A Case of Non-Small Cell Lung Cancer with Mutually Exclusive EGFR and KRAS Mutations
Abstract
1. Introduction
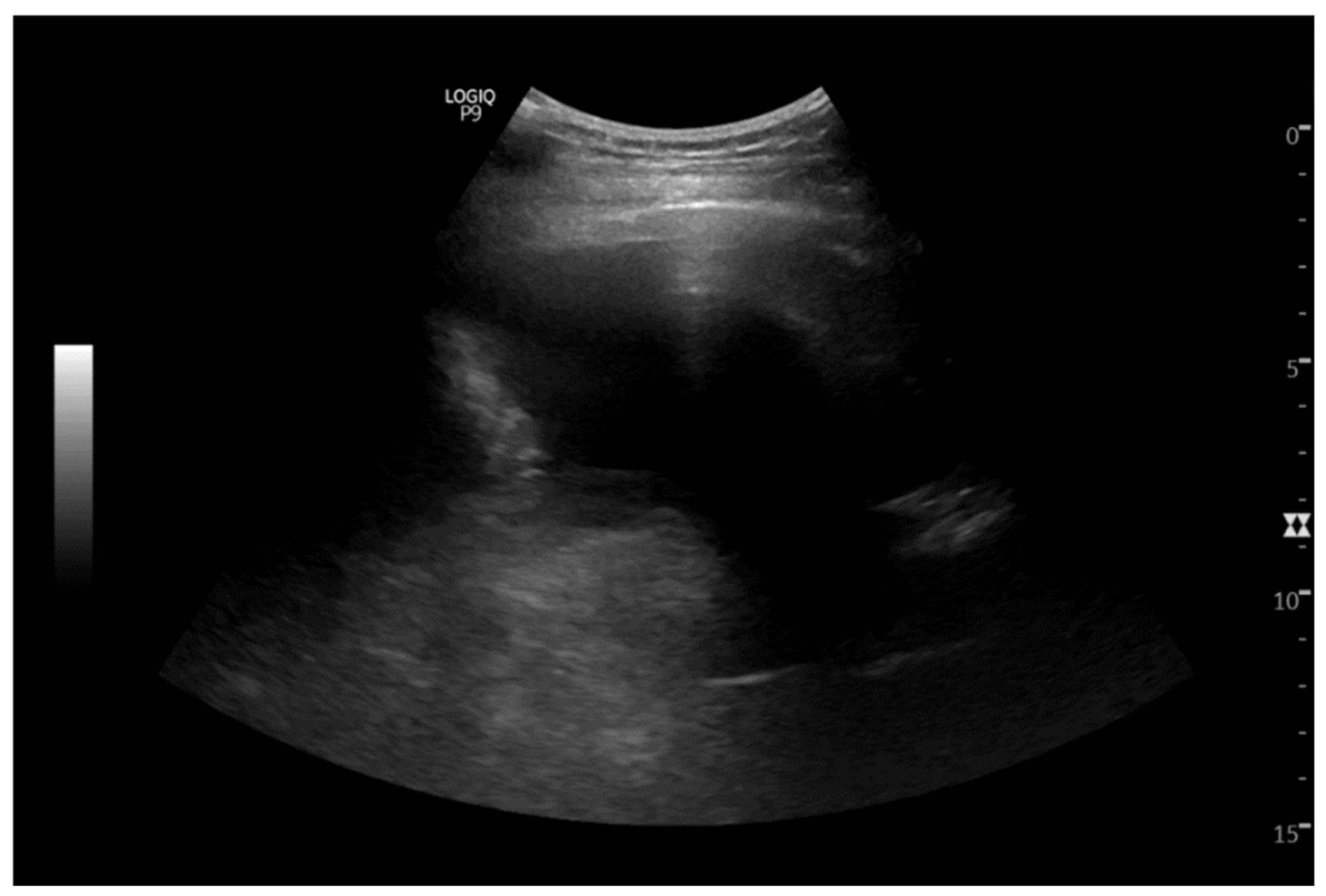
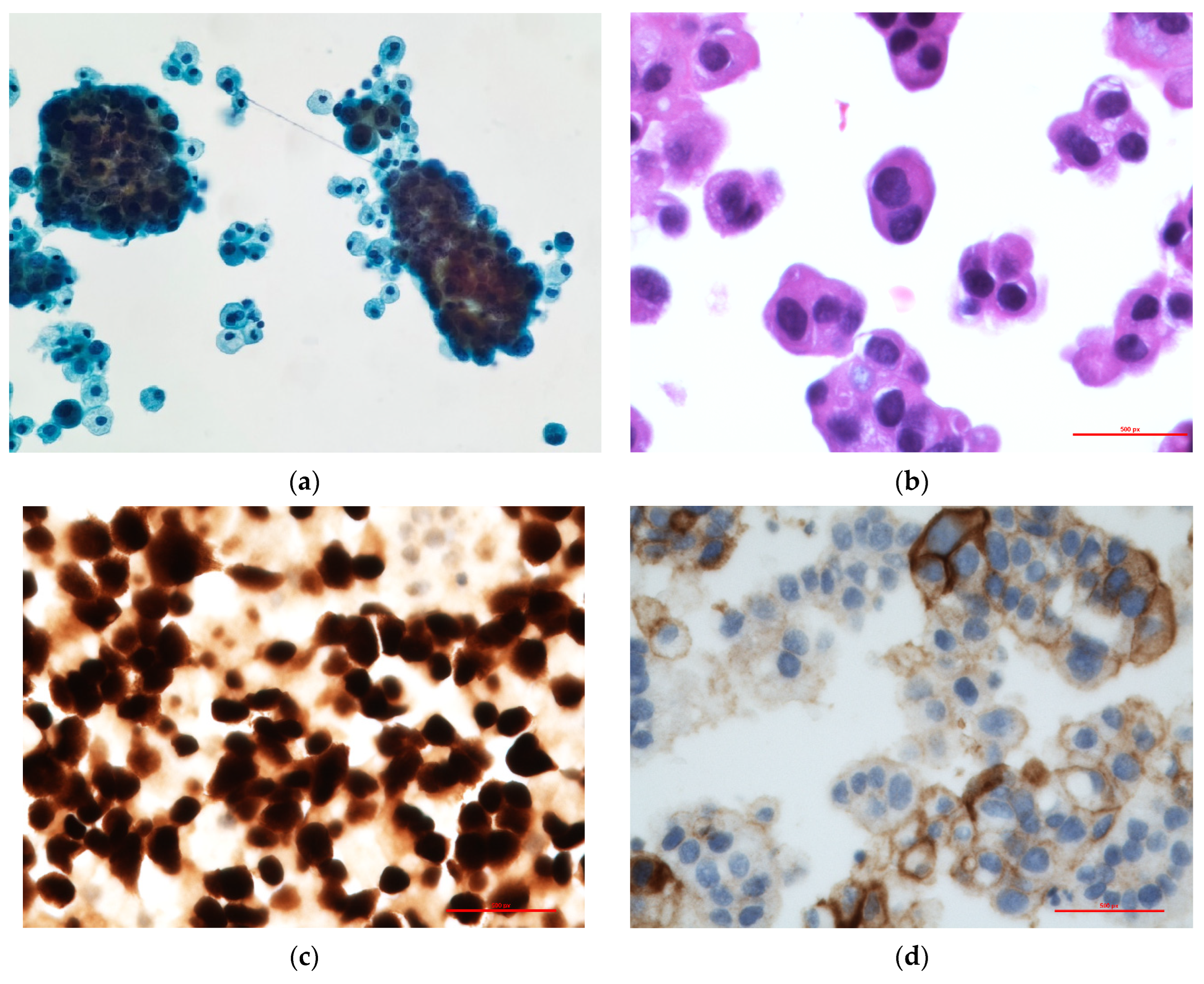
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erratum to “Cancer statistics, 2024”. CA Cancer J. Clin. 2024, 74, 203. [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Gan, X. Novel therapeutic strategies for rare mutations in non-small cell lung cancer. Sci. Rep. 2024, 14, 10317. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Melosky, B.; Park, K.; Yamamoto, N.; Yang, J.C. EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small-cell lung cancer: Outcomes in Asian populations. Future Oncol. 2021, 17, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, X.; Gao, Q.; Zhang, J.; Zheng, J.; Zhao, G.; Okuda, K.; Tartarone, A.; Jiang, M. Association between cigarette smoking history, metabolic phenotypes, and EGFR mutation status in patients with non-small cell lung cancer. J. Thorac. Dis. 2023, 15, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.L.; Broderick, S.; Zhou, Q.; Chitale, D.; Li, A.R.; Zakowski, M.F.; Kris, M.G.; Rusch, V.W.; Azzoli, C.G.; Seshan, V.E.; et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J. Thorac. Oncol. 2008, 3, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.W.; Oak, C.H.; Chang, H.K.; Suo, S.J.; Jung, M.H. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J. Intern. Med. 2009, 24, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C. P76.33 Concurrent EGFR and KRAS Mutations in Lung Adenocarcinoma: A Single Institution Case Series. J. Thorac. Oncol. 2021, 16, S600–S601. [Google Scholar] [CrossRef]
- Ju, L.; Han, M.; Zhao, C.; Li, X. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer 2016, 95, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Choughule, A.; Sharma, R.; Trivedi, V.; Thavamani, A.; Noronha, V.; Joshi, A.; Desai, S.; Chandrani, P.; Sundaram, P.; Utture, S.; et al. Coexistence of KRAS mutation with mutant but not wild-type EGFR predicts response to tyrosine-kinase inhibitors in human lung cancer. Br. J. Cancer 2014, 111, 2203–2204. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, L.; Liang, C.; Pang, J.; Yin, J.C.; Zhang, J.; Shao, Y.; Sun, C.; Guo, R. Landscape of Concomitant Driver Alterations in Classical EGFR-Mutated Non-Small Cell Lung Cancer. JCO Precis. Oncol. 2024, 8, e2300520. [Google Scholar] [CrossRef] [PubMed]
- Gini, B.; Thomas, N.; Blakely, C.M. Impact of concurrent genomic alterations in epidermal growth factor receptor (EGFR)-mutated lung cancer. J. Thorac. Dis. 2020, 12, 2883–2895. [Google Scholar] [CrossRef] [PubMed]
- Benesova, L.; Minarik, M.; Jancarikova, D.; Belsanova, B.; Pesek, M. Multiplicity of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC) patients treated with tyrosine kinase inhibitors. Anticancer Res. 2010, 30, 1667–1671. [Google Scholar] [PubMed]
- Rachiglio, A.M.; Fenizia, F.; Piccirillo, M.C.; Galetta, D.; Crinò, L.; Vincenzi, B.; Barletta, E.; Pinto, C.; Ferraù, F.; Lambiase, M.; et al. The Presence of Concomitant Mutations Affects the Activity of EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC) Patients. Cancers 2019, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Nardo, G.; Carlet, J.; Marra, L.; Bonanno, L.; Boscolo, A.; Maso, A.D.; Bragadin, A.B.; Indraccolo, S.; Zulato, E. Detection of Low-Frequency KRAS Mutations in cfDNA From EGFR-Mutated NSCLC Patients After First-Line EGFR Tyrosine Kinase Inhibitors. Front. Oncol. 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhao, C.; Li, J.; Su, C.; Chen, X.; Ren, S.; Li, X.; Zhou, C. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019, 8, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Burkhart, D.L.; Haigis, K.M. Classification of KRAS-Activating Mutations and the Implications for Therapeutic Intervention. Cancer Discov. 2022, 12, 913–923. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tushir, A.; Akhtar, I.; Seth, A. A Case of Non-Small Cell Lung Cancer with Mutually Exclusive EGFR and KRAS Mutations. Curr. Issues Mol. Biol. 2025, 47, 66. https://doi.org/10.3390/cimb47010066
Tushir A, Akhtar I, Seth A. A Case of Non-Small Cell Lung Cancer with Mutually Exclusive EGFR and KRAS Mutations. Current Issues in Molecular Biology. 2025; 47(1):66. https://doi.org/10.3390/cimb47010066
Chicago/Turabian StyleTushir, Abhimanyu, Israh Akhtar, and Anjali Seth. 2025. "A Case of Non-Small Cell Lung Cancer with Mutually Exclusive EGFR and KRAS Mutations" Current Issues in Molecular Biology 47, no. 1: 66. https://doi.org/10.3390/cimb47010066
APA StyleTushir, A., Akhtar, I., & Seth, A. (2025). A Case of Non-Small Cell Lung Cancer with Mutually Exclusive EGFR and KRAS Mutations. Current Issues in Molecular Biology, 47(1), 66. https://doi.org/10.3390/cimb47010066





