Dual-Faced Role of GDF6 in Cancer: Mechanistic Insights into Its Context-Dependent Regulation of Metastasis and Immune Evasion Across Human Malignancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Expression Profiling
2.3. Survival Analysis
2.4. Immune Landscape Characterization
2.5. Multi-Omics Integration
2.6. Functional Enrichment
2.7. Immunotherapy Analysis
2.8. Cell Culture and Functional Assays
2.9. Prognostic and Immunotherapy Stratification Analysis
3. Results
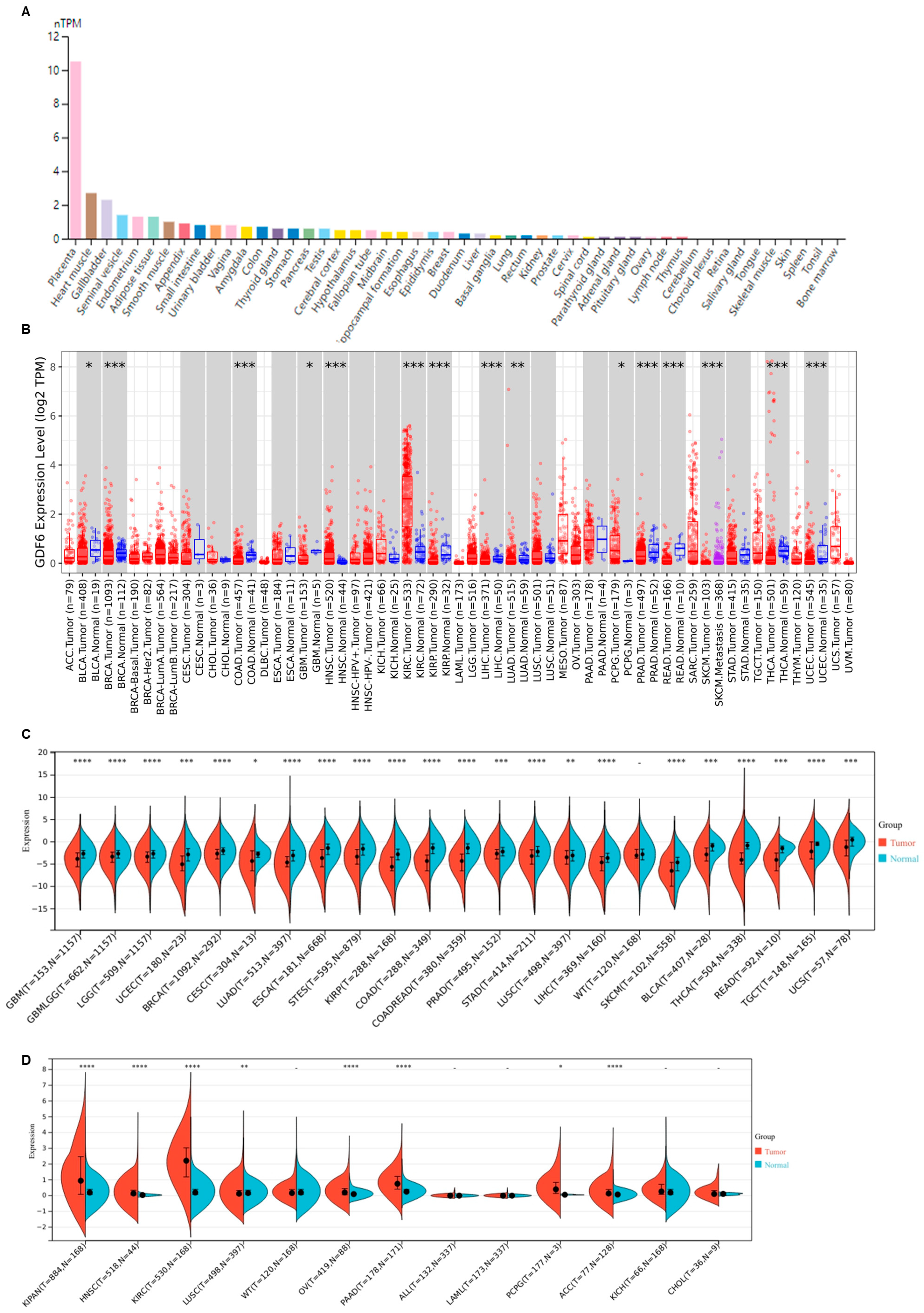
3.1. GDF6 Exhibits Aberrant Expression Across Diverse Human Malignancies
3.2. GDF6 Expression Correlates with Tumor Progression and Prognosis
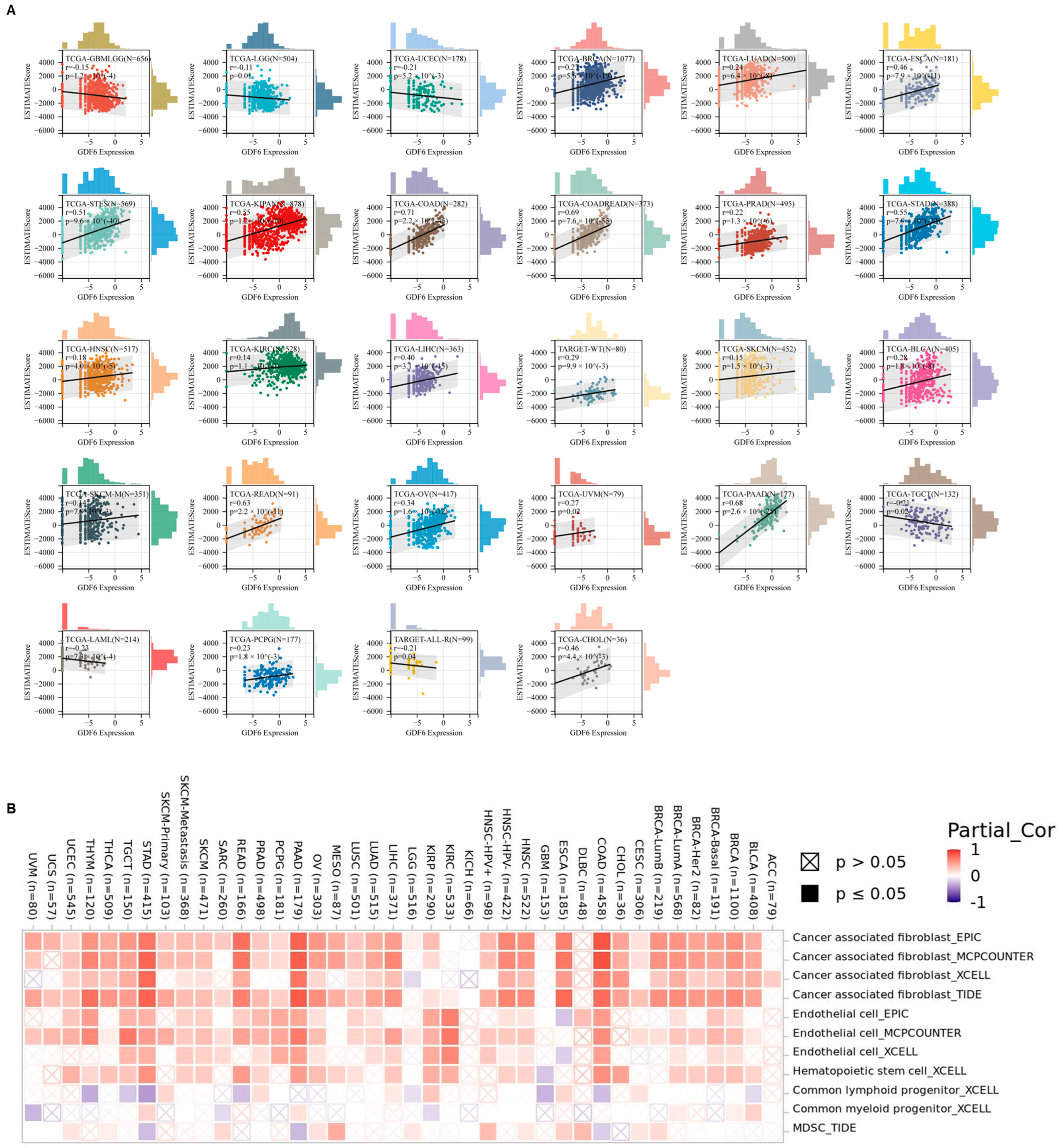
3.3. GDF6 Regulates Tumor Microenvironment and Stemness Features in Pan-Cancer Analysis
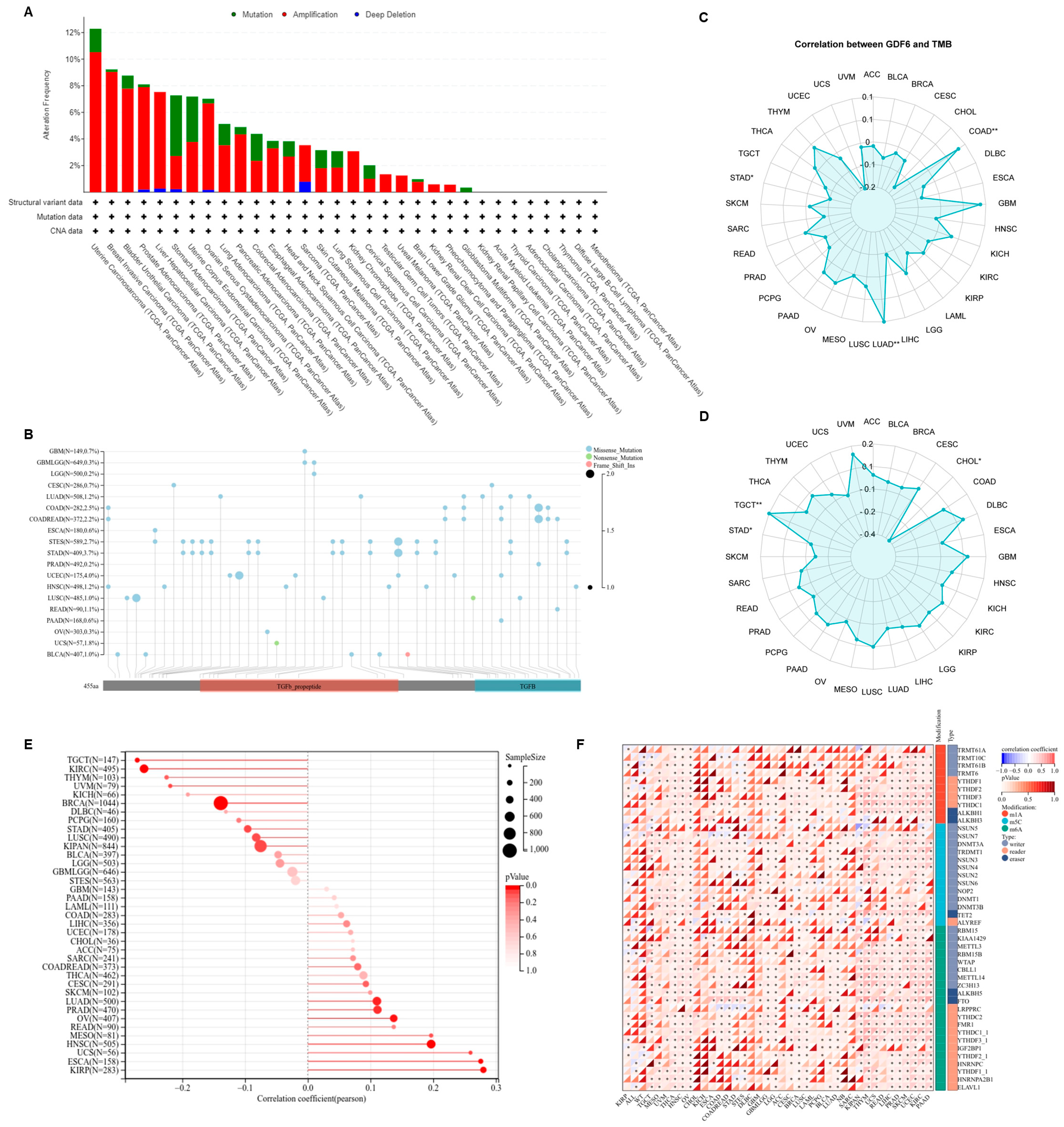
3.4. GDF6 Genomic Characteristics
3.5. Co-Expression Network, Functional Regulatory Mechanisms, and Therapeutic Sensitivity of GDF6
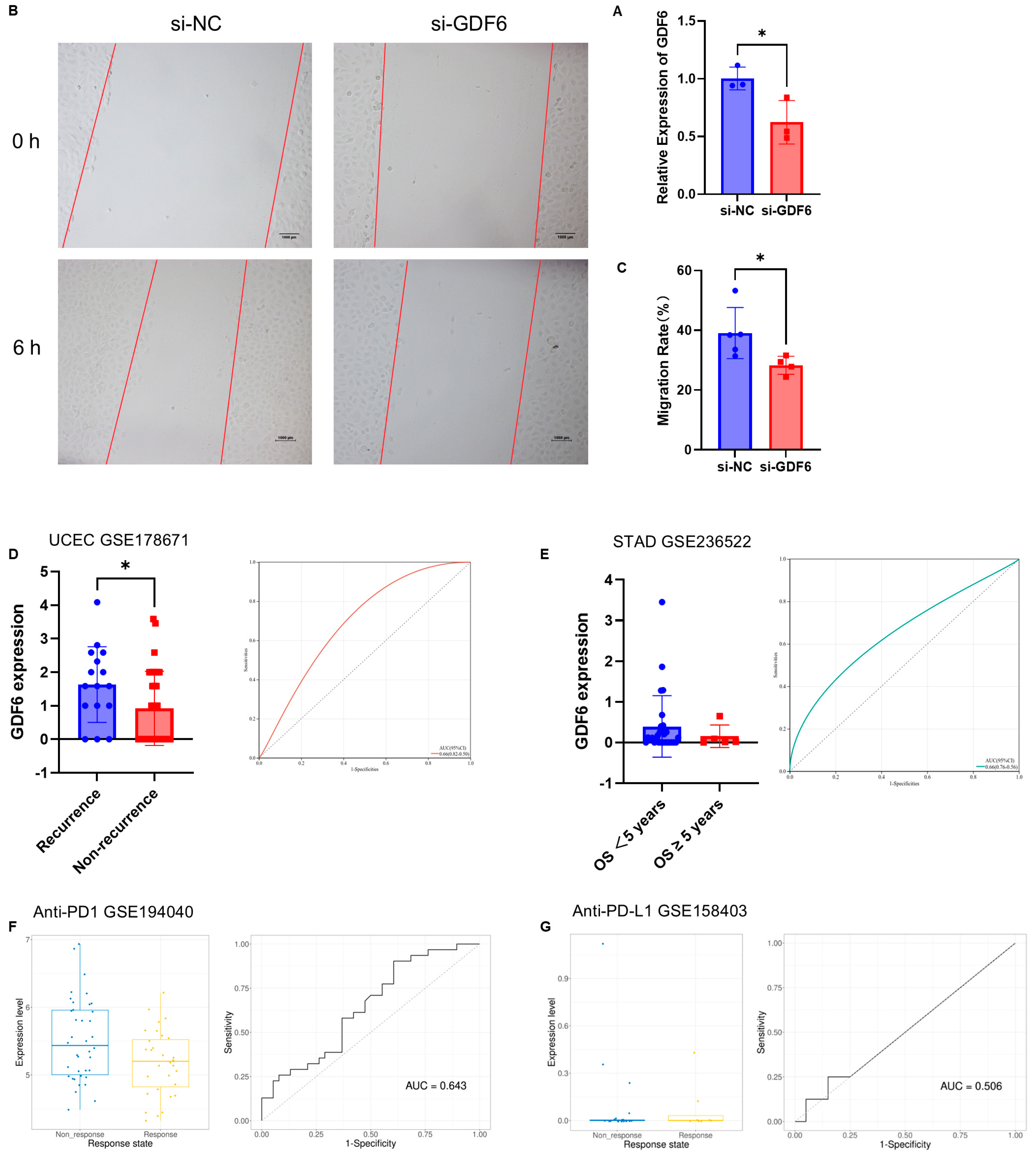
3.6. In Vitro Functional Validation and Clinical Biomarker Potential of GDF6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Vyas, R.; Gramann, A.K.; Dresser, K.; Gujja, S.; Bhatnagar, S.; Chhangawala, S.; Gomes, C.B.F.; Xi, H.S.; Lian, C.G.; et al. Ligand-activated BMP signaling inhibits cell differentiation and death to promote melanoma. J. Clin. Investig. 2018, 128, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, G.; Wang, C.; Li, N.; Yao, R.; Wang, J. Defective Joint Development and Maintenance in GDF6-Related Multiple Synostoses Syndrome. J. Bone Min. Res. 2023, 38, 568–577. [Google Scholar] [CrossRef]
- Nadolski, N.J.; Balay, S.D.; Wong, C.X.L.; Waskiewicz, A.J.; Hocking, J.C. Abnormal Cone and Rod Photoreceptor Morphogenesis in gdf6a Mutant Zebrafish. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef]
- Pant, S.D.; March, L.D.; Famulski, J.K.; French, C.R.; Lehmann, O.J.; Waskiewicz, A.J. Molecular mechanisms regulating ocular apoptosis in zebrafish gdf6a mutants. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5871–5879. [Google Scholar] [CrossRef]
- Williams, L.A.; Bhargav, D.; Diwan, A.D. Unveiling the bmp13 enigma: Redundant morphogen or crucial regulator? Int. J. Biol. Sci. 2008, 4, 318–329. [Google Scholar] [CrossRef]
- Miyazaki, K.; Miyazaki, S.; Yurube, T.; Takeoka, Y.; Kanda, Y.; Zhang, Z.; Kakiuchi, Y.; Tsujimoto, R.; Ohnishi, H.; Matsuo, T.; et al. Protective Effects of Growth Differentiation Factor-6 on the Intervertebral Disc: An In Vitro and In Vivo Study. Cells 2022, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Koinuma, D.; Miyazono, K.; Heldin, C.H. Genome-wide mechanisms of Smad binding. Oncogene 2013, 32, 1609–1615. [Google Scholar] [CrossRef]
- Schwarte-Waldhoff, I.; Schmiegel, W. Smad4 transcriptional pathways and angiogenesis. Int. J. Gastrointest. Cancer 2002, 31, 47–59. [Google Scholar] [CrossRef]
- He, C.; Chen, X. Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem. Biophys. Res. Commun. 2005, 329, 324–330. [Google Scholar] [CrossRef]
- Wu, R.; Hu, W.; Chen, H.; Wang, Y.; Li, Q.; Xiao, C.; Fan, L.; Zhong, Z.; Chen, X.; Lv, K.; et al. A Novel Human Long Noncoding RNA SCDAL Promotes Angiogenesis through SNF5-Mediated GDF6 Expression. Adv. Sci. 2021, 8, e2004629. [Google Scholar] [CrossRef] [PubMed]
- Krispin, S.; Stratman, A.N.; Melick, C.H.; Stan, R.V.; Malinverno, M.; Gleklen, J.; Castranova, D.; Dejana, E.; Weinstein, B.M. Growth Differentiation Factor 6 Promotes Vascular Stability by Restraining Vascular Endothelial Growth Factor Signaling. Arter. Thromb. Vasc. Biol. 2018, 38, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef]
- Lu, C.; Fan, X.; Zheng, M.; Zhang, S.; Wang, P.; Wang, Y.; Zhang, S. GDF6 in gastric cancer upregulated by helicobacter pylori induces epithelial-mesenchymal translation via the TGF-β/SMAD3 signaling pathway. Pathol. Res. Pract. 2024, 260, 155384. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Zhao, G.; Jin, B.; Jiang, P. A novel DNA methylation signature revealed GDF6 and RCC1 as potential prognostic biomarkers correlated with cell proliferation in clear cell renal cell carcinoma. Mol. Biol. Rep. 2023, 51, 16. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Noori, M.; Jafari-Raddani, F.; Davoodi-Moghaddam, Z.; Delshad, M.; Safiri, S.; Bashash, D. Immune checkpoint inhibitors in gastrointestinal malignancies: An Umbrella review. Cancer Cell Int. 2024, 24, 10. [Google Scholar] [CrossRef]
- Loh, J.J.; Ma, S. Hallmarks of cancer stemness. Cell Stem Cell 2024, 31, 617–639. [Google Scholar] [CrossRef]
- Tam, V.; Chopra, N.; Sima, S.; Chen, P.; Sharma, R.; Chan, D.; Diwan, A. Effects of GDF6 on active protein synthesis by cells of degenerated intervertebral disc. Eur. Spine J. 2025, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Xu, R.; Li, X.; Wang, Z.; Zhang, Y. The Physiological Functions and Therapeutic Potential of Hypoxia-Inducible Factor-1α in Vascular Calcification. Biomolecules 2024, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Schuster, V.; Dann, E.; Krogh, A.; Teichmann, S.A. multiDGD: A versatile deep generative model for multi-omics data. Nat. Commun. 2024, 15, 10031. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Liu, Y.; Li, H.; Gong, L.; Tan, X.; Tian, J.; Pi, H.; Wang, B.; Zhao, Y.; et al. The GDF6-FTO axis modulates the innate immune and inflammatory response to human respiratory syncytial virus. iScience 2024, 27, 111038. [Google Scholar] [CrossRef]
- Bacolod, M.D.; Mirza, A.H.; Huang, J.; Giardina, S.F.; Feinberg, P.B.; Soper, S.A.; Barany, F. Application of Multiplex Bisulfite PCR-Ligase Detection Reaction-Real-Time Quantitative PCR Assay in Interrogating Bioinformatically Identified, Blood-Based Methylation Markers for Colorectal Cancer. J. Mol. Diagn. 2020, 22, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Pirkkanen, J.; Tharmalingam, S.; Thome, C.; Sinex, H.C.; Benjamin, L.V.; Losch, A.C.; Borgmann, A.J.; Dhaemers, R.M.; Gordon, C.; Boreham, D.R.; et al. Genomic Loss and Epigenetic Silencing of the FOSL1 Tumor Suppressor Gene in Radiation-induced Neoplastic Transformation of Human CGL1 Cells Alters the Tumorigenic Phenotype In Vitro and In Vivo. Radiat. Res. 2023, 200, 48–64. [Google Scholar] [CrossRef]
- Ding, W.; Lv, D.; Wang, M.; Pei, D. IBSP Promotes Breast Cancer Bone Metastasis and Proliferation via BMP-SMAD Signaling Pathway. Cancer Rep. 2024, 7, e2153. [Google Scholar] [CrossRef]
- Zhou, F.; Elzi, D.J.; Jayabal, P.; Ma, X.; Chiu, Y.C.; Chen, Y.; Blackman, B.; Weintraub, S.T.; Houghton, P.J.; Shiio, Y. GDF6-CD99 Signaling Regulates Src and Ewing Sarcoma Growth. Cell Rep. 2020, 33, 108332. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Z.; Tao, B.; Yi, C.; Lin, Z.; Li, Y.; Shao, W.; Lin, J.; Chen, J. m6A target microRNAs in serum for cancer detection. Mol. Cancer. 2021, 20, 170. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zuo, X.; Li, G.; Wang, J.; Liu, J.; Wang, X.; Wang, S.; Zhang, W.; Zhang, K.; et al. CXCL12+ tumor-associated endothelial cells promote immune resistance in hepatocellular carcinoma. J. Hepatol. 2025, 82, 634–648. [Google Scholar] [CrossRef]
- Lee, Y.E.; Go, G.Y.; Koh, E.Y.; Yoon, H.N.; Seo, M.; Hong, S.M.; Jeong, J.H.; Kim, J.C.; Cho, D.; Kim, T.S.; et al. Synergistic therapeutic combination with a CAF inhibitor enhances CAR-NK-mediated cytotoxicity via reduction of CAF-released IL-6. J. Immunother. Cancer 2023, 11, e006130. [Google Scholar] [CrossRef]
- Bhandari, T.; Olson, J.; Johnson, R.S.; Nizet, V. HIF-1α influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J. Mol. Med. 2013, 91, 1199–1205. [Google Scholar] [CrossRef]
- Kling, L.; Schreiber, A.; Eckardt, K.U.; Kettritz, R. Hypoxia-inducible factors not only regulate but also are myeloid-cell treatment targets. J. Leukoc. Biol. 2021, 110, 61–75. [Google Scholar] [CrossRef]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Pertot, A.; Shirole, N.H.; Yao, Z.; Anaparthy, N.; Garvin, T.; Cox, H.; Chang, K.; Rollins, F.; Kendall, J.; et al. TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24− cancer cells. Elife. 2017, 6, e21615. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.T.; Song, L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear. Res. 2023, 434, 108783. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, J.; Cai, Y.; Fu, S.; Zhang, N.; Fu, X.; Li, L. IFN-γ-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int. J. Cancer 2018, 143, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, G.; Zhang, Z.; Gu, R.; Wang, W.; Lai, X.; Cui, Z.K.; Zeng, F.; Xu, S.; Deng, F. β2AR-HIF-1α-CXCL12 signaling of osteoblasts activated by isoproterenol promotes migration and invasion of prostate cancer cells. BMC Cancer 2019, 19, 1142. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Li, Q.; Liu, Q.; Zhang, X.; Ren, J.; Wang, C. Bisphenol A disrupts the neuronal F-actin cytoskeleton by activating the RhoA/ROCK/LIMK pathway in Neuro-2a cells. Toxicology 2024, 509, 153994. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells-a clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.L.; Quintanilha, J.C.; Danziger, N.; Li, G.; Sokol, E.; Schrock, A.B.; Ebot, E.; Bhardwaj, N.; Norris, T.; Afghahi, A.; et al. Effectiveness of PARP Inhibitor Maintenance Therapy in Ovarian Cancer by BRCA1/2 and a Scar-Based HRD Signature in Real-World Practice. Clin. Cancer Res. 2024, 30, 4644–4653. [Google Scholar] [CrossRef] [PubMed]
- Ehata, S.; Miyazono, K. Bone Morphogenetic Protein Signaling in Cancer; Some Topics in the Recent 10 Years. Front. Cell Dev. Biol. 2022, 10, 883523. [Google Scholar] [CrossRef]
- Gao, J.; Yang, T.; Han, J.; Yan, K.; Qiu, X.; Zhou, Y.; Fan, Q.; Ma, B. MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. J. Cell Biochem. 2011, 112, 1844–1856. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Wei, J.; Han, W. Dual-Faced Role of GDF6 in Cancer: Mechanistic Insights into Its Context-Dependent Regulation of Metastasis and Immune Evasion Across Human Malignancies. Curr. Issues Mol. Biol. 2025, 47, 249. https://doi.org/10.3390/cimb47040249
Zhu Q, Wei J, Han W. Dual-Faced Role of GDF6 in Cancer: Mechanistic Insights into Its Context-Dependent Regulation of Metastasis and Immune Evasion Across Human Malignancies. Current Issues in Molecular Biology. 2025; 47(4):249. https://doi.org/10.3390/cimb47040249
Chicago/Turabian StyleZhu, Qi, Jianshu Wei, and Weidong Han. 2025. "Dual-Faced Role of GDF6 in Cancer: Mechanistic Insights into Its Context-Dependent Regulation of Metastasis and Immune Evasion Across Human Malignancies" Current Issues in Molecular Biology 47, no. 4: 249. https://doi.org/10.3390/cimb47040249
APA StyleZhu, Q., Wei, J., & Han, W. (2025). Dual-Faced Role of GDF6 in Cancer: Mechanistic Insights into Its Context-Dependent Regulation of Metastasis and Immune Evasion Across Human Malignancies. Current Issues in Molecular Biology, 47(4), 249. https://doi.org/10.3390/cimb47040249






