CRISPR-Cas9 in the Tailoring of Genetically Engineered Animals
Abstract
1. Introduction
2. CRISPR-Cas9 Delivery to Animal Cells
3. CRISPR-Cas9 in Animal Genome Editing
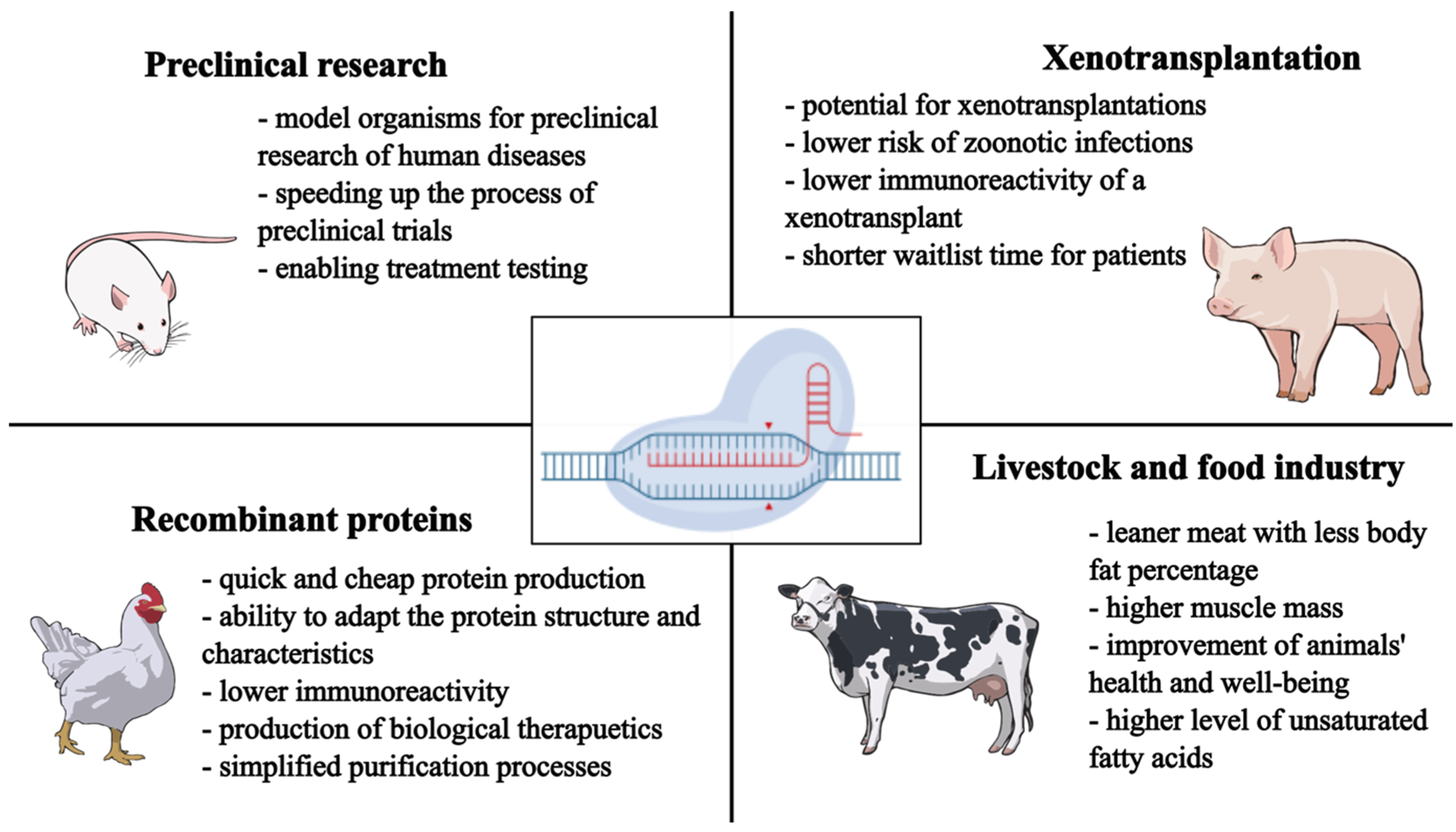
3.1. CRISPR-Cas9: Preclinical Animal Models
3.2. CRISPR-Cas: Livestock Improvement
3.3. CRISPR-Cas9: Production of Recombinant Proteins
3.4. CRISPR-Cas9 and Xenotransplantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-Gal | Galactose-α-1,3-galactose |
| AANAT | Arylalkylamine N-acetyltransferase |
| AAV | Adeno-associated virus |
| ASFV | African swine fever virus |
| ASMT | Serotonin N-acetyltransferase |
| AV | Adenovirus |
| BLG | β-Lactoglobulin |
| Cas9 | CRISPR-associated protein 9 |
| CD163 | Cluster of Differentiation 163 protein |
| CDK2 | Cyclin-dependent kinase 2 |
| CJEU | Court of Justice of the European Union |
| CMAH | Cytidine-monophospho-N-acetylneuramine |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| crRNA | CRISPR RNA |
| CSFV | Classical swine fever virus |
| CSN1S1 | Casein-alpha 1 |
| DMD | Duchenne muscular dystrophy |
| DNA | Deoxyribonucleic acid |
| DSB | Double-strand break |
| E-HEV | Hepatitis E virus |
| E2F1 | E2F Transcription Factor 1 |
| E7 | E7 protein |
| FAH | Fumarylacetoacetate hydrolase |
| FDA | Food and Drug Administration |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GMO | Genetically modified organism |
| HCC | Hepatocellular carcinoma |
| HDR | Homology-directed repair |
| HNH | Histidine–asparagine–histidine endonuclease domain |
| HPV | Human papilloma virus |
| HTI | Hereditary tyrosinemia type I |
| HTT | Huntingtin |
| IFNB1 | Interferon-β |
| IGA | Intentional genomic alteration |
| KI | Knock-in |
| KO | Knock-out |
| LALBA | α-Lactalbumin |
| LF | Lactoferrin |
| LV | Lentivirus |
| MARA | Ministry of Agriculture and Rural Affairs |
| MSTN | Myostatin |
| NHEJ | Nonhomologous end joining |
| NLS | Nuclear localization signal |
| NRAMP1 | Natural resistance-associated macrophage protein 1 |
| OVA | Ovalbumin |
| PAM | Protospacer adjacent motif |
| PBMC | Peripheral blood mononuclear cells |
| PCMV | Porcine cytomegalovirus |
| PERV | Porcine endogenous retrovirus |
| PGC1α | PPARγ coactivator 1α |
| PLHV | Porcine lymphotropic herpes virus |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PRNP | Cellular prion protein |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PRV | Pseudorabies virus |
| PUFA | Polyunsaturated fatty acid |
| RB | Retinoblastoma protein |
| RGEN | RNA-guided endonuclease |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| RuvC | Crossover junction endodeoxyribonuclease RuvC |
| SCNA | α-Synuclein |
| SCNT | Somatic cell nuclear transfer |
| SFAT-1 | Synthesized fatty acid desaturase-1 |
| sgRNA | Single-guide RNA |
| shRNA | Hairpin structure RNA |
| TALENs | Transcription activator-like effector nucleases |
| TLR22 | Toll-like receptor 22 |
| tracrRNA | Trans-activating CRISPR RNA |
| UPC1 | Uncoupling protein 1 |
| ZFNs | Zincfinger nucleases |
| α-Gal | Galactose-∝1,3-galactose |
References
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science (1979) 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-Guided Genetic Silencing Systems in Bacteria and Archaea. Nature 2012, 482, 331–338. [Google Scholar] [CrossRef]
- Lu, X.-J.; Ji, L.-J.; Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR-Cas9 Technology: Applications and Human Disease Modelling. Brief. Funct. Genom. 2017, 16, 4–12. [Google Scholar] [CrossRef]
- Hale, C.R.; Zhao, P.; Olson, S.; Duff, M.O.; Graveley, B.R.; Wells, L.; Terns, R.M.; Terns, M.P. RNA-Guided RNA Cleavage by a CRISPR RNA-Cas Protein Complex. Cell 2009, 139, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Perez Rojo, F.; Nyman, R.K.M.; Johnson, A.A.T.; Navarro, M.P.; Ryan, M.H.; Erskine, W.; Kaur, P. CRISPR-Cas Systems: Ushering in the New Genome Editing Era. Bioengineered 2018, 9, 214–221. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, X.; Zhang, Q.; Li, W.; Zu, Y. Comparison of Various Nuclear Localization Signal-Fused Cas9 Proteins and Cas9 MRNA for Genome Editing in Zebrafish. G3 Genes Genomes Genet. 2018, 8, 823–831. [Google Scholar] [CrossRef]
- Hendel, A.; Bak, R.O.; Clark, J.T.; Kennedy, A.B.; Ryan, D.E.; Roy, S.; Steinfeld, I.; Lunstad, B.D.; Kaiser, R.J.; Wilkens, A.B.; et al. Chemically Modified Guide RNAs Enhance CRISPR-Cas Genome Editing in Human Primary Cells. Nat. Biotechnol. 2015, 33, 985–989. [Google Scholar] [CrossRef]
- Allen, D.; Rosenberg, M.; Hendel, A. Using Synthetically Engineered Guide RNAs to Enhance CRISPR Genome Editing Systems in Mammalian Cells. Front. Genome Ed. 2021, 2, 617910. [Google Scholar] [CrossRef]
- Hall, B.; Cho, A.; Limaye, A.; Cho, K.; Khillan, J.; Kulkarni, A.B. Genome Editing in Mice Using CRISPR/Cas9 Technology. Curr. Protoc. Cell Biol. 2018, 81, e57. [Google Scholar] [CrossRef]
- Hossain, M.A. Chapter Two—CRISPR-Cas9: A Fascinating Journey from Bacterial Immune System to Human Gene Editing. In Progress in Molecular Biology and Translational Science; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 178, pp. 63–83. ISSN 1877-1173. [Google Scholar]
- Redman, M.; King, A.; Watson, C.; King, D. What Is CRISPR/Cas9? Arch. Dis. Child.-Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Huai, C.; Li, G.; Yao, R.; Zhang, Y.; Cao, M.; Kong, L.; Jia, C.; Yuan, H.; Chen, H.; Lu, D.; et al. Structural Insights into DNA Cleavage Activation of CRISPR-Cas9 System. Nat. Commun. 2017, 8, 1375. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of Nuclear Localization Signals and Mechanisms of Protein Import into the Nucleus. Cell Commun. Signal. 2021, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 Systems: Delivery Technologies and Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef]
- Tanihara, F.; Hirata, M.; Nguyen, N.T.; Le, Q.A.; Hirano, T.; Otoi, T. Effects of Concentration of CRISPR/Cas9 Components on Genetic Mosaicism in Cytoplasmic Microinjected Porcine Embryos. J. Reprod. Dev. 2019, 65, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Li, T.; Chen, Y.; Chen, J.; Zhang, X.; Zhang, J.; Zhang, Y.; Zhang, N.; Ma, R.; et al. Optimization of CRISPR/Cas9 Gene Editing System in Sheep (Ovis Aries) Oocytes via Microinjection. Int. J. Mol. Sci. 2025, 26, 1065. [Google Scholar] [CrossRef]
- Petersen, B.; Frenzel, A.; Lucas-Hahn, A.; Herrmann, D.; Hassel, P.; Klein, S.; Ziegler, M.; Hadeler, K.-G.; Niemann, H. Efficient Production of Biallelic 1 Knockout Pigs by Cytoplasmic Microinjection of CRISPR/Cas9 into Zygotes. Xenotransplantation 2016, 23, 338–346. [Google Scholar] [CrossRef]
- Davis, D.J.; Men, H.; Bryda, E.C. Electroporation-Mediated CRISPR/Cas9 Genome Editing in Rat Zygotes. In Transgenesis: Methods and Protocols; Saunders, T.L., Ed.; Springer US: New York, NY, USA, 2023; pp. 267–276. ISBN 978-1-0716-2990-1. [Google Scholar]
- Miao, D.; Giassetti, M.I.; Ciccarelli, M.; Lopez-Biladeau, B.; Oatley, J.M. Simplified Pipelines for Genetic Engineering of Mammalian Embryos by CRISPR-Cas9 Electroporation†. Biol. Reprod. 2019, 101, 177–187. [Google Scholar] [CrossRef]
- Qin, W.; Wang, H. Delivery of CRISPR-Cas9 into Mouse Zygotes by Electroporation. In Microinjection: Methods and Protocols; Liu, C., Du, Y., Eds.; Springer New York: New York, NY, USA, 2019; pp. 179–190. ISBN 978-1-4939-8831-0. [Google Scholar]
- Davis, D.J.; McNew, J.F.; Walls, J.N.; Bethune, C.E.; Oswalt, P.S.; Bryda, E.C. CRISPR-Cas9 Genome Editing of Rat Embryos Using Adeno-Associated Virus (AAV) and 2-Cell Embryo Electroporation. J. Vis. Exp. JoVE 2024, 205, e66069. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef]
- Fletcher, R.B.; Stokes, L.D.; Kelly, I.B., III.; Henderson, K.M.; Vallecillo-Viejo, I.C.; Colazo, J.M.; Wong, B.V.; Yu, F.; d’Arcy, R.; Struthers, M.N.; et al. Nonviral In Vivo Delivery of CRISPR-Cas9 Using Protein-Agnostic, High-Loading Porous Silicon and Polymer Nanoparticles. ACS Nano 2023, 17, 16412–16431. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.V.; Kaipa, B.R.; Ranshing, S.; Sundaresan, Y.; Millar, J.C.; Nagarajan, B.; Kiehlbauch, C.; Zhang, Q.; Jain, A.; Searby, C.C.; et al. Lentiviral Mediated Delivery of CRISPR/Cas9 Reduces Intraocular Pressure in a Mouse Model of Myocilin Glaucoma. Sci. Rep. 2024, 14, 6958. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, W.A.; Ark, J.; To, A.; Clouden, S.; Reeder, G.; Muldoon, J.J.; Chung, J.-Y.; Xie, W.H.; Allain, V.; Steinhart, Z.; et al. An Evolved AAV Variant Enables Efficient Genetic Engineering of Murine T Cells. Cell 2023, 186, 446–460.e19. [Google Scholar] [CrossRef]
- Li, Y.; Adur, M.K.; Wang, W.; Schultz, R.B.; Hale, B.; Wierson, W.; Charley, S.E.; McGrail, M.; Essner, J.; Tuggle, C.K.; et al. Effect of ARTEMIS (DCLRE1C) Deficiency and Microinjection Timing on Editing Efficiency during Somatic Cell Nuclear Transfer and in Vitro Fertilization Using the CRISPR/Cas9 System. Theriogenology 2021, 170, 107–116. [Google Scholar] [CrossRef]
- Sheets, T.P.; Park, C.-H.; Park, K.-E.; Powell, A.; Donovan, D.M.; Telugu, B.P. Somatic Cell Nuclear Transfer Followed by CRIPSR/Cas9 Microinjection Results in Highly Efficient Genome Editing in Cloned Pigs. Int. J. Mol. Sci. 2016, 17, 2031. [Google Scholar] [CrossRef]
- Kim, S.; No, J.-G.; Lee, S.; Choi, A.; Hyung, N.; Lee, J.Y.; Kwak, T.-U.; Ju, W.S.; Lee, J.-Y.; Lee, P.; et al. In Vitro Gene Editing Using Primary Cells Derived from Cas9-Expressing Pigs. J. Anim. Sci. Technol. 2025, 67, 179–192. [Google Scholar] [CrossRef]
- Laustsen, A.; Bak, R.O. Electroporation-Based CRISPR/Cas9 Gene Editing Using Cas9 Protein and Chemically Modified SgRNAs. In CRISPR Gene Editing: Methods and Protocols; Luo, Y., Ed.; Springer New York: New York, NY, USA, 2019; pp. 127–134. ISBN 978-1-4939-9170-9. [Google Scholar]
- Zhang, C.; Ren, Z.; Gong, Z. Transgenic Expression and Genome Editing by Electroporation of Zebrafish Embryos. Mar. Biotechnol. 2020, 22, 644–650. [Google Scholar] [CrossRef]
- Le, Q.A.; Tanihara, F.; Wittayarat, M.; Namula, Z.; Sato, Y.; Lin, Q.; Takebayashi, K.; Hirata, M.; Otoi, T. Comparison of the Effects of Introducing the CRISPR/Cas9 System by Microinjection and Electroporation into Porcine Embryos at Different Stages. BMC Res. Notes 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Li, Z.; Ali, A.; Quan, B.; Kang, J.; Ullah, M.; Yin, X.-J.; Shafiq, M. Comprehensive Transcriptomic Analysis of Myostatin-Knockout Pigs: Insights into Muscle Growth and Lipid Metabolism. Transgenic Res. 2025, 34, 12. [Google Scholar] [CrossRef]
- Tanihara, F.; Hirata, M.; Namula, Z.; Do, L.T.K.; Yoshimura, N.; Lin, Q.; Takebayashi, K.; Sakuma, T.; Yamamoto, T.; Otoi, T. Pigs with an INS Point Mutation Derived from Zygotes Electroporated with CRISPR/Cas9 and SsODN. Front. Cell Dev. Biol. 2023, 11, 884340. [Google Scholar] [CrossRef]
- Gim, G.-M.; Eom, K.-H.; Kwon, D.-H.; Jung, D.-J.; Kim, D.-H.; Yi, J.-K.; Ha, J.-J.; Lee, J.-H.; Lee, S.-B.; Son, W.-J.; et al. Generation of Double Knockout Cattle via CRISPR-Cas9 Ribonucleoprotein (RNP) Electroporation. J. Anim. Sci. Biotechnol. 2023, 14, 103. [Google Scholar] [CrossRef]
- Sakurai, T.; Takei, N.; Wei, Y.; Hayashi, M.; Kamiyoshi, A.; Kawate, H.; Watanabe, S.; Sato, M.; Shindo, T. Efficient Genome Editing of Two-Cell Mouse Embryos via Modified CRISPR/Cas Electroporation. Sci. Rep. 2024, 14, 30347. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nakagawa, Y. Genome Editing of Rodents by Electroporation of CRISPR/Cas9 into Frozen-Warmed Pronuclear-Stage Embryos. Cryobiology 2020, 92, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wu, S.J.; Li, Y.; Zhao, Y.; Liu, Z.M.; Deng, S.L.; Lian, Z.X. Improving the Efficiency of Precise Genome Editing with CRISPR/Cas9 to Generate Goats Overexpressing Human Butyrylcholinesterase. Cells 2023, 12, 1818. [Google Scholar] [CrossRef]
- Liu, C.; Xie, W.; Gui, C.; Du, Y. Pronuclear Microinjection and Oviduct Transfer Procedures for Transgenic Mouse Production. Methods Mol. Biol. 2013, 1027, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Schlapp, G.; Meikle, M.N.; Pórfido, J.L.; Menchaca, A.; Crispo, M. Zygote Cryobanking Applied to CRISPR/Cas9 Microinjection in Mice. PLoS ONE 2024, 19, e0306617. [Google Scholar] [CrossRef]
- Tian, H.; Niu, H.; Luo, J.; Yao, W.; Gao, W.; Wen, Y.; Cheng, M.; Lei, A.; Hua, J. Effects of CRISPR/Cas9-Mediated Stearoyl-Coenzyme A Desaturase 1 Knockout on Mouse Embryo Development and Lipid Synthesis. PeerJ 2022, 10, e13945. [Google Scholar] [CrossRef]
- Yang, D.; Liang, X.; Pallas, B.; Hoenerhoff, M.; Ren, Z.; Han, R.; Zhang, J.; Chen, Y.E.; Jin, J.-P.; Sun, F.; et al. Production of CFTR-ΔF508 Rabbits. Front. Genet. 2021, 11, 627666. [Google Scholar] [CrossRef]
- Gim, G.-M.; Kwon, D.-H.; Eom, K.-H.; Moon, J.; Park, J.-H.; Lee, W.-W.; Jung, D.-J.; Kim, D.-H.; Yi, J.-K.; Ha, J.-J.; et al. Production of MSTN-Mutated Cattle without Exogenous Gene Integration Using CRISPR-Cas9. Biotechnol. J. 2022, 17, 2100198. [Google Scholar] [CrossRef]
- Oh, J.-N.; Lee, M.; Choe, G.C.; Lee, D.-K.; Choi, K.-H.; Kim, S.-H.; Jeong, J.; Lee, C.-K. The Number of Primitive Endoderm Cells in the Inner Cell Mass Is Regulated by Platelet-Derived Growth Factor Signaling in Porcine Preimplantation Embryos. Anim. Biosci. 2023, 36, 1180–1189. [Google Scholar] [CrossRef]
- Kalds, P.; Crispo, M.; Li, C.; Tesson, L.; Anegón, I.; Chen, Y.; Wang, X.; Menchaca, A. Generation of Double-Muscled Sheep and Goats by CRISPR/Cas9-Mediated Knockout of the Myostatin Gene. Methods Mol. Biol. 2022, 2495, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Rasys, A.M.; Park, S.; Ball, R.E.; Alcala, A.J.; Lauderdale, J.D.; Menke, D.B. CRISPR-Cas9 Gene Editing in Lizards through Microinjection of Unfertilized Oocytes. Cell Rep. 2019, 28, 2288–2292.e3. [Google Scholar] [CrossRef]
- Chen, S.; Jiao, Y.; Pan, F.; Guan, Z.; Cheng, S.H.; Sun, D. Knock-In of a Large Reporter Gene via the High-Throughput Microinjection of the CRISPR/Cas9 System. IEEE Trans. Biomed. Eng. 2022, 69, 2524–2532. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Zhang, Y.; Liu, Z.; Li, J. Protocol for Conditional Mutagenesis in Zebrafish Germ Cells Using Tol2 Transposon and a CRISPR-Cas9-Based Plasmid System. STAR Protoc. 2025, 6, 103516. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.; Alston, V.; Su, B.; Khalil, K.; Elaswad, A.; Khan, M.; Johnson, A.; Xing, D.; Li, S.; Wang, J.; et al. Improved Growth and High Inheritance of Melanocortin-4 Receptor (Mc4r) Mutation in CRISPR/Cas-9 Gene-Edited Channel Catfish, Ictalurus punctatus. Mar. Biotechnol. 2022, 24, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; et al. Low Immunogenicity of LNP Allows Repeated Administrations of CRISPR-Cas9 MRNA into Skeletal Muscle in Mice. Nat. Commun. 2021, 12, 7101. [Google Scholar] [CrossRef]
- Han, J.P.; Kim, M.; Choi, B.S.; Lee, J.H.; Lee, G.S.; Jeong, M.; Lee, Y.; Kim, E.-A.; Oh, H.-K.; Go, N.; et al. In Vivo Delivery of CRISPR-Cas9 Using Lipid Nanoparticles Enables Antithrombin Gene Editing for Sustainable Hemophilia A and B Therapy. Sci. Adv. 2022, 8, eabj6901. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, W.; Song, T.; Song, M.; Liu, S.; Zhou, J.; Cheng, H.; Ding, Y. Lipid-Polymer Nanoparticles Mediate Compartmentalized Delivery of Cas9 and SgRNA for Glioblastoma Vasculature and Immune Reprogramming. Adv. Sci. 2024, 11, 2309314. [Google Scholar] [CrossRef]
- Walther, J.; Porenta, D.; Wilbie, D.; Seinen, C.; Benne, N.; Yang, Q.; de Jong, O.G.; Lei, Z.; Mastrobattista, E. Comparative Analysis of Lipid Nanoparticle-Mediated Delivery of CRISPR-Cas9 RNP versus MRNA/SgRNA for Gene Editing in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2024, 196, 114207. [Google Scholar] [CrossRef]
- Chen, X.; Gonçalves, M.A.F. V Engineered Viruses as Genome Editing Devices. Mol. Ther. 2016, 24, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.; Bozoglu, T.; Kupatt, C. AAV-Mediated Somatic Gene Editing for Cardiac and Skeletal Muscle in a Large Animal Model. In Cardiac Gene Therapy: Methods and Protocols; Ishikawa, K., Ed.; Springer US: New York, NY, USA, 2022; pp. 63–74. ISBN 978-1-0716-2707-5. [Google Scholar]
- Hakim, C.H.; Kumar, S.R.P.; Pérez-López, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; et al. Cas9-Specific Immune Responses Compromise Local and Systemic AAV CRISPR Therapy in Multiple Dystrophic Canine Models. Nat. Commun. 2021, 12, 6769. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Nakatsukasa, E.; Natsume, R.; Hamada, S.; Sakimura, K.; Watabe, A.M.; Ohtsuka, T. A Novel Technique for Large-Fragment Knock-in Animal Production without Ex Vivo Handling of Zygotes. Sci. Rep. 2023, 13, 2245. [Google Scholar] [CrossRef]
- Akter, M.S.; Hada, M.; Shikata, D.; Watanabe, G.; Ogura, A.; Matoba, S. CRISPR/Cas9-Based Genetic Screen of SCNT-Reprogramming Resistant Genes Identifies Critical Genes for Male Germ Cell Development in Mice. Sci. Rep. 2021, 11, 15438. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, B.; Zhou, J.; Zhu, H.; Niu, Y.; Ma, B.; Yu, H.; Lei, A.; Yan, H.; Shen, Q.; et al. Correction: Disruption of FGF5 in Cashmere Goats Using CRISPR/Cas9 Results in More Secondary Hair Follicles and Longer Fibers. PLoS ONE 2016, 11, e0167322. [Google Scholar] [CrossRef]
- Ryczek, N.; Hryhorowicz, M.; Zeyland, J.; Lipiński, D.; Słomski, R. CRISPR/Cas Technology in Pig-to-Human Xenotransplantation Research. Int. J. Mol. Sci. 2021, 22, 3196. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Burlak, C.; Estrada, J.L.; Li, P.; Tector, M.F.; Tector, A.J. Erythrocytes from GGTA1/CMAH Knockout Pigs: Implications for Xenotransfusion and Testing in Non-Human Primates. Xenotransplantation 2014, 21, 376–384. [Google Scholar] [CrossRef]
- Puga Yung, G.; Bongoni, A.K.; Pradier, A.; Madelon, N.; Papaserafeim, M.; Sfriso, R.; Ayares, D.L.; Wolf, E.; Klymiuk, N.; Bähr, A.; et al. Release of Pig Leukocytes and Reduced Human NK Cell Recruitment during Ex Vivo Perfusion of HLA-E/Human CD46 Double-Transgenic Pig Limbs with Human Blood. Xenotransplantation 2018, 25, e12357. [Google Scholar] [CrossRef]
- Aristizabal, A.M.; Caicedo, L.A.; Martínez, J.M.; Moreno, M.; Echeverri, G.J. Clinical Xenotransplantation, a Closer Reality: Literature Review. Cirugía Española 2017, 95, 62–72. [Google Scholar] [CrossRef]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The Current State of Animal Models in Research: A Review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Kues, W.A.; Niemann, H. The Contribution of Farm Animals to Human Health. Trends Biotechnol. 2004, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.; Tammela, T.; Cetinbas, N.M.; Akkad, A.; Roghanian, A.; Rickelt, S.; Almeqdadi, M.; Wu, K.; Oberli, M.A.; Sánchez-Rivera, F.J.; et al. In Vivo Genome Editing and Organoid Transplantation Models of Colorectal Cancer and Metastasis. Nat. Biotechnol. 2017, 35, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cui, W.; Jiang, X.; Zhang, Z.; Gnosa, S.; Ali, Z.; Jensen, L.; Jönsson, J.-I.; Blockhuys, S.; Lam, E.W.-F.; et al. The Critical Role of Dysregulated RhoB Signaling Pathway in Radioresistance of Colorectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Elkhadragy, L.; Carlino, M.J.; Jordan, L.R.; Pennix, T.; Ismail, N.; Guzman, G.; Samuelson, J.P.; Schook, L.B.; Schachtschneider, K.M.; Gaba, R.C. Development of a Genetically Tailored Implantation Hepatocellular Carcinoma Model in Oncopigs by Somatic Cell CRISPR Editing. Dis. Model. Mech. 2025, 18, dmm052079. [Google Scholar] [CrossRef]
- Gao, C.; Wu, P.; Yu, L.; Liu, L.; Liu, H.; Tan, X.; Wang, L.; Huang, X.; Wang, H. The Application of CRISPR/Cas9 System in Cervical Carcinogenesis. Cancer Gene Ther. 2022, 29, 466–474. [Google Scholar] [CrossRef]
- Couce, M.L.; Sánchez-Pintos, P.; Aldámiz-Echevarría, L.; Vitoria, I.; Navas, V.; Martín-Hernández, E.; García-Volpe, C.; Pintos, G.; Peña-Quintana, L.; Hernández, T.; et al. Evolution of Tyrosinemia Type 1 Disease in Patients Treated with Nitisinone in Spain. Medicine 2019, 98, e17303. [Google Scholar] [CrossRef]
- Das, A.M.; Ballhausen, D.; Haas, D.; Häberle, J.; Hagedorn, T.; Janson-Mutsaerts, C.; Janzen, N.; Sander, J.; Freisinger, P.; Karall, D.; et al. Diagnosis, Treatment, Management and Monitoring of Patients with Tyrosinaemia Type 1: Consensus Group Recommendations from the German-Speaking Countries. J. Inherit. Metab. Dis. 2025, 48, e12824. [Google Scholar] [CrossRef]
- Yin, H.; Xue, W.; Chen, S.; Bogorad, R.L.; Benedetti, E.; Grompe, M.; Koteliansky, V.; Sharp, P.A.; Jacks, T.; Anderson, D.G. Genome Editing with Cas9 in Adult Mice Corrects a Disease Mutation and Phenotype. Nat. Biotechnol. 2014, 32, 551–553. [Google Scholar] [CrossRef]
- Yang, S.; Chang, R.; Yang, H.; Zhao, T.; Hong, Y.; Kong, H.E.; Sun, X.; Qin, Z.; Jin, P.; Li, S.; et al. CRISPR/Cas9-Mediated Gene Editing Ameliorates Neurotoxicity in Mouse Model of Huntington’s Disease. J. Clin. Investig. 2017, 127, 2719–2724. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Kang, Y.; Yang, W.; Niu, Y.; Guo, X.; Tu, Z.; Si, C.; Wang, H.; Xing, R.; et al. Functional Disruption of the Dystrophin Gene in Rhesus Monkey Using CRISPR/Cas9. Hum. Mol. Genet. 2015, 24, 3764–3774. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Zhong, Y.-Z.; Ge, Y.-W.; Lu, K.-H.; Lu, S.-S. CRISPR/Cas9-Mediated Generation of Guangxi Bama Minipigs Harboring Three Mutations in α-Synuclein Causing Parkinson’s Disease. Sci. Rep. 2018, 8, 12420. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, J.; Huang, J.; Zhang, H.; Zhang, R.; Zhang, X.; Cao, C.; Hambly, C.; Qin, G.; Yao, J.; et al. Reconstitution of UCP1 Using CRISPR/Cas9 in the White Adipose Tissue of Pigs Decreases Fat Deposition and Improves Thermogenic Capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E9474–E9482. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, K.M.; Lee, K.; Benne, J.A.; Beaton, B.P.; Spate, L.D.; Murphy, S.L.; Samuel, M.S.; Mao, J.; O’Gorman, C.; Walters, E.M.; et al. Use of the CRISPR/Cas9 System to Produce Genetically Engineered Pigs from in Vitro-Derived Oocytes and Embryos. Biol. Reprod. 2014, 91, 78. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-Edited Pigs Are Protected from Porcine Reproductive and Respiratory Syndrome Virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Xie, Z.; Pang, D.; Yuan, H.; Jiao, H.; Lu, C.; Wang, K.; Yang, Q.; Li, M.; Chen, X.; Yu, T.; et al. Genetically Modified Pigs Are Protected from Classical Swine Fever Virus. PLoS Pathog. 2018, 14, e1007193. [Google Scholar] [CrossRef] [PubMed]
- Zezhong, Z.; Lei, X.; Yangbin, G.; Hongwei, D.; Yixuan, Z.; Xu, F.; Xiangjun, H.; Zhen, T.; Lingling, S.; Guolong, M.; et al. Testing Multiplexed Anti-ASFV CRISPR-Cas9 in Reducing African Swine Fever Virus. Microbiol. Spectr. 2024, 12, e02164-23. [Google Scholar] [CrossRef]
- Bevacqua, R.J.; Fernandez-Martín, R.; Savy, V.; Canel, N.G.; Gismondi, M.I.; Kues, W.A.; Carlson, D.F.; Fahrenkrug, S.C.; Niemann, H.; Taboga, O.A.; et al. Efficient Edition of the Bovine PRNP Prion Gene in Somatic Cells and IVF Embryos Using the CRISPR/Cas9 System. Theriogenology 2016, 86, 1886–1896.e1. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, H.; Wang, Y.; Liu, X.; Chen, L.; Li, Q.; Cui, C.; Liu, X.; Zhang, J.; Zhang, Y. Single Cas9 Nickase Induced Generation of NRAMP1 Knockin Cattle with Reduced Off-Target Effects. Genome Biol. 2017, 18, 13. [Google Scholar] [CrossRef]
- Singh, P.; Ali, S.A. Impact of CRISPR-Cas9-Based Genome Engineering in Farm Animals. Vet. Sci. 2021, 8, 122. [Google Scholar] [CrossRef]
- Chakrapani, V.; Patra, S.K.; Panda, R.P.; Rasal, K.D.; Jayasankar, P.; Barman, H.K. Establishing Targeted Carp TLR22 Gene Disruption via Homologous Recombination Using CRISPR/Cas9. Dev. Comp. Immunol. 2016, 61, 242–247. [Google Scholar] [CrossRef]
- Chakrapani, V.; Rasal, K.D.; Kumar, S.; Mohapatra, S.D.; Sundaray, J.K.; Jayasankar, P.; Barman, H.K. In Silico Analysis of NsSNPs of Carp TLR22 Gene Affecting Its Binding Ability with Poly I:C. Interdiscip. Sci. 2018, 10, 641–652. [Google Scholar] [CrossRef]
- Li, H.; Yang, G.; Ma, F.; Li, T.; Yang, H.; Rombout, J.H.W.M.; An, L. Molecular Characterization of a Fish-Specific Toll-like Receptor 22 (TLR22) Gene from Common Carp (Cyprinus carpio L.): Evolutionary Relationship and Induced Expression upon Immune Stimulants. Fish Shellfish Immunol. 2017, 63, 74–86. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, Y.; Guo, R.; Deng, M.; Deng, K.; Wang, Z.; Zhang, Y.; Wang, F. Generation of Beta-Lactoglobulin Knock-out Goats Using CRISPR/Cas9. PLoS ONE 2017, 12, e0186056. [Google Scholar] [CrossRef]
- Wang, K.; Tang, X.; Xie, Z.; Zou, X.; Li, M.; Yuan, H.; Guo, N.; Ouyang, H.; Jiao, H.; Pang, D. CRISPR/Cas9-Mediated Knockout of Myostatin in Chinese Indigenous Erhualian Pigs. Transgenic Res. 2017, 26, 799–805. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Z.; Xu, K.; Wu, T.; Ruan, J.; Zheng, X.; Bao, S.; Mu, Y.; Sonstegard, T.; Li, K. Long-Term, Multidomain Analyses to Identify the Breed and Allelic Effects in MSTN-Edited Pigs to Overcome Lameness and Sustainably Improve Nutritional Meat Production. Sci. China Life Sci. 2022, 65, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhou, Y.; Yang, J.; Li, J.; Peng, Y.; Zhang, X.; Miao, Y.; Jiang, W.; Bu, G.; Hou, L.; et al. Targeted Overexpression of PPARγ in Skeletal Muscle by Random Insertion and CRISPR/Cas9 Transgenic Pig Cloning Enhances Oxidative Fiber Formation and Intramuscular Fat Deposition. FASEB J. 2021, 35, e21308. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D.; et al. Generation of Myostatin Gene-Edited Channel Catfish (Ictalurus punctatus) via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef] [PubMed]
- Segev-Hadar, A.; Slosman, T.; Rozen, A.; Sherman, A.; Cnaani, A.; Biran, J. Genome Editing Using the CRISPR-Cas9 System to Generate a Solid-Red Germline of Nile Tilapia (Oreochromis niloticus). CRISPR J. 2021, 4, 583–594. [Google Scholar] [CrossRef]
- Cano-Garrido, O.; Rueda, F.L.; Sànchez-García, L.; Ruiz-Ávila, L.; Bosser, R.; Villaverde, A.; García-Fruitós, E. Expanding the Recombinant Protein Quality in Lactococcus Lactis. Microb. Cell Factories 2014, 13, 167. [Google Scholar] [CrossRef]
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant Pharmaceuticals from Microbial Cells: A 2015 Update. Microb. Cell Factories 2016, 15, 33. [Google Scholar] [CrossRef]
- He, Z.; Lu, R.; Zhang, T.; Jiang, L.; Zhou, M.; Wu, D.; Cheng, Y. A Novel Recombinant Human Plasminogen Activator: Efficient Expression and Hereditary Stability in Transgenic Goats and in Vitro Thrombolytic Bioactivity in the Milk of Transgenic Goats. PLoS ONE 2018, 13, e0201788. [Google Scholar] [CrossRef]
- Simons, J.P.; McClenaghan, M.; Clark, A.J. Alteration of the Quality of Milk by Expression of Sheep β-Lactoglobulin in Transgenic Mice. Nature 1987, 328, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gao, Y.; Li, G.; Xiong, Y.; Zhao, C.; Ruan, J.; Ma, Y.; Li, X.; Li, C.; Zhao, S.; et al. Enhancing the Antibacterial Activities of Sow Milk via Site-Specific Knock-in of a Lactoferrin Gene in Pigs Using CRISPR/Cas9 Technology. Cell Biosci. 2020, 10, 133. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Kontsevaya, G.V.; Shnaider, T.A.; Yunusova, A.M.; Feofanova, N.A.; Gerlinskaya, L.A.; Serova, I.A.; Serov, O.L.; Battulin, N.R. Evaluation of the α-Casein (CSN1S1) Locus as a Potential Target for a Site-Specific Transgene Integration. Sci. Rep. 2022, 12, 7983. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tao, J.; Yang, M.; He, C.; Tian, X.; Zhang, X.; Zhang, J.; Deng, S.; Feng, J.; Zhang, Z.; et al. An AANAT/ASMT Transgenic Animal Model Constructed with CRISPR/Cas9 System Serving as the Mammary Gland Bioreactor to Produce Melatonin-Enriched Milk in Sheep. J. Pineal Res. 2017, 63, e12406. [Google Scholar] [CrossRef]
- Oishi, I.; Yoshii, K.; Miyahara, D.; Tagami, T. Efficient Production of Human Interferon Beta in the White of Eggs from Ovalbumin Gene-Targeted Hens. Sci. Rep. 2018, 8, 10203. [Google Scholar] [CrossRef] [PubMed]
- Mukae, T.; Okumura, S.; Watanobe, T.; Yoshii, K.; Tagami, T.; Oishi, I. Production of Recombinant Monoclonal Antibodies in the Egg White of Gene-Targeted Transgenic Chickens. Genes 2021, 12, 38. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation—The Current Status and Prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef]
- Princeteau, M. Greffe Renale. J. Med. Bordx. 1905, 26, 549–555. [Google Scholar]
- Houdebine, L.M. Use of Transgenic Animals to Improve Human Health and Animal Production. Reprod. Domest. Anim. 2005, 40, 269–281. [Google Scholar] [CrossRef]
- Sykes, M.; Sachs, D.H. Transplanting Organs from Pigs to Humans. Sci. Immunol. 2019, 4, eaau6298. [Google Scholar] [CrossRef] [PubMed]
- Iwanczyk, Z.; Vasudev, K.; Cozzi, E.; Cooper, D.K.C. Contributions of Europeans to Xenotransplantation Research: 1. Pig Organ Xenotransplantation. Transplant Int. 2025, 38, 14041. [Google Scholar] [CrossRef] [PubMed]
- Huai, G.; Wang, Y.; Du, J.; Cheng, Z.; Xie, Y.; Zhou, J.; Tang, H.; Jiang, Y.; Xing, X.; Deng, S.; et al. The Generation and Evaluation of TKO/HCD55/HTM/HEPCR Gene-Modified Pigs for Clinical Organ Xenotransplantation. Front. Immunol. 2025, 15, 1488552. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of Human and Non-Human Primate Antibody Binding to Pig Cells Lacking GGTA1/CMAH/Β4GalNT2 Genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, C.; Xiang, X.; Li, Y.; Zhao, Y.; Li, Z.; Pan, D.; Dai, Y.; Hara, H.; Cooper, D.K.C.; et al. Production of A1,3-Galactosyltransferase and Cytidine Monophosphate-N-Acetylneuraminic Acid Hydroxylase Gene Double-Deficient Pigs by CRISPR/Cas9 and Handmade Cloning. J. Reprod. Dev. 2017, 63, 17–26. [Google Scholar] [CrossRef]
- Yang, L.; Güell, M.; Niu, D.; George, H.; Lesha, E.; Grishin, D.; Aach, J.; Shrock, E.; Xu, W.; Poci, J.; et al. Genome-Wide Inactivation of Porcine Endogenous Retroviruses (PERVs). Science 2015, 350, 1101–1104. [Google Scholar] [CrossRef]
- Douglas, C.; Maciulyte, V.; Zohren, J.; Snell, D.M.; Mahadevaiah, S.K.; Ojarikre, O.A.; Ellis, P.J.I.; Turner, J.M.A. CRISPR-Cas9 Effectors Facilitate Generation of Single-Sex Litters and Sex-Specific Phenotypes. Nat. Commun. 2021, 12, 6926. [Google Scholar] [CrossRef]
- Caplan, A.L.; Parent, B.; Shen, M.; Plunkett, C. No Time to Waste—The Ethical Challenges Created by CRISPR: CRISPR/Cas, Being an Efficient, Simple, and Cheap Technology to Edit the Genome of Any Organism, Raises Many Ethical and Regulatory Issues beyond the Use to Manipulate Human Germ Line Cells. EMBO Rep. 2015, 16, 1421–1426. [Google Scholar] [CrossRef]
- Ayanoğlu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical Issues in Genome Editing by CRISPR-Cas9 Technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-Target Effects in CRISPR/Cas9-Mediated Genome Engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Höijer, I.; Emmanouilidou, A.; Östlund, R.; van Schendel, R.; Bozorgpana, S.; Tijsterman, M.; Feuk, L.; Gyllensten, U.; den Hoed, M.; Ameur, A. CRISPR-Cas9 Induces Large Structural Variants at on-Target and off-Target Sites in Vivo That Segregate across Generations. Nat. Commun. 2022, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Wang, C.; Lee, H.K.; Yoo, K.H.; Zeng, X.; Kuhns, T.; Yang, C.M.; Mohr, T.; Liu, C.; Hennighausen, L. CRISPR/Cas9 Targeting Events Cause Complex Deletions and Insertions at 17 Sites in the Mouse Genome. Nat. Commun. 2017, 8, 15464. [Google Scholar] [CrossRef] [PubMed]
- Korablev, A.; Lukyanchikova, V.; Serova, I.; Battulin, N. On-Target CRISPR/CAS9 Activity Can Cause Undesigned Large Deletion in Mouse Zygotes. Int. J. Mol. Sci. 2020, 21, 3604. [Google Scholar] [CrossRef]
- Bortesi, L.; Zhu, C.; Zischewski, J.; Perez, L.; Bassié, L.; Nadi, R.; Forni, G.; Lade, S.B.; Soto, E.; Jin, X.; et al. Patterns of CRISPR/Cas9 Activity in Plants, Animals and Microbes. Plant Biotechnol. J. 2016, 14, 2203–2216. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Li, C.; Tu, Z.; Li, S.; Li, X.-J.; Yan, S. Application of CRISPR/Cas9 System in Establishing Large Animal Models. Front. Cell Dev. Biol. 2022, 10, 919155. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Galiano-Cogolludo, B.; Cornudella-Ardiaca, F.; Cobos-Figueroa, J.; Ousinde, O.; Bermejo-Álvarez, P. Strategies to Reduce Genetic Mosaicism Following CRISPR-Mediated Genome Edition in Bovine Embryos. Sci. Rep. 2019, 9, 14900. [Google Scholar] [CrossRef]
- Salvesen, H.A.; Grupen, C.G.; McFarlane, G.R. Tackling Mosaicism in Gene Edited Livestock. Front. Anim. Sci. 2024, 5, 1368155. [Google Scholar] [CrossRef]
- Mehravar, M.; Shirazi, A.; Nazari, M.; Banan, M. Mosaicism in CRISPR/Cas9-Mediated Genome Editing. Dev. Biol. 2019, 445, 156–162. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles Gambiae Mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef]
- Khwatenge, C.N.; Nahashon, S.N. Recent Advances in the Application of CRISPR/Cas9 Gene Editing System in Poultry Species. Front. Genet. 2021, 12, 627714. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.R.; Lee, S.S.; Miller, M.F.; Lombardi, H.A. CRISPR, Animals, and FDA Oversight: Building a Path to Success. Proc. Natl. Acad. Sci. USA 2021, 118, e2004831117. [Google Scholar] [CrossRef] [PubMed]
- Wray-Cahen, D.; Hallerman, E.; Tizard, M. Global Regulatory Policies for Animal Biotechnology: Overview, Opportunities and Challenges. Front. Genome Ed. 2024, 6, 1467080. [Google Scholar] [CrossRef] [PubMed]
- Li, T. A Chinese Model for Legal Regulation of Gene-Edited Endangered Animals and Plants. Gene 2024, 911, 148350. [Google Scholar] [CrossRef]
- Quigg, D.J.; Rifkin, J.; Justice, C.; Burger, W. Patenting of Animals—Ethical Considerations. In New Developements in Biotechnology; U.S. Government Printing Office: Washington, DC, USA, 1988; pp. 127–138. [Google Scholar]
- Ju, W.S.; Kim, S.; Lee, J.Y.; Lee, H.; No, J.; Lee, S.; Oh, K. Gene Editing for Enhanced Swine Production: Current Advances and Prospects. Animals 2025, 15, 422. [Google Scholar] [CrossRef]
- Aboelhassan, D.M.; Abozaid, H. Opportunities for CRISPR-Cas9 Application in Farm Animal Genetic Improvement. Mol. Biol. Rep. 2024, 51, 1108. [Google Scholar] [CrossRef]
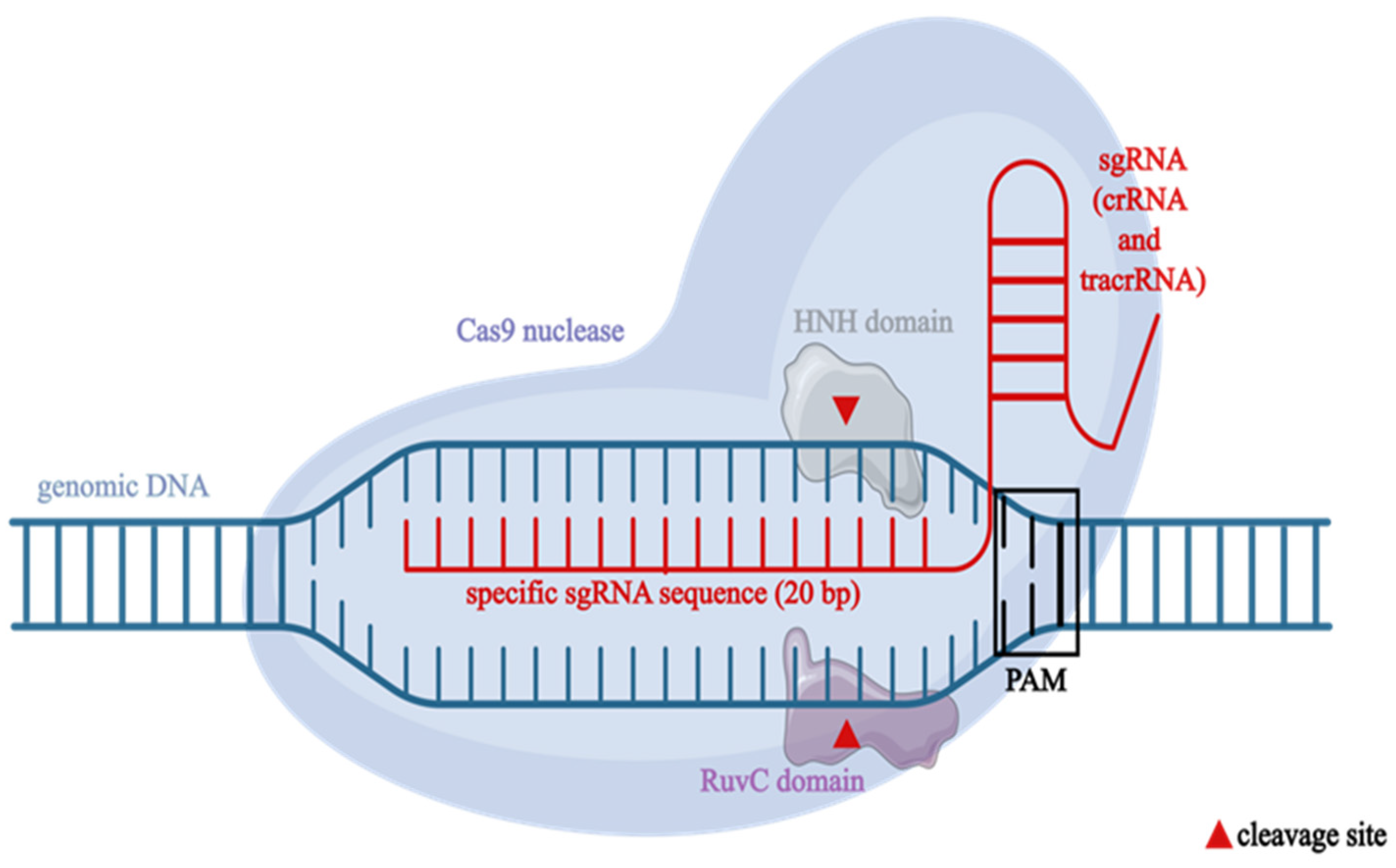
| Aspect | CRISPR-Cas9 in Bacteria | CRISPR-Cas9 in Eukaryotic Cells |
|---|---|---|
| Function | Adaptive immune system, protecting bacteria against viral infection | Genome editing tool, allowing DNA modification in living organisms |
| RNA components | CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) exist as two separate molecules | crRNA and tracrRNA are combined into a synthetic sgRNA |
| Protein localization | Cas9 functions in bacterial cytoplasm | Bioengineered Cas9 with nuclear localization signal (NLS), which allows transport into cell nucleus |
| Codon utilization | Matches bacterial codon | Codon is optimized for expression in eukaryotic cells |
| Target specificity | Targets foreign DNA based on spacer sequences | Synthetic sgRNA designed to direct Cas9 to specific DNA sequence site |
| Delivery mechanism | Naturally occurring mechanisms | Delivery through microinjection, electroporation, or viral vectors |
| Repair mechanisms | While CRISPR-Cas9 in bacteria primarily cleaves foreign DNA, creating DSBs, these breaks are subsequently repaired by the bacteria’s DNA repair mechanisms; thus, CRISPR-Cas9 indirectly triggers these repair pathways | NHEJ or HDR-driven genome modifications |
| Type | Mechanism | Mode of Action | Examples of Genetically Modified Animals |
|---|---|---|---|
| Physical | Electroporation | Short electrical pulses create transient pores in the cell membrane, enabling CRISPR-Cas9 entry [30,31]. | Mammals: Pig [20,32,33,34], Cattle [20,35], Mouse [20,21,36], Rat [19,37], Goat [38] Non-mammals: Zebrafish [31] |
| Microinjection | CRISPR-Cas9 is microinjected into fertilized egg pronuclei, and embryos are transferred to pseudopregnant surrogates for development [39]. | Mammals: Mouse [40,41], Rabbit [42], Cattle [43], Pig [18,32,44], Sheep [17,45] Non-mammals: Lizard [46], Zebrafish [47,48], Catfish [49] | |
| Chemical | Nanoparticles | Nanocarrier-mediated delivery, utilizing lipid, gold, polymer-coated nanoparticles, and exosomes, facilitates direct CRISPR-Cas9 cargo transfer to target cells [23]. | Mammals: Rat [50], Mouse [24,51,52,53,54] |
| Viral | Adenoviruses (AVs) Adeno-associated viruses (AAVs) Lentiviruses (LVs) | Viral vectors deliver CRISPR-Cas9 to host cells. Viruses are modified for safe gene delivery by disabling replication [55]. | Mammals: Pig [56], Canine [57], Mouse [25,26], Rat [22,58] |
| Somatic cell nuclear transfer (SCNT) | SCNT + microinjection | Involves screening of somatic cells for gene alterations caused by CRISPR-Cas9 microinjection. After identification, altered nuclei are transferred into enucleated oocytes [27]. | Mammals: Mouse [59], Pig [27,29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, W.; Kropacz, M.; Łach, M.; Jankowska, A. CRISPR-Cas9 in the Tailoring of Genetically Engineered Animals. Curr. Issues Mol. Biol. 2025, 47, 330. https://doi.org/10.3390/cimb47050330
Urban W, Kropacz M, Łach M, Jankowska A. CRISPR-Cas9 in the Tailoring of Genetically Engineered Animals. Current Issues in Molecular Biology. 2025; 47(5):330. https://doi.org/10.3390/cimb47050330
Chicago/Turabian StyleUrban, Wiktoria, Marta Kropacz, Maksymilian Łach, and Anna Jankowska. 2025. "CRISPR-Cas9 in the Tailoring of Genetically Engineered Animals" Current Issues in Molecular Biology 47, no. 5: 330. https://doi.org/10.3390/cimb47050330
APA StyleUrban, W., Kropacz, M., Łach, M., & Jankowska, A. (2025). CRISPR-Cas9 in the Tailoring of Genetically Engineered Animals. Current Issues in Molecular Biology, 47(5), 330. https://doi.org/10.3390/cimb47050330





