Repositioning Fluoxetine as a TRPV3 Channel Inhibitor to Alleviate Skin Inflammation and Pruritus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Cultures and Transient Transfection
2.3. Electrophysiology
2.4. Animals
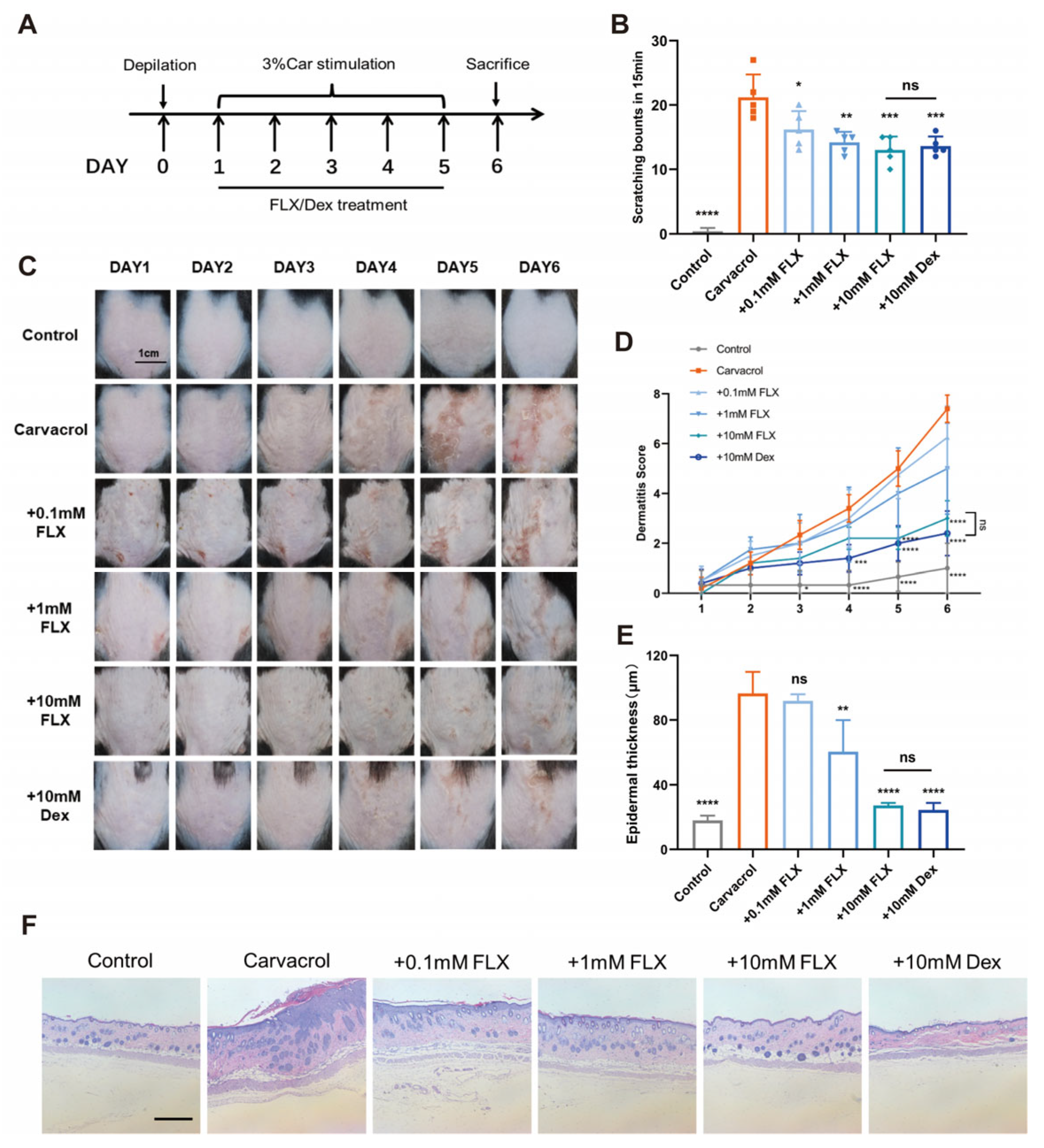
2.5. Carvacrol-Induced Mouse Dermatitis and Treatment
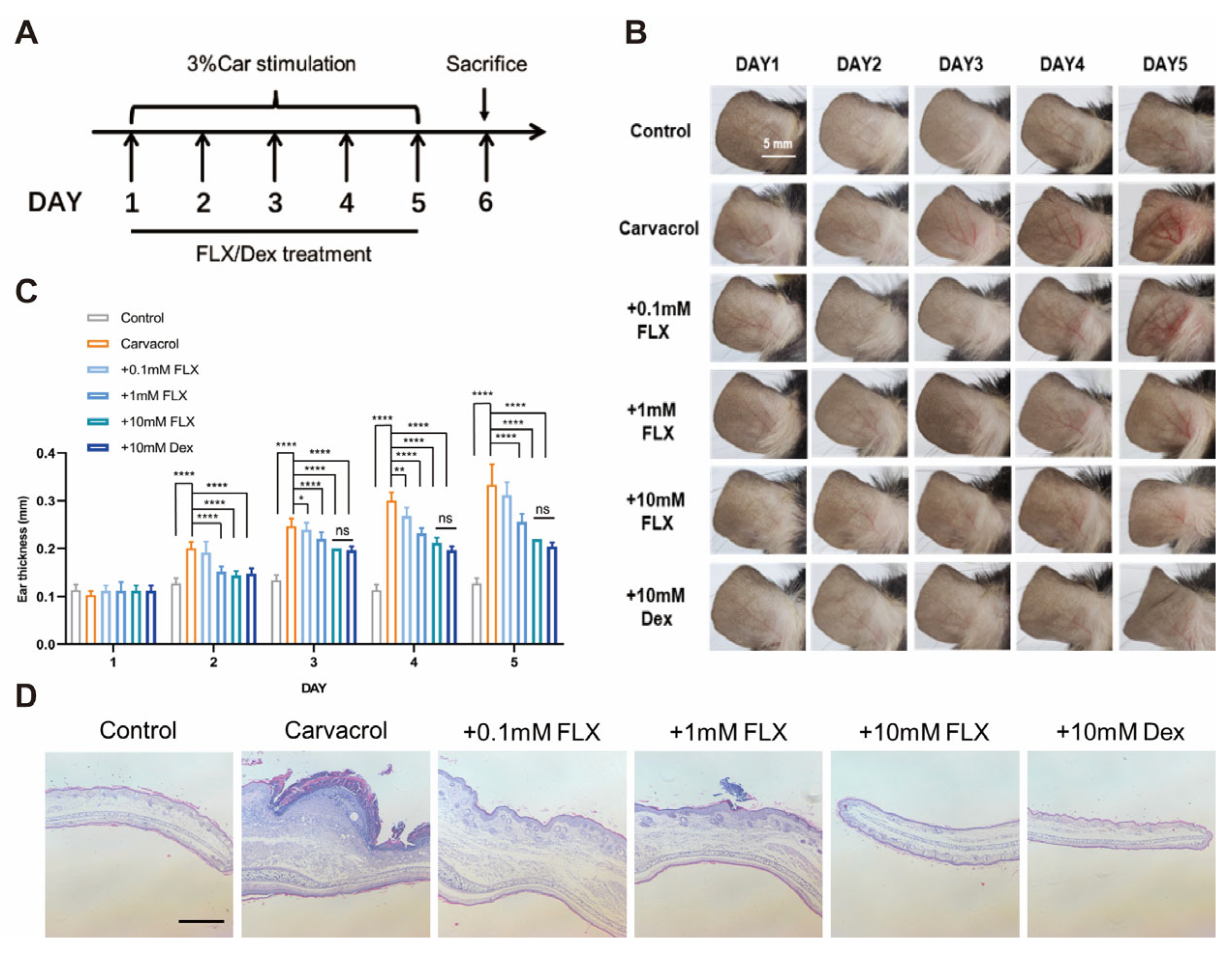
2.6. Carvacrol-Induced Mouse Ear Swelling and Treatment
2.7. Skin Lesion Scoring and Ear Thickness Measurements
2.8. Histopathological Examination
2.9. Statistical Analysis
3. Results
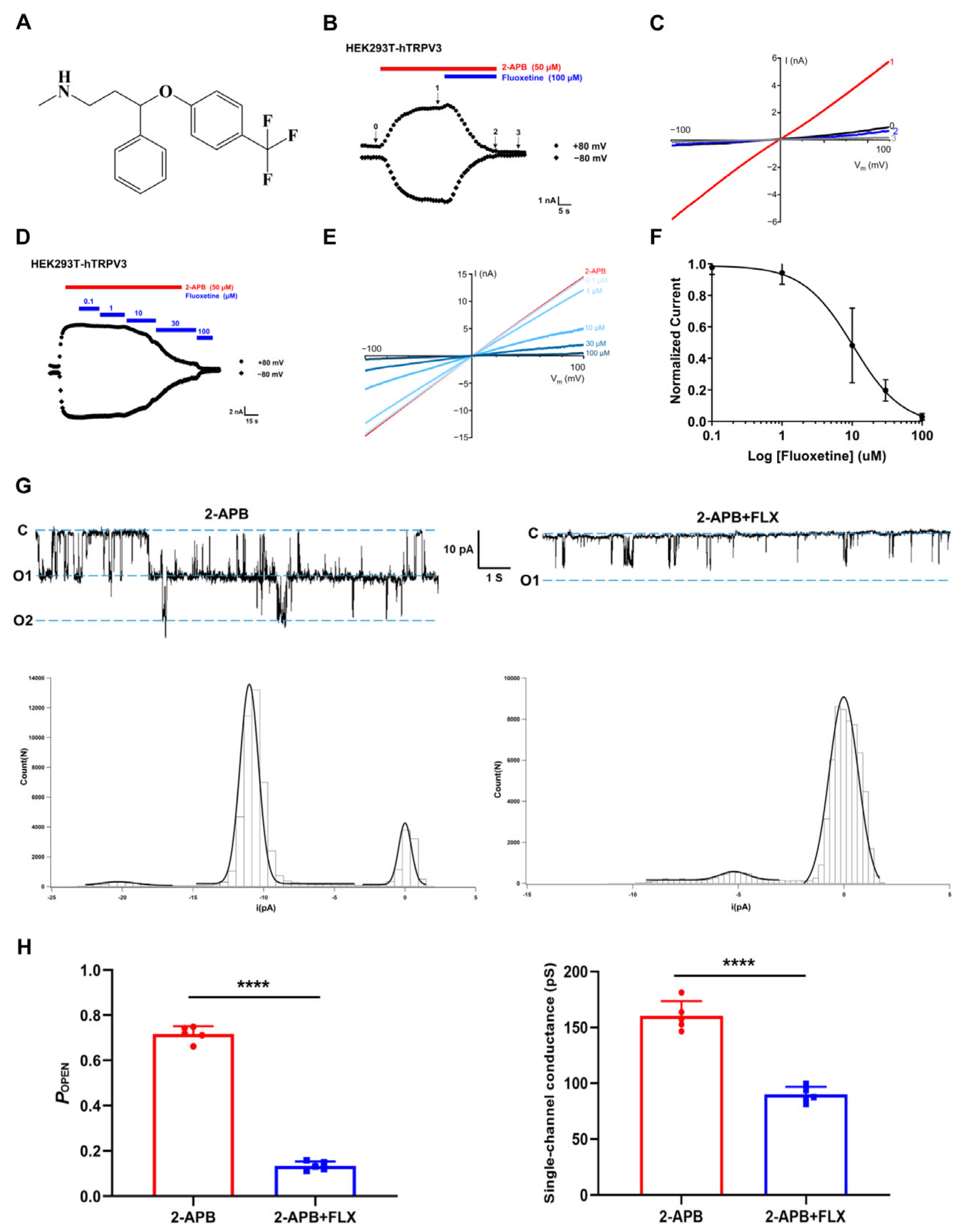
3.1. Identification of Fluoxetine as a TRPV3 Inhibitor in Patch-Clamp Recordings
3.2. Selectivity of Fluoxetine Among Thermo-TRP Channels
3.3. Fluoxetine Alleviates Carvacrol-Induced Dermatitis and Pruritus
3.4. Fluoxetine Alleviates Carvacrol-Induced Ear Swelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| 2-APB | 2-aminoethoxydiphenyl borate |
| AITC | allyl isothiocyanate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| GSK101 | GSK1016790A |
| H&E | hematoxylin and eosin |
| HEK293T | human embryonic kidney 293T |
| SD | standard deviation |
References
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Xue, C.; Chen, H.; Xue, Y.; Zhao, F.; Zhu, M.X.; Cao, Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 2021, 37, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, S.; Kim, S.E.; Chung, S.; Lee, S.E. Enhanced Thermal Sensitivity of TRPV3 in Keratinocytes Underlies Heat-Induced Pruritogen Release and Pruritus in Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 2199–2209.e6. [Google Scholar] [CrossRef]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a therapeutic target for itch. J. Investig. Dermatol. 2012, 132, 2109–2112. [Google Scholar] [CrossRef]
- Song, Z.; Chen, X.; Zhao, Q.; Stanic, V.; Lin, Z.; Yang, S.; Chen, T.; Chen, J.; Yang, Y. Hair Loss Caused by Gain-of-Function Mutant TRPV3 Is Associated with Premature Differentiation of Follicular Keratinocytes. J. Investig. Dermatol. 2021, 141, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Sun, X.; Wang, G.; Liu, Y.; Wang, K. Pharmacological Activation of Thermo-Transient Receptor Potential Vanilloid 3 Channels Inhibits Hair Growth by Inducing Cell Death of Hair Follicle Outer Root Sheath. J. Pharmacol. Exp. Ther. 2019, 370, 299–307. [Google Scholar] [CrossRef]
- Szöllősi, A.G.; Vasas, N.; Angyal, Á.; Kistamás, K.; Nánási, P.P.; Mihály, J.; Béke, G.; Herczeg-Lisztes, E.; Szegedi, A.; Kawada, N.; et al. Activation of TRPV3 Regulates Inflammatory Actions of Human Epidermal Keratinocytes. J. Investig. Dermatol. 2018, 138, 365–374. [Google Scholar] [CrossRef]
- Peters, F.; Kopp, J.; Fischer, J.; Tantcheva-Poór, I. Mutation in TRPV3 causes painful focal plantar keratoderma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e620–e622. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the Warm Temperature-Activated Ca(2+)-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel Attenuates Atopic Dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Qi, H.; Ma, Q.; Zhou, Q.; Wang, W.; Wang, K. Pharmacological Inhibition of the Temperature-Sensitive and Ca(2+)-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel by Natural Forsythoside B Attenuates Pruritus and Cytotoxicity of Keratinocytes. J. Pharmacol. Exp. Ther. 2019, 368, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, L.L.; Qi, H.; Gao, Q.; Wang, G.X.; Wei, N.N.; Wang, K. Antipruritic Effect of Natural Coumarin Osthole through Selective Inhibition of Thermosensitive TRPV3 Channel in the Skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Wei, X.; Hu, J.; Ping, C.; Gao, Y.; Xie, C.; Wang, P.; Cao, P.; Cao, Z.; et al. Therapeutic inhibition of keratinocyte TRPV3 sensory channel by local anesthetic dyclonine. Elife 2021, 10, e68128. [Google Scholar] [CrossRef]
- Neuberger, A.; Nadezhdin, K.D.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the anesthetic dyclonine. Nat. Commun. 2022, 13, 2795. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, Y.; Zhang, C.; Niu, C.; Tang, X.; Sun, X.; Wang, K. Selective inhibition of overactive warmth-sensitive Ca(2+)-permeable TRPV3 channels by antispasmodic agent flopropione for alleviation of skin inflammation. J. Biol. Chem. 2024, 300, 105595. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef]
- Fuller, R.W.; Perry, K.W.; Molloy, B.B. Effect of an uptake inhibitor on serotonin metabolism in rat brain: Studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140). Life Sci. 1974, 15, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. Case history: The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.T.; Wang, G.X.; Wei, N.N.; Wang, K. A pivotal role for the activation of TRPV3 channel in itch sensations induced by the natural skin sensitizer carvacrol. Acta Pharmacol. Sin. 2018, 39, 331–335. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Palkar, R.; Ongun, S.; Catich, E.; Li, N.; Borad, N.; Sarkisian, A.; McKemy, D.D. Cooling Relief of Acute and Chronic Itch Requires TRPM8 Channels and Neurons. J. Investig. Dermatol. 2018, 138, 1391–1399. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhao, Z.; Huang, X.; Xiong, H.; Mei, Z. Thymol activates TRPM8-mediated Ca(2+) influx for its antipruritic effects and alleviates inflammatory response in Imiquimod-induced mice. Toxicol. Appl. Pharmacol. 2020, 407, 115247. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca(2+)-permeable TRPV3 channel. J. Dermatol. Sci. 2020, 97, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, B.; Xu, W.; Wei, D.; Zhang, L.; Sun, J.; Shi, Y.; Feng, J.; Yang, F.; Zhang, H.; et al. Inhibition of transient receptor potential vanilloid 3 channels by antimalarial hydroxychloroquine alleviates TRPV3-dependent dermatitis. J. Biol. Chem. 2024, 300, 107733. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012, 165, 683–692. [Google Scholar] [CrossRef]
- Bischof, M.; Olthoff, S.; Glas, C.; Thorn-Seshold, O.; Schaefer, M.; Hill, K. TRPV3 endogenously expressed in murine colonic epithelial cells is inhibited by the novel TRPV3 blocker 26E01. Cell Calcium 2020, 92, 102310. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef]
- Pakyurek, M.; Pasol, E. Sublingually administered fluoxetine for major depression in medically compromised patients. Am. J. Psychiatry 1999, 156, 1833–1834. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chang, J.; Xu, Y.; Ge, Q.; Zhang, C. Repositioning Fluoxetine as a TRPV3 Channel Inhibitor to Alleviate Skin Inflammation and Pruritus. Curr. Issues Mol. Biol. 2025, 47, 277. https://doi.org/10.3390/cimb47040277
Zhang L, Chang J, Xu Y, Ge Q, Zhang C. Repositioning Fluoxetine as a TRPV3 Channel Inhibitor to Alleviate Skin Inflammation and Pruritus. Current Issues in Molecular Biology. 2025; 47(4):277. https://doi.org/10.3390/cimb47040277
Chicago/Turabian StyleZhang, Ling, Junjie Chang, Yimei Xu, Qi Ge, and Congxiao Zhang. 2025. "Repositioning Fluoxetine as a TRPV3 Channel Inhibitor to Alleviate Skin Inflammation and Pruritus" Current Issues in Molecular Biology 47, no. 4: 277. https://doi.org/10.3390/cimb47040277
APA StyleZhang, L., Chang, J., Xu, Y., Ge, Q., & Zhang, C. (2025). Repositioning Fluoxetine as a TRPV3 Channel Inhibitor to Alleviate Skin Inflammation and Pruritus. Current Issues in Molecular Biology, 47(4), 277. https://doi.org/10.3390/cimb47040277





