Abstract
Familial Mediterranean fever (FMF) is an autoinflammatory genetic disorder characterized by recurrent fevers and inflammation of the serous membranes in the abdomen, lungs, and joints. Currently, the standard treatment of FMF includes colchicine, which is an alkaloid, derived from Colchicum autumnale. Colchicine’s efficacy in FMF is well-established as it is used both to prevent acute attacks and reduce the risk of long-term complications. However, despite these available treatments, 5–10% of patients exhibit resistance to the drug. It has been demonstrated that polymorphisms in several genes involved in inflammation can influence treatment outcomes and the risk of FMF complications like amyloidosis. Among them, some research focused on polymorphism affecting adenosine triphosphate (ATP)-binding cassette sub-family B member 1 (ABCB1) gene encoding for P-glycoprotein. P-glycoprotein is considered a key transporter protein as it regulates the absorption, distribution, and excretion of several drugs, including colchicine. In diseases like FMF, ABCB1 polymorphisms have been shown to affect the response to colchicine, potentially leading to treatment resistance or altered toxicity. Based on this evidence, this systematic review aims to analyze available evidence on ABCB1-mediated colchicine transport and its clinical implications in FMF, showing how relevant ABCB1 variants are in response to therapy.
1. Introduction
Familial Mediterranean fever (FMF), also called “periodic peritonitis” or “Reimann syndrome”, is an autoinflammatory genetic disorder characterized by recurrent fevers and inflammation of the serous membranes in the abdomen, lungs, and joints, resulting in significant pain [1,2]. The initial episode often occurs in childhood, typically before the age of 20, and it may be accompanied by a rash or headache [1,2].
As the most prevalent of the periodic fever syndromes, FMF predominantly affects individuals of Mediterranean and Middle Eastern descent, which is reflected in its name [1].
FMF is an autosomal recessive disease [1]. The gene responsible for FMF is the MEFV gene, which is located on the short arm of chromosome 16 (16 p13.3) [2]. In the last years, approximately 300 different mutations of the MEFV gene have been identified and associated to FMF [2].
Currently, the treatment for FMF includes colchicine, an alkaloid obtained from Colchicum autumnale [2]. Colchicine reduces the frequency and severity of FMF attacks by decreasing inflammation through neutrophil chemotaxis inhibition [1,3]. Typically prescribed as a daily medication, colchicine can also prevent complications such as amyloidosis, which can arise from prolonged inflammation [2]. However, colchicine can cause several side effects such as diarrhea and vomiting, which are often dose-dependent [2]. Other uncommon side effects are myelosuppression, hepatotoxicity, nephrotoxicity, myopathy, neuropathy, and hypersensitivity reaction [1,3]. Nevertheless, other treatments have been recognized for FMF treatment, such as interleukin-1 (IL-1) inhibitors (e.g., anakinra, canakinumab), which represent the second-line drugs for patients who have colchicine-resistant FMF or who have an intolerance to colchicine [2,4].
Despite these available treatments, it is essential to take genetic polymorphisms into account in the era of personalized medicine. These variations play a vital role in understanding individual responses to therapies, particularly in inflammatory conditions like FMF. In effect, some studies focused on different polymorphisms involved in FMF and treatment response [5,6]. Among them, great attention has been focused on genetic variants affecting adenosine triphosphate (ATP)-binding cassette sub-family B member 1 (ABCB1, also known as MDR1 or P-glycoprotein), which is an important membrane transporter that plays a key role in the efflux of various substances out of cells [5,6].
Polymorphisms in the ABCB1 gene can affect how drugs, including colchicine, are processed in the body, affecting the efficacy and safety of treatment. The clinical effect of these polymorphisms can help to tailor therapies for patients with FMF, ensuring better management of their symptoms and reducing the risk of adverse effects. ABCB1 polymorphisms may influence also the interaction of colchicine with other medications, further complicating treatment regimens [7,8,9,10].
However, the lack of conclusive data on the influence of ABCB1 polymorphisms on colchicine response in FMF significantly impacts clinical practice because it limits the ability to tailor treatment and optimize therapeutic outcomes. Without a clear understanding of how genetic variations in ABCB1 affect the efficacy or toxicity of colchicine, physicians are forced to rely on standard dosage regimens, which may not be suitable for all patients. This systematic review aims to provide a comprehensive summary of the available evidence, paving the way for future research that could clarify the relationship between ABCB1 polymorphisms and colchicine response in order to design customized strategies to improve FMF clinical management. This information would allow the development of more tailored treatment strategies, improving both efficacy and safety for patients with FMF.
The success of pharmacogenetics in other inflammatory diseases, such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), where genetic factors like TPMT (thiopurine methyltransferase) have already been integrated into clinical practice [11,12], highlights the potential benefits of applying similar personalized approaches in FMF. By leveraging genetic data, clinicians can more accurately predict drug responses and avoid adverse effects, ultimately enhancing patient care.
Thus, in this study, we aimed to explore if variations in the ABCB1 gene can affect the transport and efficacy of colchicine. In particular, by systematically reviewing existing research, the study seeks to understand the impact of ABCB1 polymorphism rs1045642 on colchicine treatment outcomes for improving FMF management.
2. Methods
2.1. Search Strategy
We conducted a comprehensive literature search using the bibliographic databases Web of Science, Embase (via OVID), and PubMed (MEDLINE). To ensure a rigorous search strategy, we adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) as previously done [13]. The search was performed by A.A. and S.A.S., focusing exclusively on English-language publications and utilizing the eligibility criteria detailed in Table 1.

Table 1.
Inclusion and exclusion criteria employed for the literature search.
Two content experts (A.P.C. and E.E.) oversaw the search strategy development and the research process. The search included articles published from the inception until December 2024. No geographic restrictions were applied. Studies were considered if they focused on ABCB1 transporter linked with colchicine administration in the pathological context of FMF.
The search terms, detailed in Table 2, comprised combinations of keywords related to ABCB1, colchicine, and FMF.

Table 2.
Combinations of keywords used for the search plan.
2.2. Study Selection
We carried out our research using the PubMed (MEDLINE), Embase (OVID), and Web of Science databases to identify relevant records. After the initial search, we removed any duplicate entries. Two review authors (A.A. and S.A.S.) independently screened the titles and abstracts to exclude irrelevant studies. Full-text articles were then thoroughly examined to select those meeting our inclusion criteria. A third reviewer (A.P.C.) was involved in resolving any disagreements between the initial reviewers.
Two authors (A.A. and S.A.S.) performed data extraction from the selected studies. The information collected included study population, geographical location, year of publication, article title, author(s), and associated clinical outcomes.
2.3. Assessment of Risk of Bias
Two reviewers (A.A. and S.A.S.) independently assessed the quality of the studies included in this systematic review using the Newcastle–Ottawa Scale (NOS; see Supplementary Table S1). The studies were classified as having a low, moderate, or high risk of bias according to their NOS scores, in line with previous methodologies [14,15,16]. Discrepancies in scoring were resolved through the involvement of a third reviewer (A.P.C.). The following cutoffs were applied: scores below 4 indicated a “high risk of bias”, scores between 4 and 6 represented an “intermediate risk of bias”, and scores above 6 indicated a “low risk of bias”. The following nine criteria of the NOS score were employed: representative of the exposed cohort, selection of external control, ascertainment of exposure, outcome of interest does not present at the start of the study, comparability of cohorts (main factor and additional factor), assessment of outcomes, sufficient follow-up time, and adequacy of follow-up.
Based on these criteria, all the studies were rated as having a “low risk of bias.” The individual assessments for each study are detailed in Supplementary Table S1.
3. Results
3.1. Findings from Systematic Search
Using our established search strategy, we retrieved a total of 1157 records from PubMed (MEDLINE), Web of Science, and Embase (OVID). After removing duplicate entries, 1005 articles remained. Titles and abstracts were then screened to assess eligibility, which resulted in the exclusion of 997 articles that did not meet the criteria. This left eight articles for full-text review. Studies were excluded during the full-text review phase if they did not meet the inclusion criteria. Four did not fit the required study design, or failed to provide clear data (Supplementary Table S2).
Ultimately, as outlined in Figure 1, four studies met the inclusion criteria and were included in this systematic review.
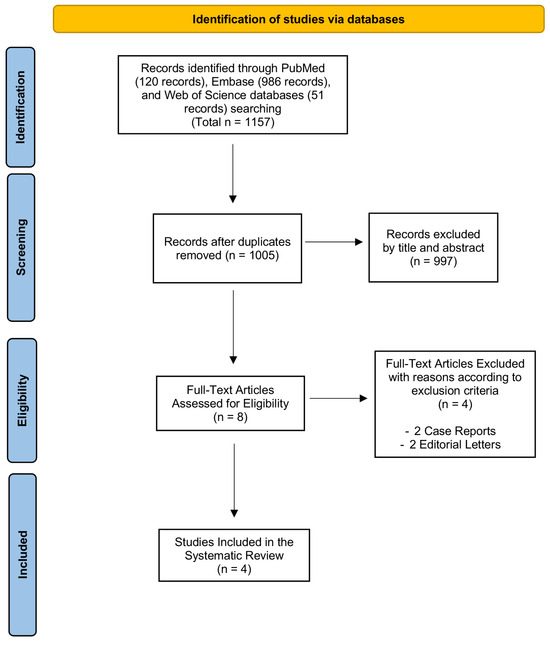
Figure 1.
PRISMA flow diagram. The PRISMA flow diagram outlines the entire selection process, starting from the identification stage and concluding with the final inclusion of articles for the systematic review.
3.2. Description of Included Studies in the Systematic Review
This systematic review collected four different studies, all rated as “low risk of bias” following NOS assessment.
In the last years, many single nucleotide polymorphisms of the ABCB1 gene have been identified. Among these, in particular, the synonymous variant c.3435T>C (p.Ile1145=) in exon 26 (rs1045642) has been reported to be associated with the function and amount of expression of ABCB1 [6,17]. In this context, Tufan and colleagues [6] evaluated the clinical relevance of this polymorphism in the ABCB1 gene for colchicine efficacy in 120 FMF patients. Among them, 98 patients were evaluated as responders and 22 as non-responders to colchicine. The distributions of ABCB1 CC, CT, and TT genotypes were considerably different between responsive and nonresponsive groups [6]. Colchicine resistance was significantly higher in patients harboring the C allele than in patients with the TT genotype. Similarly, the mean colchicine dose to prevent remission was significantly lower in the TT group compared with subjects with the C allele [6].
Ozen et al. [17] studied the association between the C3435T polymorphism and colchicine-resistant FMF patients by using total genomic DNA samples from 52 FMF patients with colchicine unresponsiveness.
The results obtained by Ozen and colleagues showed increased T allele frequency in colchicine non-responder FMF patients, suggesting that C3435T polymorphism was associated with colchicine resistance [17].
Another study by Dogruer and colleagues [18] investigated the impact of ABCB1 polymorphisms on bioavailability of colchicine in 48 FMF patients showing there was no significant gender difference. Moreover, no significant relationship was found between colchicine doses that would introduce optimal clinical response and ABCB1 polymorphisms in FMF carrier patients [18].
Later, Uludag and colleagues [5] examined the relationship between ABCB1 C3435T polymorphism and colchicine response in 50 FMF patients. After the detection of variant C3435T by real-time polymerase chain reaction, patients were divided into three groups: patients who recovered from episodes with standard colchicine treatment and had no attack in the last 1 year defined as complete; patients whose episode number and intensity were decreased with the ongoing standard treatment defined as partial; and patients whose episodes were not decreased despite the standard treatment defined as non-responders [5]. These data suggested that C3435T polymorphism enacts an important role in colchicine response in FMF, showing that good response to colchicine treatment was related to the presence of the C allele, whereas poor response was related to the T allele in FMF-treated patients [5]. The studies cited in this paragraph are summarized in Table 3. The relationship between ABCB1 C3435T polymorphism and colchicine resistance in FMF patients is shown according to PICOTS in Table 4.

Table 3.
Overview of the included studies.

Table 4.
Relationship between ABCB1 C3435T polymorphism and colchicine resistance in FMF patients according to PICOTS.
4. Discussion
In diseases like FMF, ABCB1 polymorphisms have been shown to affect the response to colchicine, potentially leading to treatment resistance or altered toxicity [19,20].
In this systematic review, data from a total of 270 FMF patients from the Mediterranean area treated with colchicine are collected. No adverse events related to the treatment have been reported.
In the general cohort, all the FMF-treated patients are genotyped for the C3435T variant to evaluate the possible association with drug response. In only three of the four selected studies, we observed a total of 94 FMF patients considered not responding or partially responding to the colchicine treatment. We observed a comparable total number of non-responsive patients between homozygous CC and TT patients (20 vs. 21) and 53 non-responsive patients in the heterozygous group for the C3435T variant in ABCB1.
The other 127 FMF patients are considered responsive to colchicine treatment.
In the study by Dogruer et al. [18], we can only report the frequency of this genotype evaluated in a group of FMF patients in treatment with colchicine.
The comprehension of the clinical impact of the variant C3435T in the ABCB1 gene remains uncertain, but this polymorphism as well as others could help in tailoring the therapy, optimizing efficacy and long-term outcome, especially for help to evaluate in a pre-emptive way the possibility to start a different therapy in case of colchicine resistance.
Canakinumab, which is a monoclonal antibody targeting IL-1β, provides an effective alternative for managing febrile attacks in these resistant cases, even in the absence of certain genetic markers associated with FMF [21]. A recent study suggests that while colchicine is effective for a majority of Japanese FMF patients, a notable subset exhibits resistance. This research underscores the importance of personalized treatment approaches based on individual patient responses and genetic backgrounds [22].
Also, three case studies where FMF patients were either unresponsive or intolerant to conventional treatments showed significant improvement with canakinumab therapy [23].
Another promising therapeutic option for FMF patients is anakinra, an interleukin-1 receptor antagonist, as reported in a randomized controlled trial evaluating the efficacy and safety of anakinra in patients with FMF who do not respond to colchicine treatment [24]. In a retrospective study of 2021, the authors reported a comparison between the two treatments; as stated, both anakinra and canakinumab are effective for FMF management, but canakinumab demonstrates a more favorable safety profile. The potential for serious side effects associated with short- and long-term anakinra usage must be considered in clinical practice [22].
Some limitations should be considered in the current review; firstly, the few included studies. The number of patients is limited, but we have to consider that FMF is an underdiagnosed genetic disorder, prevalent in countries surrounding the Mediterranean Sea, with differences in distribution reported as approximately 1–5 people per 10,000 [25]. Linked to this, we must underline that all the FMF patients in the selected studies are Turkish residents and Mediterranean natives because Turkey is the country with the greatest prevalence of FMF.
Moreover, the collected findings can be subjected to potential bias due to different variables. We included observational studies based on FMF cohorts’ patients that can differ regarding MEFV genotype and severity of the disease. Also, age, sex, and other clinical complications, as well as individual pharmacological treatments, can have an impact and confuse the results. The question if this single polymorphism C3435T in the ABCB1 gene can be associated with a different response to colchicine in FMF patients remains open. More clear evidence will be collected in a larger cohort of FMF patients, and of course, with the use of high-throughput techniques such as Next-Generation Sequencing (NGS), the approach can be more global to better define other pharmacogenetic variants associated with clinical response in colchicine treatment.
5. Conclusions
Especially in complex conditions such as inflammatory disorders, every scientific advance can help the life-long clinical management of patients to control their symptoms and prescribe safer and more effective therapeutic options. In this scenario, for FMF patients, new biological drugs targeting interleukin-1 represent a good alternative to the standard colchicine treatment.
A lot of attention is related to the genetic profiles of every FMF patient that can be associated with the severity of the disease and the drug response. In fact, 5–10% of patients exhibit colchicine resistance, and this definition is still controversial also as the underlying mechanism is not fully understood [26]. However, in this clinical setting, it remains mandatory to treat the affecting patients life-long to mitigate their chronic status of inflammation. To date, canakinumab and anakinra represent a significant therapeutic option for managing various inflammatory disorders, particularly those involving dysregulated IL-1 activity, including FMF.
For this reason, it is useful to collect data on the presence of polymorphisms that can guide therapeutic choices in a rapid and personalized manner’ in this case, we consider a synonymous variant C3435T in the ABCB1 gene associated with an altered expression of a transport protein that regulates the efflux of colchicine from cells.
In the future, more evidence resulting from integrated data about genetics, pharmacology, and clinics would help to tailor the treatments and improve FMF patients’ quality of life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb47030210/s1. Ref. [27] are cited in Supplementary Materials file.
Author Contributions
Conceptualization, E.E. and A.P.C.; methodology, S.A.S. and A.A.; software, S.A.S. and A.A.; writing—original draft preparation, S.A.S. and A.A.; writing—review and editing, A.P.C.; supervision, A.P.C. and E.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| FMF | familial Mediterranean fever |
| ABCB1 | adenosine triphosphate (ATP)-binding cassette sub-family B member 1 |
| IL-1β | interleukin-1β |
References
- Ozdogan, H.; Ugurlu, S. Familial Mediterranean Fever. Presse Med. 2019, 48, e61–e76. [Google Scholar] [CrossRef]
- Bhatt, H.; Cascella, M. Familial Mediterranean Fever. In StatPearl; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kallinich, T.; Haffner, D.; Niehues, T.; Huss, K.; Lainka, E.; Neudorf, U.; Schaefer, C.; Stojanov, S.; Timmann, C.; Keitzer, R.; et al. Colchicine use in children and adolescents with familial Mediterranean fever: Literature review and consensus statement. Pediatrics 2007, 119, e474–e483. [Google Scholar] [CrossRef]
- Yalcin-Mutlu, M.; Icacan, O.C.; Yildirim, F.; Temiz, S.A.; Fagni, F.; Schett, G.; Tascilar, K.; Minopoulou, I.; Burul, G.; Bes, C. IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort. J. Clin. Med. 2024, 13, 3375. [Google Scholar] [CrossRef]
- Uludag, A.; Silan, C.; Atik, S.; Akurut, C.; Uludag, A.; Silan, F.; Ozdemir, O. Relationship between response to colchicine treatment and MDR1 polymorphism in familial Mediterranean fever patients. Genet Test Mol. Biomark. 2014, 18, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.; Babaoglu, M.O.; Akdogan, A.; Yasar, U.; Calguneri, M.; Kalyoncu, U.; Karadag, O.; Hayran, M.; Ertenli, A.I.; Bozkurt, A.; et al. Association of drug transporter gene ABCB1 (MDR1) 3435C to T polymorphism with colchicine response in familial Mediterranean fever. J. Rheumatol. 2007, 34, 1540–1544. [Google Scholar] [PubMed]
- Schwier, N.C.; Cornelio, C.K.; Boylan, P.M. A systematic review of the drug–drug interaction between statins and colchicine: Patient characteristics, etiologies, and clinical management strategies. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Kone-Paut, I.; Gül, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum 2017, 47, 115–120. [Google Scholar] [CrossRef]
- Giat, E.; Ben-Zvi, I.; Lidar, M.; Livneh, A. The preferential use of anakinra in various settings of FMF: A review applied to an updated treatment-related perspective of the disease. Int. J. Mol. Sci. 2022, 23, 3956. [Google Scholar] [CrossRef]
- Koochaki, R.; Amini, E.; Zarehossini, S.; Zareh, D.; Haftcheshmeh, S.M.; Jha, S.K.; Kesharwani, P.; Shakeri, A.; Sahebkar, A. Alkaloids in Cancer therapy: Targeting the tumor microenvironment and metastasis signaling pathways. Fitoterapia 2024, 179, 106222. [Google Scholar] [CrossRef]
- Elawi, A.M.; Irshaid, Y.M.; Ismail, S.I.; Mustafa, K.N. Thiopurine S-methytransferase gene polymorphism in rheumatoid arthritis. Arch. Med. Res. 2013, 44, 105–109. [Google Scholar] [CrossRef]
- Carvalho, A.T.; Esberard, B.C.; Froes, R.S.; Rapozo, D.C.; Grinman, A.B.; Simao, T.A.; Santos, J.C.; Carneiro, A.J.; Ribeiro-Pinto, L.F.; de Souza, H.S. Thiopurine-methyltransferase variants in inflammatory bowel disease: Prevalence and toxicity in Brazilian patients. World J. Gastroenterol. 2014, 20, 3327–3334. [Google Scholar] [CrossRef]
- Capra, A.P.; Ardizzone, A.; Crupi, L.; Calapai, F.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Efficacy of Palmitoylethanolamide and Luteolin Association on Post-Covid Olfactory Dysfunction: A Systematic Review and Meta-Analysis of Clinical Studies. Biomedicines 2023, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ardizzone, A.; Panto, G.; Paterniti, I.; Campolo, M.; Crupi, L.; Squeri, R.; Esposito, E. The Prognostic Value of Pentraxin-3 in COVID-19 Patients: A Systematic Review and Meta-Analysis of Mortality Incidence. Int. J. Mol. Sci. 2023, 24, 3537. [Google Scholar] [CrossRef]
- Capra, A.P.; Ardizzone, A.; Briuglia, S.; La Rosa, M.A.; Mondello, S.; Campolo, M.; Esposito, E. A Systematic Review and Meta-Analysis of the Association between the FV H1299R Variant and the Risk of Recurrent Pregnancy Loss. Biology 2022, 11, 1608. [Google Scholar] [CrossRef]
- Crupi, L.; Ardizzone, A.; Calapai, F.; Scuderi, S.A.; Benedetto, F.; Esposito, E.; Capra, A.P. The Impact of COVID-19 on Amputation and Mortality Rates in Patients with Acute Limb Ischemia: A Systematic Review and Meta-Analysis. Diseases 2024, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Ozen, F.; Silan, C.; Uludag, A.; Candan, F.; Silan, F.; Ozdemir, S.; Atik, S.; Ozdemir, O. Association between ABCB1 (MDR1) gene 3435 C > T polymorphism and colchicine unresponsiveness of FMF patients. Ren. Fail. 2011, 33, 899–903. [Google Scholar] [CrossRef]
- Dogruer, D.; Tug, E.; Bes, C.; Soy, M. Lack of an effect of CYP3A4 and MDR1 gene polymorphisms on colchicine pharmacogenetics in the treatment of Familial Mediterranean fever. Genet. Mol. Res. 2013, 12, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Berg, S.; Wekell, P.; Fasth, A.; Hawkins, P.N.; Lachmann, H. Autoinflammatory disorders. Prim. Immunodefic. Dis. Defin. Diagn. Manag. 2017, 393–435. [Google Scholar]
- Şahin, A.; Derin, M.E.; Albayrak, F.; Karakaş, B.; Karagöz, Y. Assessment of effectiveness of anakinra and canakinumab in patients with colchicine-resistant/unresponsive familial Mediterranean fever. Adv. Rheumatol. 2020, 60, 12. [Google Scholar] [CrossRef]
- Yoshida, S.; Sumichika, Y.; Saito, K.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; Asano, T.; Sato, S.; Migita, K. Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study. J. Clin. Med. 2023, 12, 6272. [Google Scholar] [CrossRef] [PubMed]
- Tsinti, M.; Tsitsami, E. Indications and efficacy of canakinumab in Familial Mediterranean Fever: Report of three cases. Pediatr. Rheumatol. 2014, 12, P253. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Kukuy, O.; Giat, E.; Pras, E.; Feld, O.; Kivity, S.; Perski, O.; Bornstein, G.; Grossman, C.; Harari, G.; et al. Anakinra for Colchicine-Resistant Familial Mediterranean Fever: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2017, 69, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Lancieri, M.; Bustaffa, M.; Palmeri, S.; Prigione, I.; Penco, F.; Papa, R.; Volpi, S.; Caorsi, R.; Gattorno, M. An Update on Familial Mediterranean Fever. Int. J. Mol. Sci. 2023, 24, 9584. [Google Scholar] [CrossRef]
- Erden, A.; Batu, E.D.; Sari, A.; Sonmez, H.E.; Armagan, B.; Demir, S.; Firat, E.; Bilginer, Y.; Bilgen, S.A.; Karadag, O.; et al. Which definition should be used to determine colchicine resistance among patients with familial Mediterranean fever? Clin. Exp. Rheumatol. 2018, 36, 97–102. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).