Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of THP-1 Human Pre-Monocytes
2.2. Caffeine/LPS Exposure and Experimental Groups
2.3. Gene Expression Measurements
2.4. Immunoassay Measurement
2.5. Statistics
3. Results
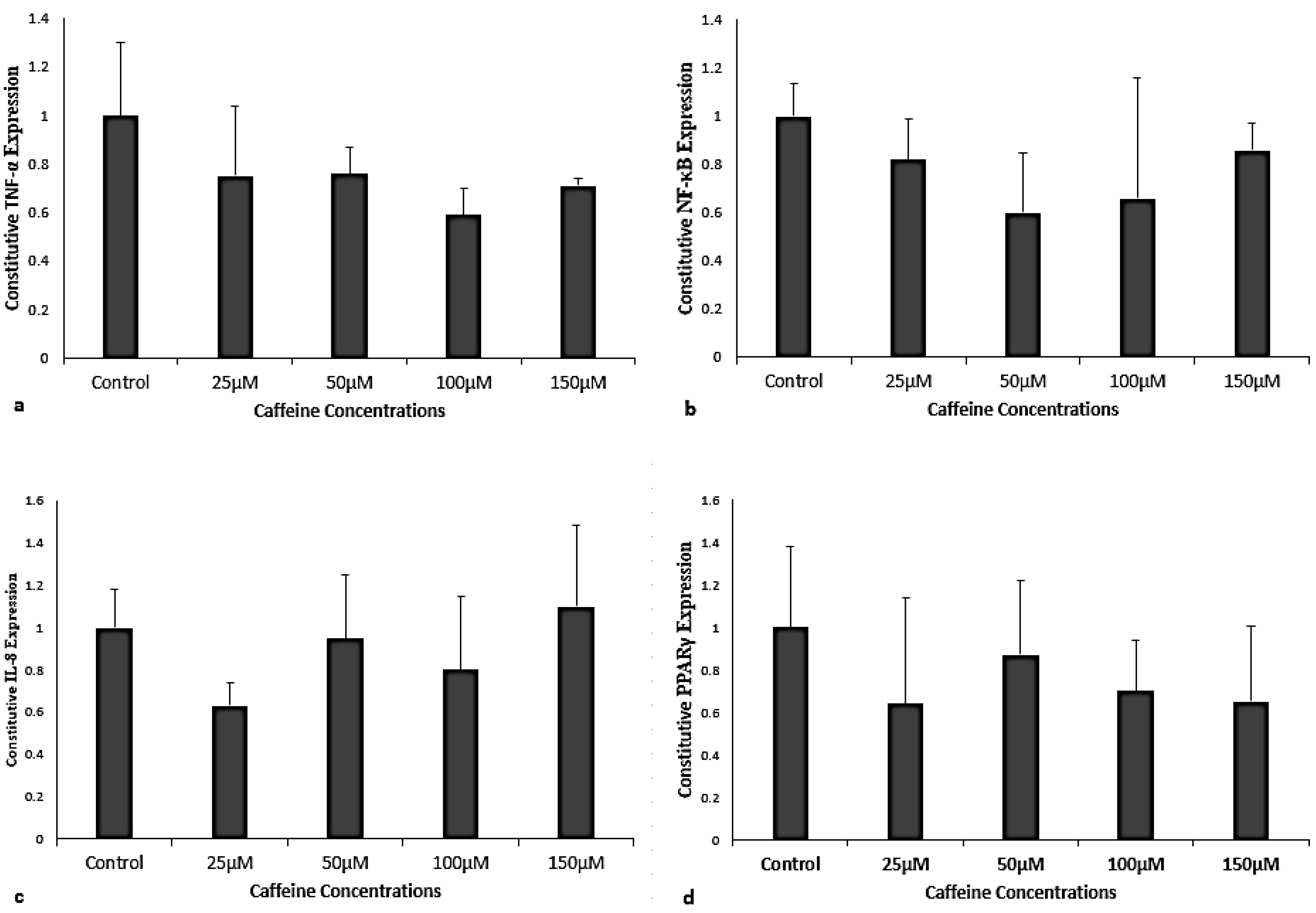
3.1. Caffeine Effects on Constitutive Gene Expression of TNF-α, NF-κB, IL-8, and PPARγ
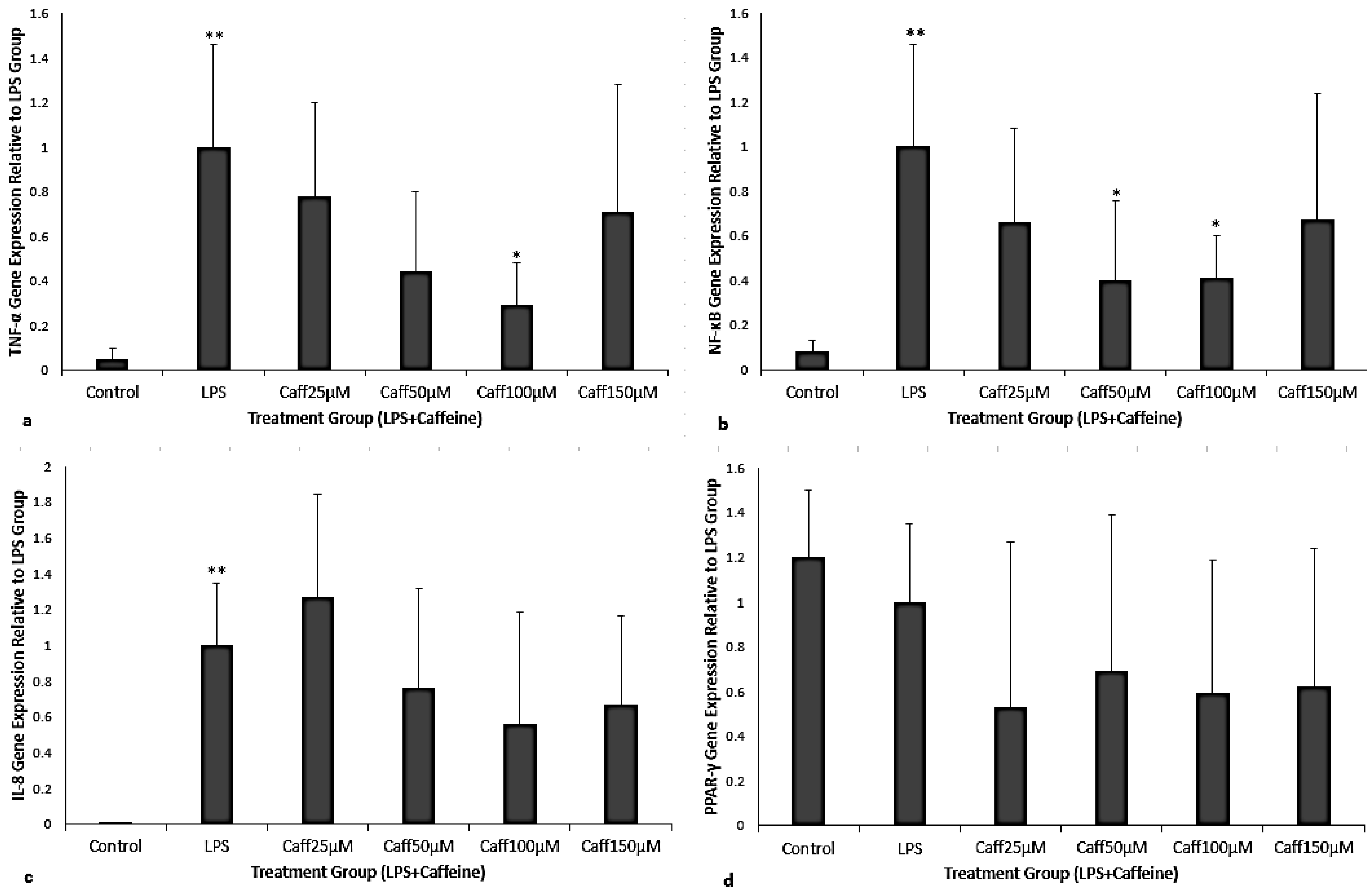
3.2. Caffeine Administered After LPS Exposure (Treatment)
3.3. Caffeine Administered Prior to LPS Exposure (Prophylaxis)
3.4. Immunoassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NICU | Neonatal intensive care unit |
| BPD | Bronchopulmonary dysplasia |
| LPS | Lipopolysaccharide |
| RT-PCR | Reverse transcriptase-polymerase chain reaction |
| PMA | Phorbol myristate acetate |
References
- Abu-Shaweesh, J.M.; Martin, R.J. Caffeine use in the neonatal intensive care unit. Semin. Fetal Neonatal Med. 2017, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Seshia, M.; McMillan, D.D.; Barrington, K.; Yang, J.; Lee, S.K.; Shah, P.S.; Canadian Neonatal Network. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015, 169, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E. Caffeine for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2179–2181. [Google Scholar] [CrossRef] [PubMed]
- Vizentin, V.K.; de Sá Pacheco, I.M.; Azevêdo, T.F.V.B.; de Mesquita, C.F.; Pereira, R.A. Early versus Late Caffeine Therapy Administration in Preterm Neonates: An Updated Systematic Review and Meta-Analysis. Neonatology 2024, 121, 7–16. [Google Scholar] [CrossRef]
- Miller, J.D.; Benjamin, J.T.; Kelly, D.R.; Frank, D.B.; Prince, L.S. Chorioamnionitis stimulates angiogenesis in saccular stage fetal lungs via CC chemokines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L637–L645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, J.J.; Kuypers, E.; Nitsos, I.; Jane Pillow, J.; Polglase, G.R.; Kemp, M.W.; Newnham, J.P.; Cleutjens, J.P.; Frints, S.G.; Kallapur, S.G.; et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2012, 303, L778–L787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Köroğlu, O.A.; MacFarlane, P.M.; Balan, K.V.; Zenebe, W.J.; Jafri, A.; Martin, R.J.; Prabha, K. Anti-inflammatory effect of caffeine is associated with improved lung function after lipopolysaccharide-induced amnionitis. Neonatology 2014, 106, 235–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blackwell, T.S.; Hipps, A.N.; Yamamoto, Y.; Han, W.; Barham, W.J.; Ostrowski, M.C.; Yull, F.E.; Prince, L.S. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J. Immunol. 2011, 187, 2740–2747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagatomo, T.; Jiménez, J.; Richter, J.; De Baere, S.; Vanoirbeek, J.; Naulaers, G.; Allegaert, K.; Croubels, S.; Deprest, J.A.; Toelen, J. Caffeine Prevents Hyperoxia-Induced Functional and Structural Lung Damage in Preterm Rabbits. Neonatology 2016, 109, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P.; Deitch, E.A.; Vizi, E.S. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007, 113, 264–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blackburn, M.R.; Vance, C.O.; Morschl, E.; Wilson, C.N. Adenosine receptors and inflammation. Handb. Exp. Pharmacol. 2009, 193, 215–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iris, M.; Tsou, P.S.; Sawalha, A.H. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin. Immunol. 2018, 192, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Valdez, R.; Ahlawat, R.; Wills-Karp, M.; Gauda, E.B. Mechanisms of modulation of cytokine release by human cord blood monocytes exposed to high concentrations of caffeine. Pediatr. Res. 2016, 80, 101–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chavez-Valdez, R.; Wills-Karp, M.; Ahlawat, R.; Cristofalo, E.A.; Nathan, A.; Gauda, E.B. Caffeine modulates TNF-alpha production by cord blood monocytes: The role of adenosine receptors. Pediatr. Res. 2009, 65, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Chavez Valdez, R.; Ahlawat, R.; Wills-Karp, M.; Nathan, A.; Ezell, T.; Gauda, E.B. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. J. Pediatr. 2011, 158, 57–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitkin, C.R.; Bonfield, T.L. Balancing anti-inflammatory and anti-oxidant responses in murine bone marrow derived macrophages. PLoS ONE. 2017, 12, e0184469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J Clin Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobson, N.R.; Patel, R.M. The Role of Caffeine in Noninvasive Respiratory Support. Clin. Perinatol. 2016, 43, 773–782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Widdrington, J.D.; Gomez-Duran, A.; Pyle, A.; Ruchaud-Sparagano, M.-H.; Scott, J.; Baudouin, S.V.; Rostron, A.J.; Lovat, P.F.; Chinnery, S.V.; Simpson, A.J. Exposure of Monocytic Cells to Lipopolysaccharide Induces Coordinated Endotoxin Tolerance, Mitochondrial Biogenesis, Mitophagy, and Antioxidant Defenses. Front. Immunol. 2018, 9, 2217. [Google Scholar] [CrossRef]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, Peripheral Blood Mononuclear Cells, and THP-1 Cells Exhibit Different Cytokine Expression Patterns following Stimulation with Lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef]
- Kumar, V.H.S.; Lipshultz, S.E. Caffeine and Clinical Outcomes in Premature Neonates. Children 2019, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats. Oxidative Med. Cell. Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Bührer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef]
- Chou, W.C.; Kao, M.C.; Yue, C.T.; Tsai, P.S.; Huang, C.J. Caffeine Mitigates Lung Inflammation Induced by Ischemia-Reperfusion of Lower Limbs in Rats. Mediat. Inflamm. 2015, 2015, 361638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Auwerx, J. The human leukemia cell line, THP-1: A multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef] [PubMed]
- López-Zambrano, M.; Rodriguez-Montesinos, J.; Crespo-Avilan, G.E.; Muñoz-Vega, M.; Preissner, K.T. Thrombin Promotes Macrophage Polarization into M1-Like Phenotype to Induce Inflammatory Responses. Thromb. Haemost. 2020, 120, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Lopez, M.L.; McCurdy, S.; Fischer, S.; Meiler, S.; Baumer, Y.; Galuska, S.P.; Preissner, K.T.; Boisvert, W.A. Regulation of monocyte/macrophage polarisation by extracellular RNA. Thromb. Haemost. 2015, 113, 473–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenberg, J.M.; Poindexter, B.B.; Shaw, P.A.; Bellamy, S.L.; Keller, R.L.; Moore, P.E.; McPherson, C.; Ryan, R.M. Respiratory medication use in extremely premature (<29 weeks) infants during initial NICU hospitalization: Results from the prematurity and respiratory outcomes program. Pediatr. Pulmonol. 2020, 55, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child Fetal. Neonatal. Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovács, E.G.; Alatshan, A.; Budai, M.M.; Czimmerer, Z.; Bíró, E.; Benkő, S. Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients 2021, 13, 2409. [Google Scholar] [CrossRef] [PubMed]
- Toobian, D.; Ghosh, P.; Katkar, G.D. Parsing the Role of PPARs in Macrophage Processes. Front. Immunol. 2021, 12, 783780. [Google Scholar] [CrossRef]
- Sherman, D.J.; Tovbin, J.; Lazarovich, T.; Avrech, O.; Reif, R.; Hoffmann, S.; Caspi, E.; Boldur, I. Chorioamnionitis caused by gram-negative bacteria as an etiologic factor in preterm birth. Eur. J. Clin. Microbiol. Infect Dis. 1997, 16, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Puopolo, K.M.; Hansen, N.I.; Sánchez, P.J.; Bell, E.F.; Carlo, W.A.; Cotten, C.M.; D’Angio, C.T.; Kazzi, S.N.J.; Poindexter, B.B.; et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020, 174, e200593. [Google Scholar] [CrossRef]
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef]
- el Manouni el Hassani, S.; Berkhout, D.J.C.; Niemarkt, H.J.; Mann, S.; de Boode, W.P.; Cossey, V.; Hulzebos, C.V.; Van Kaam, A.H.; Kramer, B.W.; van Lingen, R.A.; et al. Risk Factors for Late-Onset Sepsis in Preterm Infants: A Multicenter Case-Control Study. Neonatology 2019, 116, 42–51. [Google Scholar] [CrossRef]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Htun, Z.T.; Raffay, T.M.; Martin, R.J.; MacFarlane, P.M.; Bonfield, T.L. Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression. Curr. Issues Mol. Biol. 2025, 47, 248. https://doi.org/10.3390/cimb47040248
Htun ZT, Raffay TM, Martin RJ, MacFarlane PM, Bonfield TL. Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression. Current Issues in Molecular Biology. 2025; 47(4):248. https://doi.org/10.3390/cimb47040248
Chicago/Turabian StyleHtun, Zeyar T., Thomas M. Raffay, Richard J. Martin, Peter M. MacFarlane, and Tracey L. Bonfield. 2025. "Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression" Current Issues in Molecular Biology 47, no. 4: 248. https://doi.org/10.3390/cimb47040248
APA StyleHtun, Z. T., Raffay, T. M., Martin, R. J., MacFarlane, P. M., & Bonfield, T. L. (2025). Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression. Current Issues in Molecular Biology, 47(4), 248. https://doi.org/10.3390/cimb47040248





