, T.V.; Jimenez, M.; Borges, L.; Hatanaka, E.; Braga, P.; Monteiro, F.R.; Amaral, J.B.; et al. Salivary Proteome, Inflammatory, and NETosis Biomarkers in Older Adult Practitioners and Nonpractitioners of Physical Exercise. Oxidative Med. Cell. Longev. 2022, 2022, 3725056. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Schena, F.; Gelati, M.; Danese, E.; Cervellin, G.; Guidi, G.C.; Lippi, G. The Concentration of High-Sensitivity Troponin I, Galectin-3 and NT-proBNP Substantially Increase after a 60-Km Ultramarathon. Clin. Chem. Lab. Med. (CCLM) 2014, 52, 267–272. [Google Scholar] [CrossRef]
- Billebeau, G.; Vodovar, N.; Sadoune, M.; Launay, J.-M.; Beauvais, F.; Cohen-Solal, A. Effects of a Cardiac Rehabilitation Programme on Plasma Cardiac Biomarkers in Patients with Chronic Heart Failure. Eur. J. Prev. Cardiol. 2017, 24, 1127–1135. [Google Scholar] [CrossRef]
- Fernandes-Silva, M.M.; Guimarães, G.V.; Rigaud, V.O.; Lofrano-Alves, M.S.; Castro, R.E.; De Barros Cruz, L.G.; Bocchi, E.A.; Bacal, F. Inflammatory Biomarkers and Effect of Exercise on Functional Capacity in Patients with Heart Failure: Insights from a Randomized Clinical Trial. Eur. J. Prev. Cardiol. 2017, 24, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Hättasch, R.; Spethmann, S.; De Boer, R.A.; Ruifrok, W.P.; Schattke, S.; Wagner, M.; Schroeckh, S.; Durmus, T.; Schimke, I.; Sanad, W.; et al. Galectin-3 Increase in Endurance Athletes. Eur. J. Prev. Cardiol. 2014, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhen, J.; Liu, S.; Ren, L.; Zhao, G.; Liang, J.; Xu, A.; Li, C.; Wu, J.; Cheung, B.M.Y. Association between Sleep Patterns and Galectin-3 in a Chinese Community Population. BMC Public. Health 2024, 24, 1323. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schäffler, A.; Aslanidis, C.; Schölmerich, J.; et al. Serum Galectin-3 Is Elevated in Obesity and Negatively Correlates with Glycosylated Hemoglobin in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef]
- Suthahar, N.; Meems, L.M.G.; Groothof, D.; Bakker, S.J.L.; Gansevoort, R.T.; Van Veldhuisen, D.J.; De Boer, R.A. Relationship between Body Mass Index, Cardiovascular Biomarkers and Incident Heart Failure. Eur. J. Heart Fail. 2021, 23, 396–402. [Google Scholar] [CrossRef]
- Florido, R.; Kwak, L.; Echouffo-Tcheugui, J.B.; Zhang, S.; Michos, E.D.; Nambi, V.; Goldberg, R.B.; Hoogeveen, R.C.; Lazo, M.; Gerstenblith, G.; et al. Obesity, Galectin-3, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e023238. [Google Scholar] [CrossRef]
- Zeytinli Aksit, M.; Demet Arslan, F.; Karakoyun, I.; Aydin, C.; Turgut, E.; Parildar, H.; Gokbalci, U.; Isbilen Basok, B.; Duman, C.; Emiroglu, M. Galectin-3 Levels and Inflammatory Response in Patients Undergoing Bariatric Surgery. Cytokine 2022, 151, 155793. [Google Scholar] [CrossRef]
- Fryk, E.; Rodrigues Silva, V.R.; Strindberg, L.; Strand, R.; Ahlström, H.; Michaëlsson, K.; Kullberg, J.; Lind, L.; Jansson, P.-A. Metabolic Profiling of Galectin-1 and Galectin-3: A Cross-Sectional, Multi-Omics, Association Study. Int. J. Obes. 2024, 48, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Wang, A.; Xu, T.; Zhong, C.; Zheng, X.; Zhu, Z.; Peng, Y.; Peng, H.; Li, Q.; Ju, Z.; et al. Co-Effect of Serum Galectin-3 and High-Density Lipoprotein Cholesterol on the Prognosis of Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tan, J.; Hong, Y. Effect of Inhibition of Galectin-3 Expression on Lipid Accumulation and Atherosclerosis Lesions in a Rat Model of Carotid Atherosclerosis. J. Biol. Regul. Homeost. Agents 2024, 38, 3215–3223. [Google Scholar] [CrossRef]
- Winter, M.; Wiesbauer, F.; Alimohammadi, A.; Blessberger, H.; Pavo, N.; Schillinger, M.; Huber, K.; Wojta, J.; Lang, I.M.; Maurer, G.; et al. Soluble Galectin-3 Is Associated with Premature Myocardial Infarction. Eur. J. Clin. Investig. 2016, 46, 386–391. [Google Scholar] [CrossRef]
- Melin, E.O.; Dereke, J.; Hillman, M. Female Sex, High Soluble CD163, and Low HDL-Cholesterol Were Associated with High Galectin-3 Binding Protein in Type 1 Diabetes. Biol. Sex. Differ. 2019, 10, 51. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 Induces Endothelial Cell Morphogenesis and Angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef]
- Pang, Z.-D.; Sun, X.; Bai, R.-Y.; Han, M.-Z.; Zhang, Y.-J.; Wu, W.; Zhang, Y.; Lai, B.-C.; Zhang, Y.; Wang, Y.; et al. YAP-Galectin-3 Signaling Mediates Endothelial Dysfunction in Angiotensin II-Induced Hypertension in Mice. Cell. Mol. Life Sci. 2023, 80, 38. [Google Scholar] [CrossRef]
- Hao, W.-R.; Cheng, C.-H.; Liu, J.-C.; Chen, H.-Y.; Chen, J.-J.; Cheng, T.-H. Understanding Galectin-3’s Role in Diastolic Dysfunction: A Contemporary Perspective. Life 2024, 14, 906. [Google Scholar] [CrossRef]
- Hsu, B.-G.; Wang, C.-H.; Lai, Y.-H.; Tsai, J.-P. Serum Galectin-3 Level Is Positively Associated with Endothelial Dysfunction in Patients with Chronic Kidney Disease Stage 3 to 5. Toxins 2021, 13, 532. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, D.; Chen, R.; Ying, C.; Wang, C.; Guo, J.; Zhang, G. Galectin-3 Predicts Left Ventricular Remodeling of Hypertension. J. Clin. Hypertens. 2016, 18, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L. Galectins in Endothelial Cell Biology and Angiogenesis: The Basics. Biomolecules 2021, 11, 1386. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Gluba-Brzózka, A.; Michalska-Kasiczak, M.; Misztal, M.; Rysz, J.; Banach, M. The Multi-Biomarker Approach for Heart Failure in Patients with Hypertension. Int. J. Mol. Sci. 2015, 16, 10715–10733. [Google Scholar] [CrossRef] [PubMed]
- Ianoș, R.D.; Cozma, A.; Lucaciu, R.L.; Hangan, A.C.; Negrean, V.; Mercea, D.C.; Ciulei, G.; Pop, C.; Procopciuc, L.M. Role of Circulating Biomarkers in Diabetic Cardiomyopathy. Biomedicines 2024, 12, 2153. [Google Scholar] [CrossRef]
- Lebedev, D.A.; Lyasnikova, E.A.; Vasilyeva, E.Y.; Likhonosov, N.P.; Sitnikova, M.Y.; Babenko, A.Y. Association between Markers of Fibrosis and Heart Failure Incidence in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9589185. [Google Scholar] [CrossRef]
- Flores-Ramírez, R.; Azpiri-López, J.R.; González-González, J.G.; Ordaz-Farías, A.; González-Carrillo, L.E.; Carrizales-Sepúlveda, E.F.; Vera-Pineda, R. Global Longitudinal Strain as a Biomarker in Diabetic Cardiomyopathy. A Comparative Study with Gal-3 in Patients with Preserved Ejection Fraction. Arch. Cardiol. México 2017, 87, 278–285. [Google Scholar] [CrossRef]
- Jin, Q.; Lou, Y.; Li, T.; Chen, H.; Liu, Q.; He, X. Serum Galectin-3: A Risk Factor for Vascular Complications in Type 2 Diabetes Mellitus. Chin. Med. J. 2013, 126, 2109–2115. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Cheung, C.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 and Risk of Cardiovascular Events and All-cause Mortality in Type 2 Diabetes. Diabetes Metab. Res. 2019, 35, e3093. [Google Scholar] [CrossRef]
- Kuzan, A.; Królewicz, E.; Kustrzeba-Wójcicka, I.; Lindner-Pawłowicz, K.; Sobieszczańska, M. How Diabetes and Other Comorbidities of Elderly Patients and Their Treatment Influence Levels of Glycation Products. Int. J. Environ. Res. Public Health 2022, 19, 7524. [Google Scholar] [CrossRef]
- Vora, A.; De Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef]
- Yilmaz, H.; Cakmak, M.; Inan, O.; Darcin, T.; Akcay, A. Increased Levels of Galectin-3 Were Associated with Prediabetes and Diabetes: New Risk Factor? J. Endocrinol. Investig. 2015, 38, 527–533. [Google Scholar] [CrossRef]
- Blasetti Fantauzzi, C.; Iacobini, C.; Menini, S.; Vitale, M.; Sorice, G.P.; Mezza, T.; Cinti, S.; Giaccari, A.; Pugliese, G. Galectin-3 Gene Deletion Results in Defective Adipose Tissue Maturation and Impaired Insulin Sensitivity and Glucose Homeostasis. Sci. Rep. 2020, 10, 20070. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Dávila-Román, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; De Las Fuentes, L.; Joseph, S.M.; Vader, J.; Hernandez, A.F.; et al. Cardiovascular Phenotype in HFpEF Patients With or Without Diabetes. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [CrossRef]
- Andersen, M.L.; Moyses-Oliveira, M.; Tufik, S. Unlocking the Role of Galectin-3: Implications for Sleep Disorders and Health. Sleep Med. 2024, 124, 110–114. [Google Scholar] [CrossRef]
- Pusuroglu, H.; Somuncu, U.; Bolat, I.; Akgul, O.; Ornek, V.; Yıldırım, H.A.; Akkaya, E.; Karakurt, H.; Yıldırım, A.; Savaş, A.U. Galectin-3 Is Associated with Coronary Plaque Burden and Obstructive Sleep Apnoea Syndrome Severity. Kardiol. Pol. 2017, 75, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hanis, C.L.; Redline, S.; Ballantyne, C.M.; Hamzeh, I.; Aguilar, D. Sleep Apnea and Galectin-3: Possible Sex-Specific Relationship. Sleep Breath. 2019, 23, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Amirkhani, N.; Sharifkashani, S.; Behnoush, A.H. Role of Galectin-3 as a Biomarker in Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Breath. 2023, 27, 2273–2282. [Google Scholar] [CrossRef]
- Zong, D.; Liu, X.; Shen, C.; Liu, T.; Ouyang, R. Involvement of Galectin-3 in Neurocognitive Impairment in Obstructive Sleep Apnea via Regulating Inflammation and Oxidative Stress through NLRP3. Sleep Med. 2023, 101, 1–10. [Google Scholar] [CrossRef]
| Goal | Health Goal Category | Definition | |
|---|---|---|---|
| HEALTH BEHAVIORS | Current smoking | POOR | Current |
| INTERMEDIATE | Former ≤ 12 months | ||
| IDEAL | Never or quit > 12 months | ||
| Healthy diet score | POOR | 0–1 components a | |
| INTERMEDIATE | 2–3 components a | ||
| IDEAL | 4–5 components a | ||
| Physical activity | POOR | Complete absence of weekly exercise | |
| INTERMEDIATE | Engaging in 1–149 min per week of moderate-intensity activity, or 1–74 min per week of vigorous-intensity exercise, or a combination of moderate and vigorous efforts not exceeding 149 min in total. | ||
| IDEAL | A weekly total of ≥150 min of moderate-intensity physical activity, or ≥75 min of vigorous-intensity exercise, or an equivalent combination of both intensity levels | ||
| Body mass index | POOR | ≥30 kg/m2 | |
| INTERMEDIATE | 25–29.9 kg/m2 | ||
| IDEAL | <25 kg/m2 | ||
| BIOLOGICAL METRICS | Total cholesterol | POOR | ≥240 mg/dL |
| INTERMEDIATE | 200–239 mg/dL or under effective pharmacological control c | ||
| IDEAL | <200 mg/dL b | ||
| Blood pressure | POOR | SBP ≥ 140 mmHg or DBP ≥ 90 mmHg | |
| INTERMEDIATE | SBP 120–139 mmHg or DBP 80–89 mmHg or under effective pharmacological control d | ||
| IDEAL | <120/<80 mmHg b | ||
| Fasting plasma glucose | POOR | ≥126 mg/dL | |
| INTERMEDIATE | 100–125 mg/dL or treated to goal e | ||
| IDEAL | <100 mg/dL b |
| Feature/Function | Description | Ref. |
|---|---|---|
| Secretion | Lacks a classical signal sequence; secreted via non-classical pathways (e.g., through exosomes) | [5,8,10] |
| Role in Apoptosis | Exhibits antiapoptotic activity—binds Bax, Bcl-2, CD95/Fas, stabilizes the mitochondrial membrane, and inhibits cytochrome c release | [25,26,27,28,29,30,31,32,33] |
| Influence on Proliferation | Overexpression in the cytoplasm promotes tumor growth, while nuclear expression may inhibit cell division | [34,35,36] |
| Nuclear Function | Plays a role in mRNA splicing and gene expression regulation | [8,37] |
| Interaction with Receptors | Can stabilize and regulate receptor activity (e.g., EGFR, integrins, immunoglobulins) | [8] |
| Cell Adhesion and Migration | Enhances intercellular and cell–matrix interactions by crosslinking glycoconjugates | [38,39,40] |
| Role in Angiogenesis | Stimulates blood vessel formation through interactions with receptors on endothelial cells | [41,42] |
| Immune Modulation | Binds bacterial LPS, facilitates phagocytosis, and regulates dendritic cell maturation as well as T lymphocyte apoptosis | [4] |
| Pro-inflammatory Effect | Activates macrophages and neutrophils, and recruits leukocytes | |
| Anti-inflammatory Effect | May induce T lymphocyte apoptosis and inhibit the secretion of pro-inflammatory cytokines | [5,43,44] |
| Galectin Network Formation | Oligomerization of gal-3 on the cell surface can organize membrane signaling microdomains via LLPS | [45,46] |
| Process | Description of the Mechanism | Ref. |
|---|---|---|
| Secretion by macrophages | In response to tissue injury, galectin-3 is released by macrophages and acts in a paracrine manner | [5] |
| Fibroblast proliferation | It stimulates fibroblasts to transform into myofibroblasts and to increase collagen synthesis | |
| Impact on the heart | It promotes myocardial fibrosis, leading to increased stiffness and cardiac dysfunction | |
| Clinical application | A potential biomarker of fibrosis and inflammation, particularly in HF | |
| Activation of pro-fibrotic pathways | It induces TGF-β, angiotensin II, and endothelin, thereby enhancing fibrosis | [49,50,51] |
| Inhibition of metalloproteinases | It limits extracellular matrix degradation, promoting collagen deposition | [52,53,54] |
| Phagocytosis of necrotic cells | It stimulates macrophages to clear damaged tissues, which may help reduce inflammation | [7] |
| Chronic inflammation | Prolonged exposure to galectin-3 may sustain macrophage activation and chronic inflammation | |
| Role in cancer | It promotes invasion and metastasis by enhancing cancer cell adhesion | [5,48] |
| Impact on other organs | Fibrosis in the kidneys, liver, and lungs is involved in the pathogenesis of diabetic nephropathy and idiopathic pulmonary fibrosis | [7,47] |
| Functioning as a DAMP/alarmin | Galectin-3 signals the presence of tissue damage by stimulating macrophages to release pro-inflammatory cytokines | [4] |
| Study Objective | Model of Exposure | Impact on Gal-3 Concentrations | Molecular Mechanisms | Biological Effects Associated with gal-3 | Ref. |
|---|---|---|---|---|---|
| Smoking vs. gal-3 levels | Population cohort | Weak negative correlation, not significant (p = 0.876) | Not evaluated | No independent effect of smoking | [74] |
| Role of gal-3 in EMT (cigarette smoke) | AECs + CSE (5%) | ↑ gal-3, ↑ RUNX-2 | ROS → RUNX-2 → gal-3↑; reversed by NAC, GB1107 | EMT (↓ E-cadherin, ↑ vimentin), ↑ migration, invasion | [75] |
| Nicotine and gal-3 in breast cancer | MCF-7 + nicotine (1–100 µM) | ↑ gal-3 (up to 5×) | α9nAChR → STAT3 → gal-3↑; mitochondrial stabilization | ↑ survival, migration, chemoresistance | [76] |
| Smoking and gal-3 in COPD | COPD patients during exacerbation/recovery | ↑ gal-3 in smokers vs. former/never smokers (p < 0.05) | Correlation: hsCRP, pro-BNP; smoking = independent predictor (β=0.458) | Inflammation, fibrosis, COPD progression | [77] |
| CSE and gal-3/autophagy | EPCs + CSE (8%) | ↑ gal-3 mRNA (6.7×), protein (5.9×) | ROS → gal-3 → AMPK↑, mTOR↓; reversed by gal-3 shRNA | ↑ autophagy, ↓ migration, ↓ angiogenesis | [78] |
| Gal-3 in MUC1-C/EGFR-dependent EMT | AECs + tobacco smoke | ↑ gal-3 in MUC1-C/EGFR complexes | Gal-3 stabilizes MUC1-C/EGFR; glycosylation-dependent | EMT, ↓ E-cadherin, Src/Jnk activation | [79] |
| Waterpipe smoke and gal-3 | Rats exposed to waterpipe smoke | ↑ gal-3 in aorta (p < 0.001) | ↑ TNF-α, IL-1β, VCAM-1, ICAM-1, NF-κB activation, ↓SIRT1 | Inflammation, endothelial dysfunction, DNA damage | [82] |
| Nicotine, DEX and gal-3 | Rats + nicotine (0.5 mg/kg) | ↑ gal-3 in lung (p < 0.001) | ↑ IL-1β, IL-6, MDA, TOS; ↓ SOD, GSH-Px; DEX reduces effect | Inflammation, oxidative stress, alveolar damage | [81] |
| Research Model/Population | Type of Dietary Intervention | Study Objective | Effect on Gal-3 and Related Pathways | Ref. |
|---|---|---|---|---|
| Mice on HFD + HepG2 cells | HFD; palmitic acid | Effect of HFD on gal-3, TLR4, NLRP3 in NASH | HFD: ↑ gal-3, TLR4, NLRP3; ↑ IL-1β, TNF-α, IL-6; suppression: β-lactose, TAK-242 | [83] |
| Rats fed HFD | HFD + MCP (gal-3 inhibitor) | Role of gal-3 in cardiac lipotoxicity | HFD: ↑ gal-3 (heart); MCP ↓ TG, LPC, oxidative stress, mitochondrial damage | [85] |
| Lgals3−/− mice on atherogenic diet | High-fat, high-cholesterol diet | Role of gal-3 in NASH pathogenesis | gal-3 absence → ↓ steatosis, inflammation, fibrosis; ↓ ALEs | [84] |
| Wistar rats on HCD | HCD (fats, sugars, salt) | Cardiac changes and gal-3 expression | HCD: ↑ gal-3; correlates with fibrosis, hypertrophy, inflammation | [86] |
| LGALS3−/− vs. WT mice on HFD | HFD | gal-3 role in adipose metabolism, insulin resistance | gal-3 deficiency: ↑ inflammation, insulin resistance; ↑ M1 macrophages, VAT | [87] |
| Animal models, literature review | Western diet, processed meat | Effect of dietary elastin on vascular remodeling | Elastin peptides may activate gal-3 pathways; no data for healthy diet | [88] |
| 67 participants (IF vs. control) | IF | Changes in gal-3 and metabolic markers | IF: ↑ gal-3; improved HOMA-IR, ↓ glucose, insulin | [89] |
| Mice on HFD ± IF | IF vs. HFD | IF effect on gal-3 and WAT inflammation | IF: ↓ gal-3 (serum, WAT); ↓ M1 macrophages, ↓ crown-like structures; ↑ insulin sensitivity | [90] |
| IF effect on gal-3 in liver | IF: ↓ gal-3 (liver); ↓ inflammation, macrophage activation, fibrosis (via LCN2, STAT3) | [91] |
| Population/Model | Type of Physical Activity | Study Objective | Effect on gal-3 | Conclusions | Ref. |
|---|---|---|---|---|---|
| Healthy, trained men (runners, cyclists) | Acute HIIT sessions | Assessment of the effect of HIIT on gal-3 concentration and endothelial markers | ↑ gal-3 by 39.5%; correlation with circulating endothelial cells | ↑ gal-3 as a response to endothelial stress; potential fibrosis-related mechanism | [93] |
| Amateur marathon runners | Marathon running | Evaluation of changes in cardiac biomarkers | ↑ gal-3 after the run (from 8.53 to 10.65 ng/mL), returned to baseline after 2 weeks | Transient ↑ gal-3 as an adaptive response | [94] |
| Trained runners | Half-marathon | Dynamics of cardiac biomarkers | ↑ gal-3 by 33%, normalized within 24 h | ↑ gal-3 reflects adaptation, not cardiac injury | [95] |
| Marathon runners, ultramarathon runners, 10 km runners | Running 42/67/10 km | Dynamics of gal-3 and ST2 after exercise | ↑ gal-3 after exertion, returned to baseline within 3 h | ↑ gal-3 after running but quickly returns to baseline | [96] |
| Amateur marathon runners | Marathon running | Assessment of changes in RV function and biomarkers | ↑ gal-3 after the run; correlated with ↓ RVEF and VO2max | gal-3 as a marker of cardiac stress and reduced performance | [97] |
| Mice (VCID model) | 3 h of daily activity | Assessment of neuroinflammatory mechanisms involving gal-3 | ↓ gal-3 expression in white matter | Exercise reduces neuroinflammatory gal-3 expression | [98] |
| TGF-β1+/+ mice on a HCD | Aerobic exercise | Effect of exercise and statins on neuroinflammation | ↓ gal-3 expression in microglia | Exercise ↓ gal-3-related neuroinflammatory pathways | [99] |
| Patients with CAD after COVID-19 | 8 weeks of HIIT or combined exercise | Evaluation of changes in inflammatory and metabolic markers | ↓ gal-3 in both training groups | Exercise ↓ gal-3; combined training is more effective | [100] |
| Postmenopausal women | HIIT vs. MIACT over 8 weeks | Gal-3 gene expression and lipid profile | ↓ gal-3 gene expression (HIIT: −94%, MIACT: −85%) | Physical activity ↓ gal-3 expression; HIIT shows stronger effect | [101] |
| Patients with CHF and healthy individuals | No physical intervention | Association of gal-3 with physical performance | ↑ gal-3 in patients with CHF; inverse correlation with SPPB and hand grip strength | ↑ gal-3 concentration associated with poorer physical performance | [102] |
| Older adults, physically active vs. inactive | Daily physical activity | Assessment of differences in salivary biomarkers | ↑ gal-3 binding protein in physically active individuals | Findings relate to LGALS3BP, not gal-3 itself | [103] |
| Trained athletes | 60 km ultramarathon | Dynamics of cardiac biomarkers | ↑ gal-3 ×2.4; decreased after 1 h | Transient ↑ gal-3 does not indicate permanent damage | [104] |
| Patients with CHF with LVEF ≤ 45% | 4–6 months of CR | Evaluation of changes in biomarkers after CR | ↓ gal-3 by 6.3% | CR ↓ gal-3; confirms anti-inflammatory effect of exercise | [105] |
| Patients with CHF with LVEF ≤ 40% | 12 weeks of aerobic training | Assessment of the effect of gal-3 on training response | ↑ gal-3 = no improvement in VO2 peak; ↓ gal-3 = improvement in VO2 peak | ↓ gal-3 predisposes to better training response | [106] |
| Marathon runners + mouse model | 30 km run + animal experiment | Source of gal-3 after exercise | ↑ gal-3 after the run; expression primarily in skeletal muscle | gal-3 originates mainly from skeletal muscle, not the heart—clinically important for interpretation | [107] |
| Study Objective | Study Type and Population | Quantitative Findings (Association with BMI/WC/VAT) | Conclusions Regarding Gal-3 and BMI/Obesity Relationship | Ref. |
|---|---|---|---|---|
| Assessment of the relationship between gal-3 and metabolic and sleep parameters | Cross-sectional; Chinese population, n = 904 | BMI: R = 0.07, p = 0.03; β = 0.04, p = 0.15; WC: R = 0.19, p < 0.001; β = 0.12, p = 0.005 | Stronger association with abdominal obesity than with overall BMI | [108] |
| Profile of gal-1 and -3 in relation to adipogenesis and insulin resistance | Cross-sectional; n = 502, POEM study, Sweden | Gal-3: β = 0.07, 95% CI: −0.01–0.16; p = 0.095 | Gal-3 does not significantly correlate with BMI or visceral/subcutaneous fat | [113] |
| Association between BMI, risk of HF, and gal-3 | Prospective cohort; n = 8687, ARIC, USA | OR for gal-3 ≥ 75th percentile: 2.32 (95% CI: 1.88–2.86; p < 0.001) for BMI ≥ 35 | Strong association between BMI and elevated gal-3; significantly increased HF risk | [111] |
| Gal-3 and inflammatory markers in individuals after bariatric surgery | Interventional; n = 100, Turkey; assessment 0–6 months post-surgery | BMI vs. gal-3: r = 0.375, p < 0.001; pre-surgery: 17.6 vs. 14.1 ng/mL (p = 0.016) | Gal-3 elevated in individuals with BMI ≥ 40; no reduction after surgery | [112] |
| Evaluation of cardiac biomarkers based on BMI and HF risk | Prospective cohort; n = 8202, PREVEND, Netherlands | Gal-3: 11.7 vs. 10.2 ng/mL (BMI ≥ 30 vs. < 25); p < 0.001 | Gal-3 increases with BMI but does not independently predict HF compared to other markers | [110] |
| Sources of gal-3 synthesis; association with T2DM and adipose tissue | Cross-sectional + tissue analysis; n = 83, Germany | BMI vs. gal-3: r = 0.357, p = 0.001; VAT > SAT; PVS > SVS > HVS | VAT is the main site of gal-3 synthesis; correlation with IL-6, leptin, and resistin | [109] |
| Biological Mechanism/Process | Clinical or Physiological Consequence | Type of Evidence/Research Model | Ref. |
|---|---|---|---|
| ↑ gal-3 in response to pro-inflammatory stimuli (angiotensin II, oxidized LDL, advanced glycation end products, interleukin-1β) | Activation of the inflammatory cascade and vascular remodeling | In vivo model (murine), in vitro | [119,120] |
| Activation of the Src pathway → YAP → gal-3 expression in the endothelium | Promotion of endothelial dysfunction, disturbances in vascular tone | HUVECs, murine model of hypertension | [119] |
| ↓ eNOS, ↓ NO, ↑ ROS, ↑ NADPH oxidase subunits NOX2/p47phox | Impaired vascular relaxation, oxidative stress, endothelial injury | Animal model + cellular studies | |
| ↑ Expression of VCAM-1, IL-6, CD68 | Localized vascular inflammation, recruitment of immune cells | Murine model + immunohistochemistry | |
| Interaction of gal-3 with integrins, modulation of VEGFR2, angiogenesis | Endothelial phenotype alteration, microcirculatory disturbances | In vitro (HUVEC), Matrigel model | [118,124] |
| Differentiation of VSMCs into an osteoblast-like phenotype | Vascular calcification, increased vascular stiffness | Studies on cell lines and atherosclerosis models (apolipoprotein E-deficient, Apoe−/−) | [120,123] |
| Interactions with the ECM, including hyaluronic acid and fibronectin | Vascular wall remodeling, loss of elasticity | Molecular studies, ECM analysis | |
| ↑ Production of type I and III collagen by VSMCs and myofibroblasts | Fibrosis of the vascular media, wall thickening | In vitro, histological studies of blood vessels | [123] |
| ↑ Arterial stiffness (pulse wave velocity, vascular remodeling) | ↑ vascular resistance, ↑ SBP | Clinical and experimental data | [122,123] |
| ↑ Afterload → LVH, HFpEF | Left ventricular remodeling, increased left ventricular mass | ECHO assessment + enzyme-linked immunosorbent assay (ELISA, clinical) | [122] |
| Study Objective | Population/Model | Results Regarding Glucose/HbA1c | Results Regarding Galectin-3 | Conclusions Regarding Glucose Concentration | Ref. |
|---|---|---|---|---|---|
| Review of the role of gal-3 in diabetic cardiomyopathy | Review article | No quantitative data | Gal-3 increases in diabetes and obesity | Relationship with glucose only suggested | [126] |
| Correlation of gal-3 with HFpEF in patients with T2DM treated with SGLT2i | 102 patients with T2DM | HbA1c 8.5% vs. 8.2%, p = 0.39 (no sig. difference) | Increased gal-3 concentration in patients with HFpEF (12.64 vs. 9.82 ng/mL; p = 0.012) | Gal-3 is an independent predictor of HFpEF, no correlation with glycemia | [127] |
| Gal-3 and subclinical cardiac dysfunction in T2DM | 121 individuals (T2DM + controls) | No correlation with HbA1c | Higher gal-3 in patients (p = 0.003) | Gal-3 is a marker of subclinical changes, not linked to current glycemia | [128] |
| Gal-3 and vascular complications in patients with T2DM | 284 patients with T2DM | HbA1c correlates with gal-3 (r = 0.217, p = 0.018); FBG–no sig. correlation | Higher gal-3 in the presence of complications | Gal-3 moderately correlates with HbA1c, not with FBG | [129] |
| Gal-3 and cardiovascular risk and mortality in T2DM | 1495 patients with T2DM | Higher HbA1c and glucose in deceased patients | Gal-3 weakly correlates with HbA1c (r = 0.06, p = 0.04) | Gal-3 is an independent risk predictor, weak correlation with HbA1c | [130] |
| Gal-3 and metabolic parameters in older adults | Geriatric population | HbA1c r = 0.267 (p = 0.031); glucose r = 0.39 (p < 0.0001) | Higher gal-3 in patients with T2DM, lower with metformin use | Gal-3 correlates with glucose and HbA1c, reduced by metformin | [131] |
| Gal-3 and the risk and development of T2DM | Dallas Heart Study, n > 3000 | FBG/HbA1c not reported | Gal-3 strongly associated with the presence and incidence of T2DM | Gal-3 is a risk marker for T2DM independent of BMI | [132] |
| Gal-3 and the HFpEF phenotype in patients with T2DM | 216 patients with HFpEF | No data on HbA1c/glucose | Higher gal-3 in patients with diabetes (p < 0.001) | Indirect association with hyperglycemia, no quantitative data | [135] |
| Gal-3 and HbA1c in patients with T2DM | 100 patients with T2DM | HbA1c inversely correlated with gal-3 (r = −0.323; p = 0.001) | Lower gal-3 with better glycemic control and metformin use | Gal-3 may reflect metabolic improvement | [109] |
| Gal-3 and prediabetes and newly diagnosed T2DM | 174 individuals (controls, prediabetes, T2DM) | FBG r = 0.787; OGTT 2h r = 0.833; HbA1c not reported | Gal-3 progressively increases from healthy individuals to T2DM | Strong correlation with glucose and HOMA-IR, good diagnostic marker | [133] |
| The role of gal-3 in glucose homeostasis–animal model | Lgal3−/− mice vs. WT | Impaired glucose tolerance, ↑FBG, ↓insulin | Absence of gal-3 = impaired insulin secretion and resistance | Gal-3 influences glucose metabolism independently of diet | [134] |
| Study Objective | Main Findings | Conclusions Regarding Gal-3 | Limitations | Ref. |
|---|---|---|---|---|
| Sleep quality/duration vs. gal-3 levels | Sleep disturbances correlated with ↑ gal-3 concentration (OR 1.68; 95% CI: 1.05–2.68); no association with sleep duration | Gal-3 reflects the impact of poor sleep quality on inflammatory and neuroinflammatory processes | Cross-sectional design; subjective sleep assessment | [108] |
| Mechanisms linking gal-3 with OSA and sleep disorders | ↑ gal-3 concentration in patients with OSA; gal-3 reduction after CPAP therapy | Gal-3 as a neuroinflammatory mediator and indicator of treatment effectiveness | No quantitative data; narrative review | [136] |
| gal-3 vs. OSA severity and coronary atherosclerosis | Gal-3 ↑ with OSA severity (p < 0.001); predictor of OSA severity (OR = 2.329) and atherosclerosis | Gal-3 as a biomarker of chronic inflammation and cardiovascular risk in OSA | No intervention data; cross-sectional design | [137] |
| gal-3 levels in OSA: sex differences | Significantly ↑ gal-3 concentration in women with moderate/severe OSA (p < 0.001); no correlation in men | Gal-3 as a potential indicator of cardiovascular risk in women with OSA | Uneven sex distribution; no causal data | [138] |
| gal-3 and neurocognitive consequences of OSA, review | Gal-3 associated with inflammation and microglial activation in OSA; gal-3 reduction after CPAP | Gal-3 as a marker of neuroinflammatory consequences of OSA and response to therapy | No quantitative data on sleep duration/quality | [139] |
| Impact of OSA and CPAP therapy on gal-3 and cognition | ↑ gal-3 concentration in patients with OSA; reduction after CPAP; correlation with AHI, hypoxia, and cognitive functions | Gal-3 as a link between sleep, neuroinflammation, and cognitive dysfunction | No data on the direct impact of sleep duration on gal-3 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martuszewski, A.; Paluszkiewicz, P.; Poręba, R.; Gać, P. Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks. Curr. Issues Mol. Biol. 2025, 47, 332. https://doi.org/10.3390/cimb47050332
Martuszewski A, Paluszkiewicz P, Poręba R, Gać P. Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks. Current Issues in Molecular Biology. 2025; 47(5):332. https://doi.org/10.3390/cimb47050332
Chicago/Turabian StyleMartuszewski, Adrian, Patrycja Paluszkiewicz, Rafał Poręba, and Paweł Gać. 2025. "Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks" Current Issues in Molecular Biology 47, no. 5: 332. https://doi.org/10.3390/cimb47050332
APA StyleMartuszewski, A., Paluszkiewicz, P., Poręba, R., & Gać, P. (2025). Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks. Current Issues in Molecular Biology, 47(5), 332. https://doi.org/10.3390/cimb47050332








