Fisetin Mitigates Chronic Lung Injury Induced by Benzo(a)Pyrene by Regulation of Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Development of Chronic Lung Injury Model and Fisetin Treatment
2.3. Animal Sacrifice, Lung Isolation, and Blood Collection
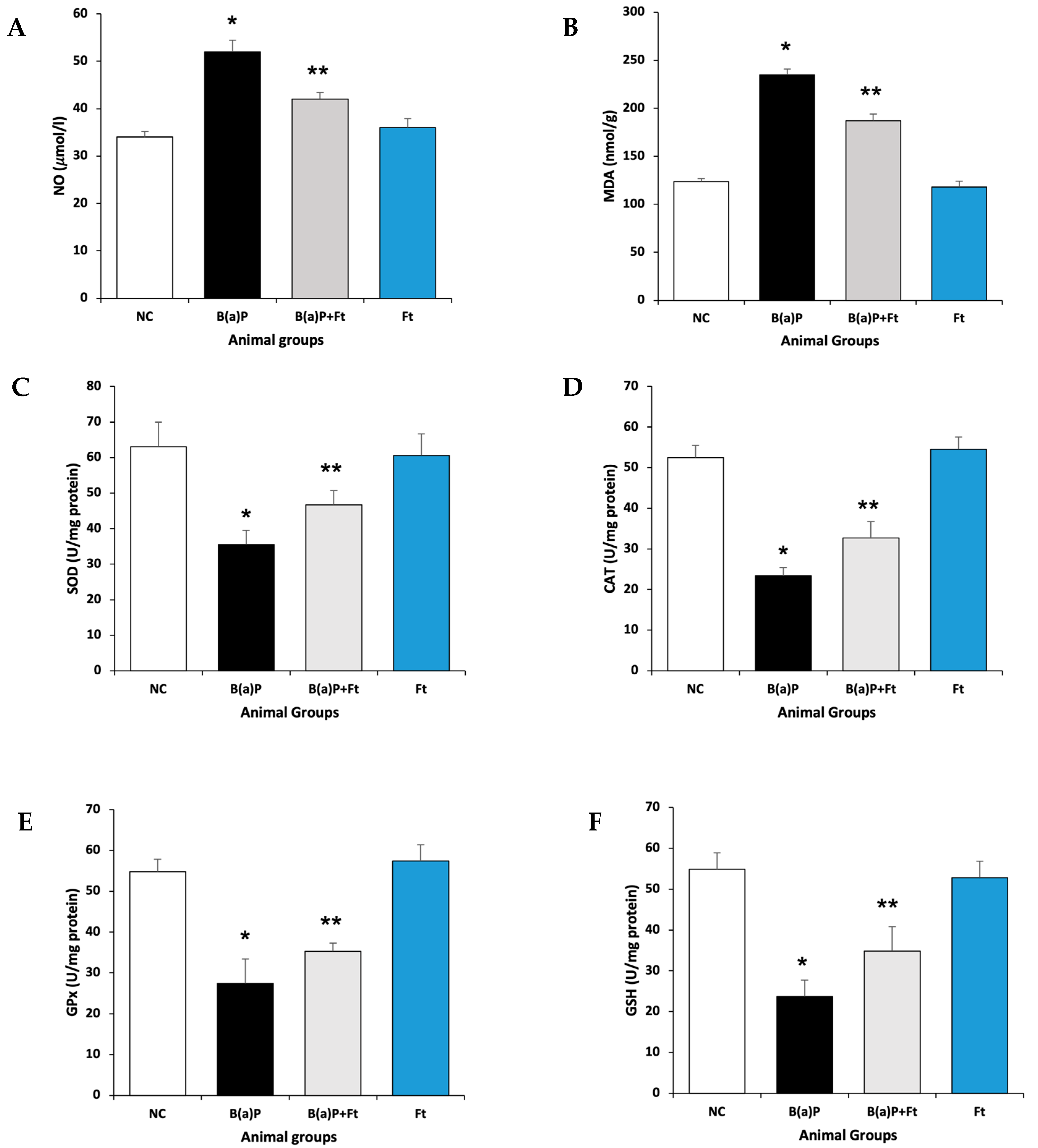
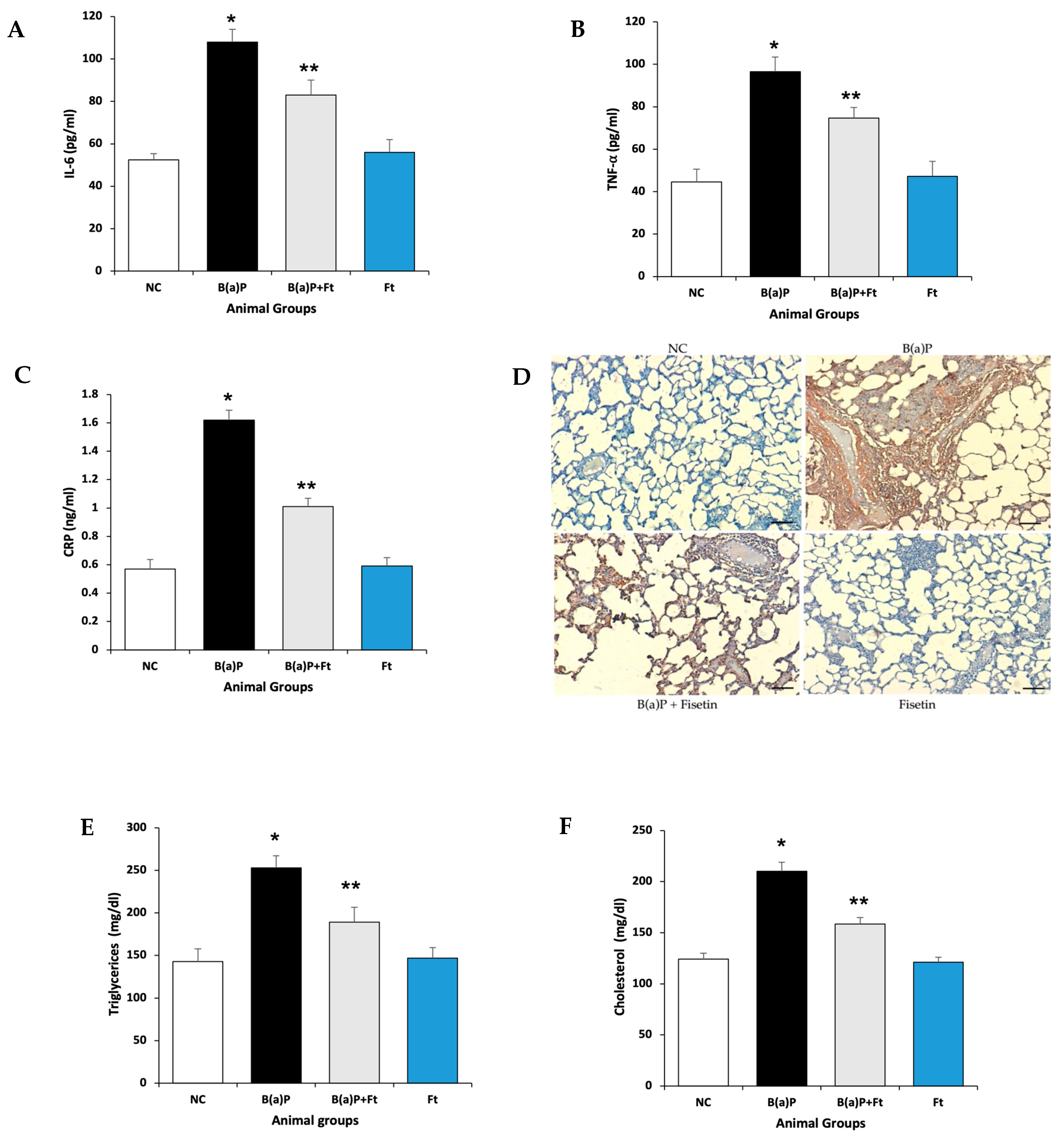
2.4. Measurement of Oxidative Stress and Inflammatory Marker Levels
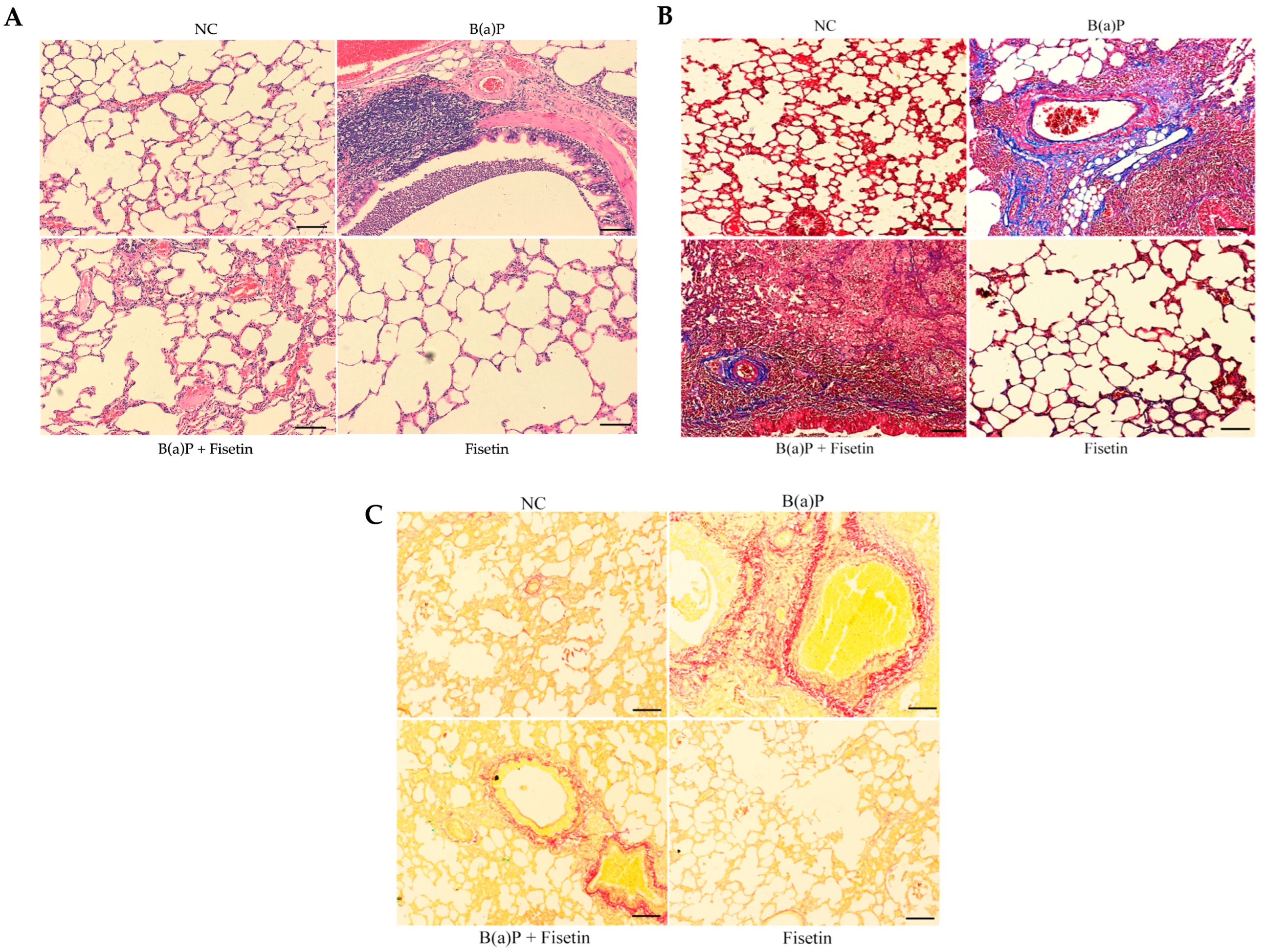
2.5. Histopathology Analysis
2.6. Immunohistochemistry
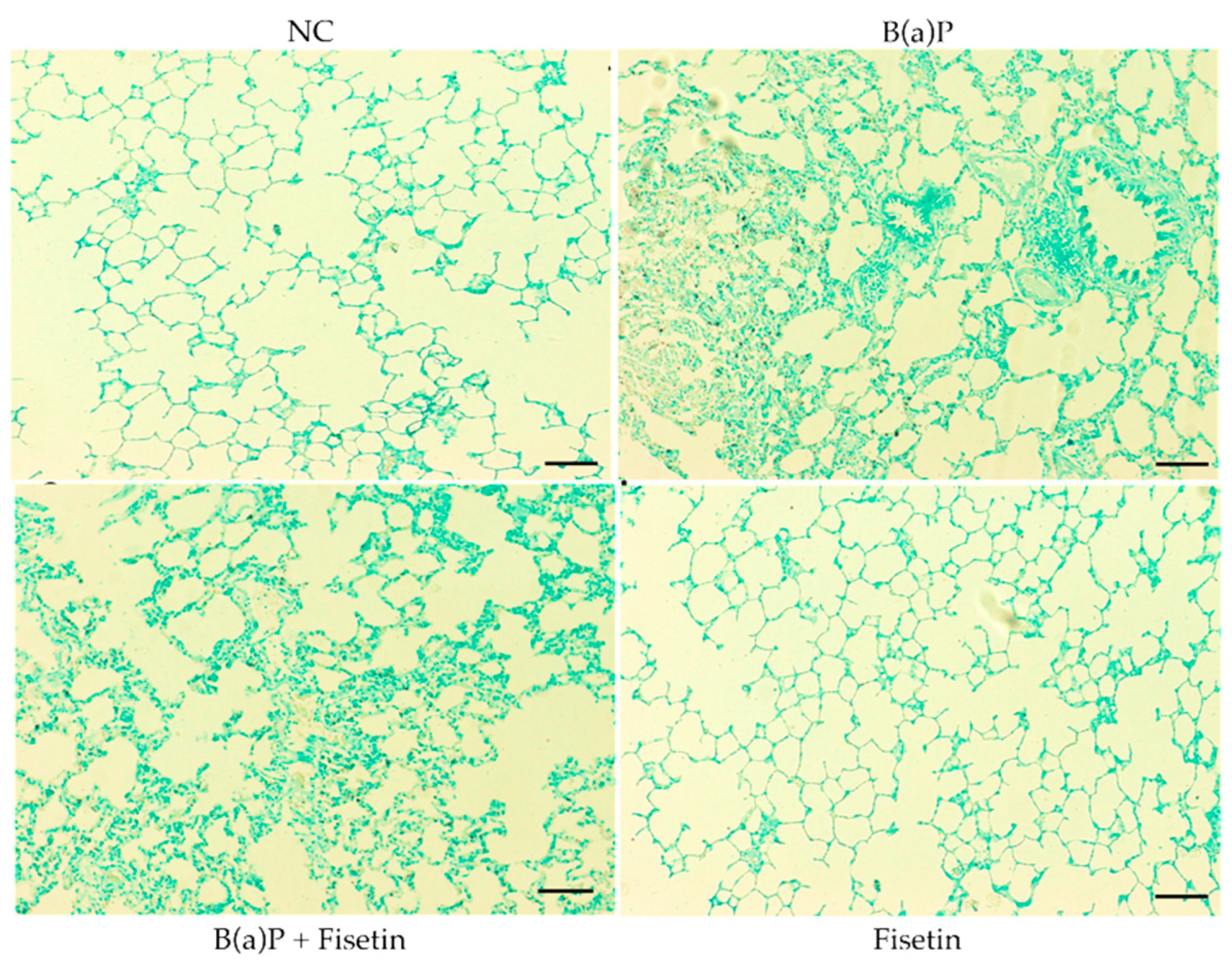
2.7. Evaluate Apoptosis Through TUNEL Assay (Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay)
2.8. Statistical Analysis
3. Results
3.1. Fisetin Attenuates Oxidative Stress Following Benzo(a)Pyrene Treatment
3.2. Fisetin Treatment Downregulates Inflammation and the Increase in Lipids Induced by B(a)P
3.3. Fisetin Alleviates Lung Tissue Damage Induced by Benzo(a)Pyrene
3.4. Fisetin Is Protective Against Apoptosis Caused by Benzo(a)Pyrene
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRD | Chronic Respiratory Disease |
| B(a)P | Benzo(a)Pyrene |
| Ft | Fisetin |
| COPD | Chronic Obstructive Pulmonary Disease |
| IHME | Institute for Health Metrics and Evaluation |
| PAHs | Polycyclic aromatic hydrocarbons |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| TLRs | Toll-Like Receptors |
| EGFR2 | Epidermal growth factor |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| SOD | Superoxide dismutases |
| CAT | Catalase |
| GPx | Glutathione peroxide |
| GSH | Glutathione |
| LPS | Lipopolysaccharide |
References
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Chronic Respiratory Diseases Collaborators; Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.-H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; et al. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S. Prevalence, incidence, morbidity and mortality rates of COPD in Saudi Arabia: Trends in burden of COPD from 1990 to 2019. PLoS ONE 2022, 17, e0268772. [Google Scholar] [CrossRef]
- Alomary, S.A.; Al Madani, A.J.; Althagafi, W.A.; Adam, I.F.; Elsherif, O.E.; Al-Abdullaah, A.A.; Al-Jahdali, H.; Jokhdar, H.A.; Alqahtani, S.H.; Nahhas, M.A.; et al. Prevalence of asthma symptoms and associated risk factors among adults in Saudi Arabia: A national survey from Global Asthma Network Phase I. World Allergy Organ. J. 2022, 15, 100623. [Google Scholar] [CrossRef]
- Humans IWGotEoCRt. Tobacco Smoke and Involuntary Smoking; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, and International Agency for Research on Cancer: Lyon, France, 2004; Volume 83, pp. 1–1438. [Google Scholar]
- Jiang, H.; Liu, Z.; Wei, P.; Zhang, F.; Wang, S.; Ou-Yang, W.B.; Li, X.; Pan, X.-B. Global, regional and national burdens of cardiovascular disease attributable to secondhand smoke from 1990–2019: An age-period-cohort analysis. Open Heart 2025, 12, e003079. [Google Scholar] [CrossRef]
- Al Moamary, M.S.; Al Ghobain, M.O.; Al Shehri, S.N.; Gasmelseed, A.Y.; Al-Hajjaj, M.S. Predicting tobacco use among high school students by using the global youth tobacco survey in Riyadh, Saudi Arabia. Ann. Thorac. Med. 2012, 7, 122–129. [Google Scholar] [CrossRef]
- Moradi-Lakeh, M.; El Bcheraoui, C.; Tuffaha, M.; Daoud, F.; Al Saeedi, M.; Basulaiman, M.; Memish, Z.A.; AlMazroa, M.A.; Al Rabeeah, A.A.; Mokdad, A.H. Tobacco consumption in the Kingdom of Saudi Arabia, 2013: Findings from a national survey. BMC Public Health 2015, 15, 611. [Google Scholar] [CrossRef]
- Berg, C.D.; Schiller, J.H.; Boffetta, P.; Cai, J.; Connolly, C.; Kerpel-Fronius, A.; Kitts, A.B.; Lam, D.C.; Mohan, A.; Myers, R.; et al. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J. Thorac. Oncol. 2023, 18, 1277–1289. [Google Scholar] [CrossRef]
- DeMarini, D.M.; Linak, W.P. Mutagenicity and carcinogenicity of combustion emissions are impacted more by combustor technology than by fuel composition: A brief review. Environ. Mol. Mutagen. 2022, 63, 135–150. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Shiizaki, K.; Kawanishi, M.; Yagi, T. Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ. 2017, 39, 14. [Google Scholar] [CrossRef] [PubMed]
- Omidian, K.; Rafiei, H.; Bandy, B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem. Toxicol. 2017, 106 Pt A, 165–174. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chen, Y.C.; Yu, A.L.; Yu, J. Benzo[a]pyrene induces fibrotic changes and impairs differentiation in lung stem cells. Ecotoxicol. Environ. Saf. 2021, 210, 111892. [Google Scholar] [CrossRef] [PubMed]
- Rylance, J.; Kankwatira, A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gao, X.; Dong, Q.; Sodergren, E.; Weinstock, G.M.; et al. Household air pollution and the lung microbiome of healthy adults in Malawi: A cross-sectional study. BMC Microbiol. 2016, 16, 182. [Google Scholar] [CrossRef]
- Li, C.; Zhihong, H.; Wenlong, L.; Xiaoyan, L.; Qing, C.; Wenzhi, L.; Siming, X.; Shengming, L. The Nucleotide-Binding Oligomerization Domain-Like Receptor Family Pyrin Domain-Containing 3 Inflammasome Regulates Bronchial Epithelial Cell Injury and Proapoptosis after Exposure to Biomass Fuel Smoke. Am. J. Respir. Cell Mol. Biol. 2016, 55, 815–824. [Google Scholar] [CrossRef]
- Krimmer, D.; Ichimaru, Y.; Burgess, J.; Black, J.; Oliver, B. Exposure to biomass smoke extract enhances fibronectin release from fibroblasts. PLoS ONE 2013, 8, e83938. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; et al. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018, 53, 81–95. [Google Scholar] [CrossRef]
- Chiruta, C.; Schubert, D.; Dargusch, R.; Maher, P. Chemical modification of the multitarget neuroprotective compound fisetin. J. Med. Chem. 2012, 55, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Chang, P.C.; Shen, Y.C.; Lin, C.; Tsai, C.F.; Chen, J.H.; Yeh, W.-L.; Wu, L.-H.; Lin, H.-Y.; Liu, Y.-S.; et al. Regulatory effects of fisetin on microglial activation. Molecules 2014, 19, 8820–8839. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jiang, Z.Y.; Sun, B.; Fu, J.; Li, T.Z. Fisetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury via TLR4-Mediated NF-kappaB Signaling Pathway in Rats. Inflammation 2016, 39, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Patra, J.K.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, X.; Huang, J.; Wu, M.; Zhang, S.; Wang, D.; Liu, S.; Fan, H. Fisetin Alleviated Bleomycin-Induced Pulmonary Fibrosis Partly by Rescuing Alveolar Epithelial Cells From Senescence. Front. Pharmacol. 2020, 11, 553690. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S. Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp. Ther. Med. 2018, 15, 2667–2673. [Google Scholar] [CrossRef]
- Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023, 16, 196. [Google Scholar] [CrossRef]
- Hussain, T.; Al-Attas, O.S.; Alamery, S.; Ahmed, M.; Odeibat, H.A.M.; Alrokayan, S. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J. Food Biochem. 2019, 43, e12962. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Khan, A.A.; Almatroodi, S.A. Quercetin, a Plant Flavonol Attenuates Diabetic Complications, Renal Tissue Damage, Renal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Metabolites 2023, 13, 130. [Google Scholar] [CrossRef]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective Effects of Thymoquinone, an Active Compound of Nigella sativa, on Rats with Benzo(a)pyrene-Induced Lung Injury through Regulation of Oxidative Stress and Inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef]
- Rahmani, A.; Alzohairy, M.; Khadri, H.; Mandal, A.K.; Rizvi, M.A. Expressional evaluation of vascular endothelial growth factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int. J. Clin. Exp. Pathol. 2012, 5, 195–202. [Google Scholar] [PubMed]
- Ansorge, N.; Dannecker, C.; Jeschke, U.; Schmoeckel, E.; Heidegger, H.H.; Vattai, A.; Burgmann, M.; Czogalla, B.; Mahner, S.; Fuerst, S. Regulatory T Cells with Additional COX-2 Expression Are Independent Negative Prognosticators for Vulvar Cancer Patients. Int. J. Mol. Sci. 2022, 23, 4662. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Bhardwaj, N.; Rajaura, S.; Chandra, H.; Singh, A.; Babu, R.; Gupta, N.J. Benzo (A) pyrene exposure alters alveolar epithelial and macrophage cells diversity and induces antioxidant responses in lungs. Toxicol. Rep. 2024, 13, 101777. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. The cytokine storm of COVID-19: A spotlight on prevention and protection. Expert Opin. Ther. Targets 2020, 24, 723–730. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Wu, Y.; Potempa, L.A.; El Kebir, D.; Filep, J.G. C-reactive protein and inflammation: Conformational changes affect function. Biol. Chem. 2015, 396, 1181–1197. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef]
- Broedl, U.C.; Jin, W.; Rader, D.J. Endothelial lipase: A modulator of lipoprotein metabolism upregulated by inflammation. Trends Cardiovasc. Med. 2004, 14, 202–206. [Google Scholar] [CrossRef]
- Negri, E.M.; Montes, G.S.; Saldiva, P.H.; Capelozzi, V.L. Architectural remodelling in acute and chronic interstitial lung disease: Fibrosis or fibroelastosis? Histopathology 2000, 37, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.-I.; Ryu, S.-Y.; Do, S.-H.; Lee, C.-S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharmacal Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Kang, O.H.; Lee, J.H.; Kwon, D.Y. Apigenin inhibits release of inflammatory mediators by blocking the NF-kappaB activation pathways in the HMC-1 cells. Immunopharmacol. Immunotoxicol. 2011, 33, 473–479. [Google Scholar] [CrossRef]
- Deng, C.; Dang, F.; Gao, J.; Zhao, H.; Qi, S.; Gao, M. Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon 2018, 4, e00898. [Google Scholar] [CrossRef]
- Zarkovic, K.; Jakovcevic, A.; Zarkovic, N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017, 111, 110–126. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Cha, J.W.; Zheng, J.; Yao, C.W.; Chae, S.; Hyun, J.W. Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. Vitr. Cell. Dev. Biol.-Anim. 2014, 50, 66–74. [Google Scholar] [CrossRef]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef]
- Zhao, H.; Eguchi, S.; Alam, A.; Ma, D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L155–L162. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhou, Y.; Zhu, Y.; Fei, M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem. Int. 2018, 118, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, J.H.; Chang, C.N.; Lu, D.Y.; Chang, P.C.; Wang, S.L.; Yeh, W.-L. Fisetin inhibits cell migration via inducing HO-1 and reducing MMPs expression in breast cancer cell lines. Food Chem. Toxicol. 2018, 120, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.T.; Mizokami, S.S.; Ferraz, C.R.; Manchope, M.F.; Borghi, S.M.; Fattori, V.; Calixto-Campos, C.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A. The granulopoietic cytokine granulocyte colony-stimulating factor (G-CSF) induces pain: Analgesia by rutin. Inflammopharmacology 2019, 27, 1285–1296. [Google Scholar] [CrossRef]
- Patil, R.H.; Babu, R.L.; Naveen Kumar, M.; Kiran Kumar, K.M.; Hegde, S.M.; Nagesh, R.; Ramesh, G.T.; Sharma, S.C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 2016, 39, 138–147. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1beta-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1alpha pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Dholakiya, S.L.; Benzeroual, K.E. Protective effect of diosmin on LPS-induced apoptosis in PC12 cells and inhibition of TNF-alpha expression. Toxicol. Vitr. 2011, 25, 1039–1044. [Google Scholar] [CrossRef]
- Erlinger, T.P.; Miller, E.R., 3rd; Charleston, J.; Appel, L.J. Inflammation modifies the effects of a reduced-fat low-cholesterol diet on lipids: Results from the DASH-sodium trial. Circulation 2003, 108, 150–154. [Google Scholar] [CrossRef]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A., Jr.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwanian, W.M. Fisetin Mitigates Chronic Lung Injury Induced by Benzo(a)Pyrene by Regulation of Inflammation and Oxidative Stress. Curr. Issues Mol. Biol. 2025, 47, 209. https://doi.org/10.3390/cimb47030209
Alwanian WM. Fisetin Mitigates Chronic Lung Injury Induced by Benzo(a)Pyrene by Regulation of Inflammation and Oxidative Stress. Current Issues in Molecular Biology. 2025; 47(3):209. https://doi.org/10.3390/cimb47030209
Chicago/Turabian StyleAlwanian, Wanian M. 2025. "Fisetin Mitigates Chronic Lung Injury Induced by Benzo(a)Pyrene by Regulation of Inflammation and Oxidative Stress" Current Issues in Molecular Biology 47, no. 3: 209. https://doi.org/10.3390/cimb47030209
APA StyleAlwanian, W. M. (2025). Fisetin Mitigates Chronic Lung Injury Induced by Benzo(a)Pyrene by Regulation of Inflammation and Oxidative Stress. Current Issues in Molecular Biology, 47(3), 209. https://doi.org/10.3390/cimb47030209



