Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance
Abstract
1. Introduction
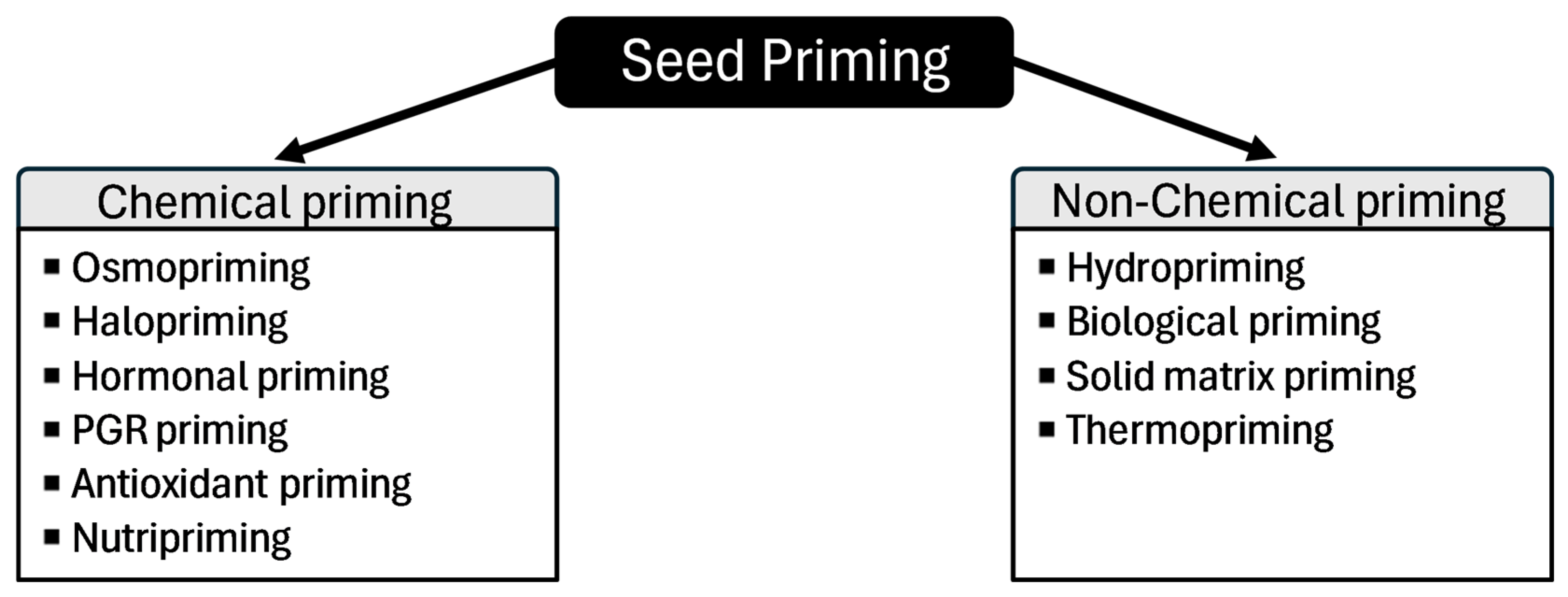
2. Seed Priming Techniques
2.1. Non-Chemical Priming
2.2. Chemical Priming
2.2.1. Osmopriming
2.2.2. Halopriming
2.2.3. Hormonal Priming
2.2.4. Plant Growth Regulator Priming
2.2.5. Nutripriming
2.2.6. Other Chemical Priming
3. Commonly Used SPAs and Their Effects
3.1. Salicylic Acid
3.2. Zinc
3.3. Gibberellic Acid
3.4. Potassium Nitrate
3.5. Selenium
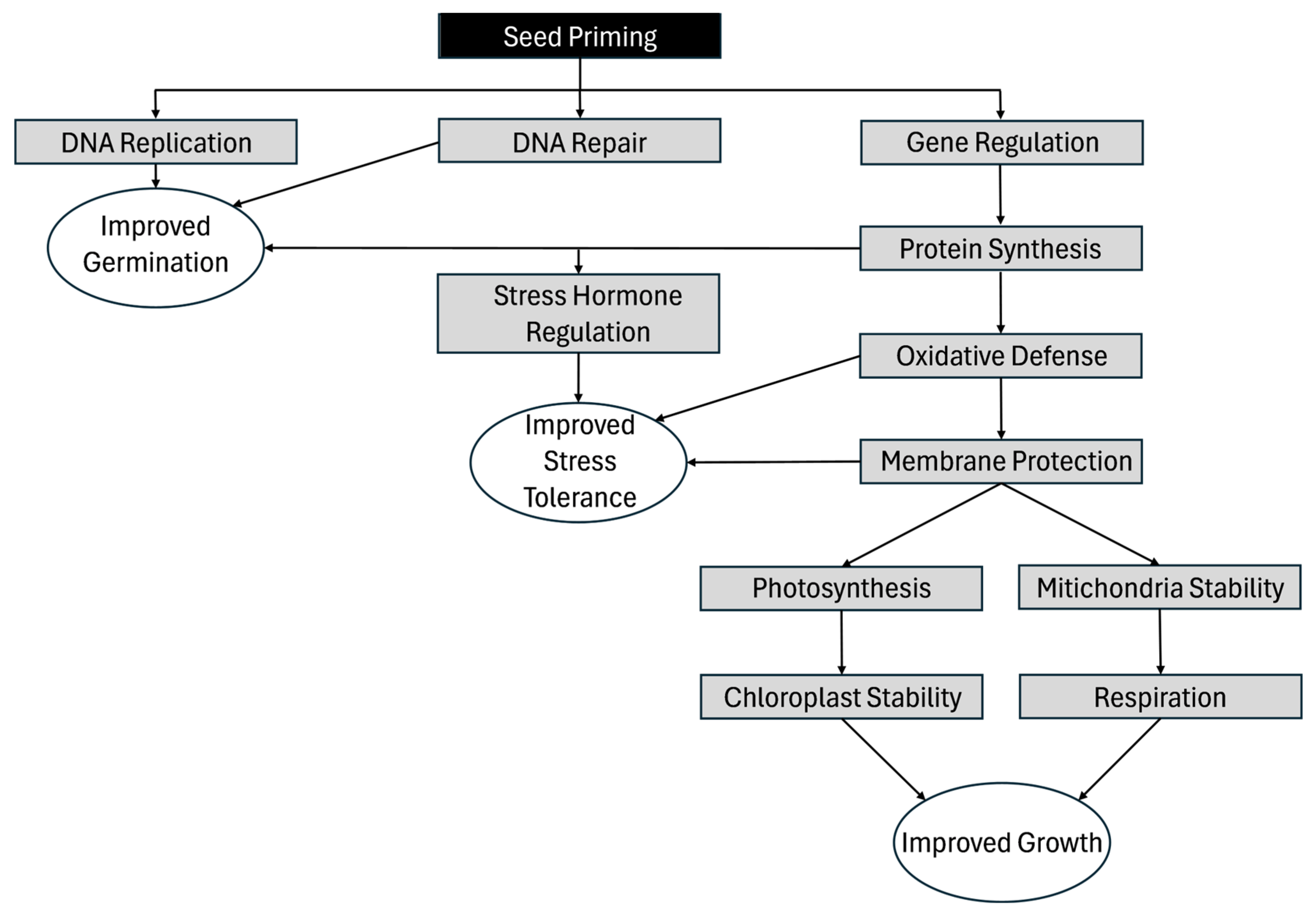
4. SPA Modes of Action
4.1. Overview
4.2. Transcriptomic and Translatomic Changes
4.3. Proteomic Changes
4.4. Metabolomic Changes
4.5. Epigenetics and Plant Memory
5. Nanoparticle Priming Agents
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.R.; Skeg, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, S.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Spinoni, J.; Barbosa, P.; De Jager, A.; McCormick, N.; Naumann, G.; Vogt, J.V.; Magni, D.; Masante, D.; Mazzeschi, M. A new global database of meteorological drought events from 1951 to 2016. J. Hydrol. Reg. Stud. 2019, 22, 100593. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Gong, Y.-H.; Zhang, F.; Ren, J.; Bai, X.-K.; Zheng, Y. Which Temperature and Precipitation Extremes Best Explain the Variation of Warm versus Cold Years and Wet versus Dry Years? J. Clim. 2018, 31, 45–59. [Google Scholar] [CrossRef]
- Lehmann, J.; Coumou, D.; Frieler, K. Increased record-breaking precipitation events under global warming. Clim. Change 2015, 132, 501–515. [Google Scholar] [CrossRef]
- Van Oort, P.A.J.; Zwart, S.J. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Glob. Change Biol. 2018, 24, 1029–1045. [Google Scholar] [CrossRef]
- Davenport, F.; Funk, C.; Galu, G. How will East African maize yields respond to climate change and can agricultural development mitigate this response? Clim. Change 2018, 147, 491–506. [Google Scholar] [CrossRef]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Change 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Change 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Mariem, S.B.; Soba, D.; Zhou, B.; Loladze, I.; Morales, F.; Aranjuelo, I. Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects. Plants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Gebeyaw, M. Role of Seed Priming on Seed Quality: A Review. Int. J. Plant Breed. Crop Sci. 2020, 7, 779–784. [Google Scholar]
- MacDonald, M.T.; Kannan, R.; Jayaseelan, R. Ascorbic Acid Preconditioning Effect on Broccoli Seedling Growth and Photosynthesis under Drought Stress. Plants 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Lada, R.R.; Stiles, A.; Blake, T.J. The effects of natural and synthetic seed preconditioning agents (SPAs) in hastening seedling emergence and enhancing yield and quality of processing carrots. Sci. Hortic. 2005, 106, 25–37. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Robinson, A.R.; Hoyle, J. The Benefits of Ambiol® in Promoting Germination, Growth, and Drought Tolerance can be Passed on to Next-Generation Tomato Seedlings. J. Plant Growth Regul. 2010, 29, 357–365. [Google Scholar] [CrossRef]
- Simma, B.; Polthanee, A.; Goggi, A.S.; Siri, B.; Promkhambut, A.; Caragea, P.C. Wood vinegar seed priming improves yield and suppresses weeds in dryland direct-seeding rice under rainfed production. Agron. Sustain. Dev. 2017, 37, 56. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Robinson, A.R.; Hoyle, J. Seed Preconditioning with Natural and Synthetic Antioxidants Induces Drought Tolerance in Tomato Seedlings. HortScience 2009, 44, 1323–1329. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Khatun, M. Seed Priming Methods: Application in Field Crops and Future Perspectives. Asian J. Res. Crop Sci. 2020, 5, 8–19. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-Sowing Seed Treatment—A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield Under Saline and Non-Saline Conditions. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 88, pp. 223–271. ISBN 978-0-12-000786-8. [Google Scholar]
- Paul, S.; Dey, S.; Kundu, R. Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 2022, 97, 215–234. [Google Scholar] [CrossRef]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Sci. Hortic. 2019, 255, 161–168. [Google Scholar] [CrossRef]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; van Genuchten, M.T. Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.). Agric. Water Manag. 2020, 231, 106022. [Google Scholar] [CrossRef]
- Anwar, M.P.; Jahan, R.; Rahman, M.R.; Islam, A.K.M.M.; Uddin, F.M.J. Seed priming for increased seed germination and enhanced seedling vigor of winter rice. IOP Conf. Ser. Earth Environ. Sci. 2021, 756, 012047. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Impact of engineered nanomaterials either alone or loaded with NPK on growth and productivity of French bean plants: Seed priming vs foliar application. S. Afr. J. Bot. 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Majda, C.; Khalid, D.; Aziz, A.; Rachid, B.; Badr, A.-S.; Lotfi, A.; Mohamed, B. Nutri-priming as an efficient means to improve the agronomic performance of molybdenum in common bean (Phaseolus vulgaris L.). Sci. Total Environ. 2019, 661, 654–663. [Google Scholar] [CrossRef]
- Bezhin, K.; Santel, H.-J.; Gerhards, R. The Effect of Sugar Beet Seed Priming on Sugar Beet Yield and Weed Suppressive Ability. J. Plant Sci. 2018, 6, 146–156. [Google Scholar]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.; Siddique, K. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4614-4692-7. [Google Scholar]
- Savaedi, Z.; Parmoon, G.; Moosavi, S.A.; Bakhshande, A. The role of light and Gibberellic Acid on cardinal temperatures and thermal time required for germination of Charnushka (Nigella sativa) seed. Ind. Crop. Prod. 2019, 132, 140–149. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Cross-Stress Toler. Stress Mem. Plants 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef] [PubMed]
- Catiempo, R.L.; Photchanachai, S.; Bayogan, E.R.V.; Wongs-Aree, C. Impact of hydropriming on germination and seedling establishment of sunflower seeds at elevated temperature. Plant Soil Environ. 2021, 67, 491–498. [Google Scholar] [CrossRef]
- Caseiro, R.; Bennett, M.; Marcos-Filho, J. Comparison of three priming techniques for onion seed lots differing in initial seed quality. Seed Sci. Technol. 2004, 32, 356–375. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Chadordooz-Jeddi, A.; Nasrullahzadeh, S.; Moghaddam, M. Influence of hydro-priming duration on field performance of pinto bean (Phaseolus vulgaris L.) cultivars. Afr. J. Agric. Res. 2010, 5, 893–897. [Google Scholar]
- Hardegree, S.P. Optimization of Seed Priming Treatments to Increase Low-Temperature Germination Rate. J. Range Manag. 1996, 49, 87. [Google Scholar] [CrossRef]
- Min, T.-G.; Seo, B.-M. Optimum conditions for tobacco seed priming by PEG 6000. Korean J. Crop Sci. 1999, 44, 263–266. [Google Scholar]
- Callan, N.W.; Mathre, D.; Miller, J.B. Biopriming seed treatment for biological control of Pythium ultimum preemergence damping-off in sh-2 sweet corn. Plant Dis. 1990, 74, 368–372. [Google Scholar] [CrossRef]
- Taylor, A.G.; Harman, G.E.; Nielsen, P.A. Biological Seed Treatments using Trichoderma harzianum for Horticultural Crops. HortTechnology 1994, 4, 105–109. [Google Scholar] [CrossRef]
- Migahid, M.M.; Elghobashy, R.M.; Bidak, L.M.; Amin, A.W. Priming of Silybum marianum (L.) Gaertn seeds with H2O2 and magnetic field ameliorates seawater stress. Heliyon 2019, 5, e01886. [Google Scholar] [CrossRef]
- Afzal, I.; Saleem, S.; Skalicky, M.; Javed, T.; Bakhtavar, M.A.; Ul Haq, Z.; Kamran, M.; Shahid, M.; Sohail Saddiq, M.; Afzal, A.; et al. Magnetic Field Treatments Improves Sunflower Yield by Inducing Physiological and Biochemical Modulations in Seeds. Molecules 2021, 26, 2022. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.; Wahid, A.; Ahmad, N. Changes in Nutrient-Homeostasis and Reserves Metabolism During Rice Seed Priming: Consequences for Seedling Emergence and Growth. Agric. Sci. China 2010, 9, 191–198. [Google Scholar] [CrossRef]
- Chatterjee, N.; Sarkar, D.; Sankar, A.; Pal, S.; Singh, H.B.; Singh, R.K.; Bohra, J.S.; Rakshit, A. On-farm seed priming interventions in agronomic crops. Acta Agric. Slov. 2018, 111, 715–735. [Google Scholar] [CrossRef]
- Darwin, C. Effect of salt-water on the germination of seeds. Gard. Chron. Agric. Gaz. 1855, 47, 773. [Google Scholar]
- Darwin, C. Does sea water kill seeds? Gard. Chron. Agric. Gaz. 1855, 242, 356–357. [Google Scholar]
- Ells, J.E. The influence of treating tomato seed with nutrient solutions on emergence rate and seedling growth. Proc. Am. Soc. Hortic. Sci. 1963, 83, 684–687. [Google Scholar]
- Singh, A.; Dahiru, R.; Musa, M.; Sani Haliru, B. Effect of Osmopriming Duration on Germination, Emergence, and Early Growth of Cowpea (Vigna unguiculata (L.) Walp.) in the Sudan Savanna of Nigeria. Int. J. Agron. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Afifa, R.I.; Islam, N.; Choudhury, S. Effects of Priming on Onion Seed Germination and Field Performance during Summer Sowing. J. Sci. Res. Rep. 2024, 30, 252–258. [Google Scholar] [CrossRef]
- Moradi, A.; Younesi, O. Effects of Osmo- and Hydro-priming on Seed Parameters of Grain Sorghum (Sorghum bicolor L.). Aust. J. Basic Appl. Sci. 2009, 3, 1696–1700. [Google Scholar]
- Moradi, L.; Siosemardeh, A. Combination of seed priming and nutrient foliar application improved physiological attributes, grain yield, and biofortification of rainfed wheat. Front. Plant Sci. 2023, 14, 1287677. [Google Scholar] [CrossRef]
- Paul, A.; Mondal, S.; Chakraborty, K.; Biswas, A.K. Moving forward to understand the alteration of physiological mechanism by seed priming with different halo-agents under salt stress. Plant Mol. Biol. 2024, 114, 24. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J.A.D. The Five “Classical” Plant Hormones. Plant Cell 1997, 7, 1197–1210. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Kumari, S.; Nazir, F.; Khanna, R.R.; Gupta, R.; Chhillar, H. Defensive Role of Plant Hormones in Advancing Abiotic Stress-Resistant Rice Plants. Rice Sci. 2023, 30, 15–35. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Nunes Da Silva, M.; Carvalho, S.M.P.; Rodrigues, A.M.; Gómez-Cadenas, A.; António, C.; Vasconcelos, M.W. Defence-related pathways, phytohormones and primary metabolism are key players in kiwifruit plant tolerance to Pseudomonas syringae pv. actinidiae. Plant Cell Environ. 2022, 45, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Singh, H.B. (Eds.) Advances in Seed Priming; Springer: Singapore, 2018; ISBN 9789811300318. [Google Scholar]
- Zulfiqar, F.; Ashraf, M. Bioregulators: Unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol. Biol. 2021, 105, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Siddique, K.H.M. Micronutrient application through seed treatments: A review. J. Soil Sci. Plant Nutr. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Hortic. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Iqbal, S.; Farooq, M.; Cheema, S.A. Boron seed priming improves the seedling emergence, growth, grain yield and grain biofortification of bread wheat. Int. J. Agric. Biol. 2017, 19, 177–182. [Google Scholar] [CrossRef]
- Sadeghizadeh, M.; Zarea, M.J. Effects of seed priming with zinc on germination, nursery seedling growth and paddy fields yield of two rice (Oryza sativa L.) cultivars. J. Crop Sci. Biotechnol. 2022, 25, 313–324. [Google Scholar] [CrossRef]
- Sharma, P.; Gautam, A.; Kumar, V.; Guleria, P. MgO nanoparticles mediated seed priming inhibits the growth of lentil (Lens culinaris). Vegetos 2022, 35, 1128–1141. [Google Scholar] [CrossRef]
- Ling, Y.; Zhao, Y.; Cheng, B.; Tan, M.; Zhang, Y.; Li, Z. Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress. Plants 2022, 11, 2015. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Effect of chitosan seed priming on mungbean seedlings subjected to different levels of water potential. Acta Physiol. Plant. 2023, 45, 6. [Google Scholar] [CrossRef]
- Kaushal, K.; Rajani, K.; Kumar, R.R.; Ranjan, T.; Kumar, A.; Ahmad, M.F.; Kumar, V.; Kumar, V.; Kumar, A. Physio-biochemical responses and crop performance analysis in chickpea upon botanical priming. Sci. Rep. 2024, 14, 9342. [Google Scholar] [CrossRef] [PubMed]
- Shumaila; Ullah, S.; Shah, W.; Hafeez, A.; Ali, B.; Khan, S.; Ercisli, S.; Al-Ghamdi, A.A.; Elshikh, M.S. Biochar and Seed Priming Technique with Gallic Acid: An Approach toward Improving Morpho-Anatomical and Physiological Features of Solanum melongena L. under Induced NaCl and Boron Stresses. ACS Omega 2023, 8, 28207–28232. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hao, X.; Wang, T.; Li, H.; Shi, X.; Liu, Y.; Luo, H. Seed Priming with Gibberellin Regulates the Germination of Cotton Seeds Under Low-Temperature Conditions. J. Plant Growth Regul. 2023, 42, 319–334. [Google Scholar] [CrossRef]
- Oğuz, M.Ç.; Oğuz, E.; Güler, M. Seed priming with essential oils for sustainable wheat agriculture in semi-arid region. PeerJ 2023, 11, e15126. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H.; Abbey, L. Seed priming with pyroligneous acid mitigates aluminum stress, and promotes tomato seed germination and seedling growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- Silva, P.C.C.; Azevedo Neto, A.D.; Gheyi, H.R.; Ribas, R.F.; Silva, C.R.R.; Cova, A.M.W. Seed priming with H2O2 improves photosynthetic efficiency and biomass production in sunflower plants under salt stress. Arid Land Res. Manag. 2022, 36, 283–297. [Google Scholar] [CrossRef]
- Hossinifarahi, M.; Moazen, H.A.; Amiri, A.; Jowkar, M.M.; Mottaghipisheh, J. Evaluation of Seed Priming and Culture Media to Improve the Germination Performance and Quality of Sweet Pepper and Eggplant Seedlings. Int. J. Hortic. Sci. Technol. 2022, 9, 415–428. [Google Scholar]
- Dawoud, T.M.; Akhtar, N.; Okla, M.K.; Shah, A.N.; Shah, A.A.; Abdel-Mawgoud, M.; AbdElgayed, G.; Al-Hashimi, A.; AbdElgawad, H. Seed Priming with Pomegranate Peel Extract Improves Growth, Glucosinolates Metabolism and Antimicrobial Potential of Brassica oleraceae Varieties. J. Plant Growth Regul. 2023, 42, 3043–3055. [Google Scholar] [CrossRef]
- El Sayed, A.I.; Rafudeen, M.S.; Ganie, S.A.; Hossain, M.S.; Gomaa, A.M. Seed priming with cypress leaf extract enhances photosynthesis and antioxidative defense in zucchini seedlings under salt stress. Sci. Hortic. 2022, 293, 110707. [Google Scholar] [CrossRef]
- Ignatenko, A.A.; Talanova, V.V.; Repkina, N.S.; Titov, A.F. Effect of Salicylic Acid on Antioxidant Enzymes and Cold Tolerance of Cucumber Plants. Russ. J. Plant Physiol. 2021, 68, 491–498. [Google Scholar] [CrossRef]
- Alam, A.U.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Seed Priming Enhances Germination and Morphological, Physio-Biochemical, and Yield Traits of Cucumber under Water-Deficit Stress. J. Soil Sci. Plant Nutr. 2023, 23, 3961–3978. [Google Scholar] [CrossRef]
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic Elucidation of Salicylic Acid and Sulphur-Induced Defence Systems, Nitrogen Metabolism, Photosynthetic, and Growth Potential of Mungbean (Vigna radiata) Under Salt Stress. J. Plant Growth Regul. 2021, 40, 1000–1016. [Google Scholar] [CrossRef]
- Alam, A.U.; Ullah, H.; Himanshu, S.K.; Praseartkul, P.; Tisarum, R.; Cha-um, S.; Datta, A. Seed Priming and Foliar Application of Salicylic Acid is Equally Beneficial in Mitigating Drought Stress in Cucumber. J. Soil Sci. Plant Nutr. 2023, 23, 6299–6316. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Thuenprom, N.; Tisarum, R.; Cha-um, S.; Datta, A. Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Anwar, S.; Iqbal, M.; Raza, S.H.; Iqbal, N. Efficacy of Seed Preconditiong with Salicylic and Ascorbic Acid in Increasing Vigor of Rice (Oryza sativa L.) Seedling. Pak. J. Bot. 2013, 45, 157–162. [Google Scholar]
- Ceritoglu, M.; Erman, M.; Çığ, F.; Ceritoglu, F.; Uçar, Ö.; Soysal, S.; El Sabagh, A. Enhancement of Root System Architecture, Seedling Growth, and Germination in Lentil under Salinity Stress by Seed Priming with Siliconand Salicylic Acid. Pol. J. Environ. Stud. 2023, 32, 4481–4491. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Liu, Z.; Tian, J.; Liang, L.; Qiu, Y.; Wang, G.; Du, Q.; Cheng, D.; Cai, H.; et al. A high activity zinc transporter OsZIP9 mediates zinc uptake in rice. Plant J. 2020, 103, 1695–1709. [Google Scholar] [CrossRef]
- Dang, K.; Mu, J.; Tian, H.; Gao, D.; Zhou, H.; Guo, L.; Shao, X.; Geng, Y.; Zhang, Q. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol. 2024, 24, 464. [Google Scholar] [CrossRef]
- Rahman, A.; Islam, R.; Azim, A.; Skalicky, M.; Hossain, A. Chapter 5—Role of Zinc for Abiotic Stress Tolerance in Plants. In Zinc in Plants; Tripathi, D.K., Singh, V.P., Pandey, S., Sharma, S., Chauhan, D.K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 95–148. ISBN 978-0-323-91314-0. [Google Scholar]
- Imran, M.; Mahmood, A.; Neumann, G.; Boelt, B. Zinc Seed Priming Improves Spinach Germination at Low Temperature. Agriculture 2021, 11, 271. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ahmad, R.; Basra, S.M.A. Seed priming with zinc improves the germination and early seedling growth of wheat. Seed Sci. Technol. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn application through seed priming improves productivity and grain nutritional quality of silage corn. Saudi J. Biol. Sci. 2022, 29, 103456. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Shah, H. ‘On-farm’ seed priming with zinc sulphate solution—A cost-effective way to increase the maize yields of resource-poor farmers. Field Crop. Res. 2007, 102, 119–127. [Google Scholar] [CrossRef]
- Sharma, M.; Parmar, D.K.; Sharma, S.K. On-farm seed priming with zinc nutrition: A cost effective way to increase the yield of resource poor farmers. J. Plant Nutr. 2021, 44, 2371–2384. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, M.; Masood, A. Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions. Curr. Sci. 2011, 100, 998–1007. [Google Scholar]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant Development and Crop Yield: The Role of Gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhao, D.-D.; Ning, Q.-R.; Wei, J.-P.; Li, Y.; Wang, M.-M.; Liu, X.-L.; Jiang, C.-J.; Liang, Z.-W. A Multi-year Beneficial Effect of Seed Priming with Gibberellic Acid-3 (GA3) on Plant Growth and Production in a Perennial Grass, Leymus chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef]
- Gnawali, A.; Subedi, R. Gibberellic acid priming enhances maize seed germination under low water potential. Indones. J. Agric. Sci. 2021, 22, 17. [Google Scholar] [CrossRef]
- Jyoti, B.; Gaurav, S.S.; Pant, U. Use of growth regulators as priming agent for improvement of seed vigour in tomato (Lycopersicum esculentum). J. Appl. Nat. Sci. 2016, 8, 84–87. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crop. Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Tsegay, B.A.; Andargie, M. Seed Priming with Gibberellic Acid (GA3) Alleviates Salinity Induced Inhibition of Germination and Seedling Growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. J. Crop Sci. Biotechnol. 2018, 21, 261–267. [Google Scholar] [CrossRef]
- Younesi, O.; Moradi, A. Effect Of Priming Of Seeds Of Medicago Sativa ‘Bami’ With Gibberellic Acid On Germination, Seedlings Growth And Antioxidant Enzymes Activity Under Salinity Stress. J. Hortic. Res. 2015, 22, 167–174. [Google Scholar] [CrossRef]
- Chunthaburee, S.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Alleviation of Salt Stress in Seedlings of Black Glutinous Rice by Seed Priming with Spermidine and Gibberellic Acid. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 405–413. [Google Scholar] [CrossRef]
- Thongtip, A.; Mosaleeyanon, K.; Korinsak, S.; Toojinda, T.; Darwell, C.T.; Chutimanukul, P.; Chutimanukul, P. Promotion of seed germination and early plant growth by KNO3 and light spectra in Ocimum tenuiflorum using a plant factory. Sci. Rep. 2022, 12, 6995. [Google Scholar] [CrossRef]
- Ruttanaruangboworn, A.; Chanprasert, W.; Tobunluepop, P.; Onwimol, D. Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). J. Integr. Agric. 2017, 16, 605–613. [Google Scholar] [CrossRef]
- Ahmadvand, G.; Soleimani, F.; Pouya, M. Effect of Seed Priming with Potassium Nitrate on Germination and Emergence Traits of Two Soybean Cultivars under Salinity Stress Conditions. IDOSI Publ. 2012, 12, 769–774. [Google Scholar]
- Moaaz Ali, M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Choudhury, A.; Bordolui, S.K. Inducement of Seed Priming with Potassium Nitrate on quality Performance of Chickpea (Cicer arietinum L.). Biol. Forum—Int. J. 2022, 14, 4. [Google Scholar]
- Dhillon, B.S.; Kumar, V.; Sagwal, P.; Kaur, N.; Singh Mangat, G.; Singh, S. Seed Priming with Potassium Nitrate and Gibberellic Acid Enhances the Performance of Dry Direct Seeded Rice (Oryza sativa L.) in North-Western India. Agronomy 2021, 11, 849. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Cha-um, S.; Tisarum, R.; Datta, A. Effect of seed priming with potassium nitrate on growth, fruit yield, quality and water productivity of cantaloupe under water-deficit stress. Sci. Hortic. 2021, 288, 110354. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Abou El-hamd, N.; Ahmed, E.Z. Red Kidney Bean (Phaseolus vulgaris L.) Germination and Seedling Growth as affected by Selenium, Nano- Selenium and Sulfur. J. Plant Physiol. Pathol. 2021, 9, 261. [Google Scholar]
- Shafiq, S.; Adeel, M.; Raza, H.; Iqbal, R. Effects of foliar application of selenium in maize (Zea mays L.) under cadmium toxicity. Biol. Forum–Int. J. 2019, 11, 2. [Google Scholar]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium seed priming enhanced the growth of salt-stressed Brassica rapa L. through improving plant nutrition and the antioxidant system. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Geng, M.; Wahid, A.; ur Rehman, H. Seed Priming with Selenium: Consequences for Emergence, Seedling Growth, and Biochemical Attributes of Rice. Biol. Trace Elem. Res. 2015, 166, 236–244. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) Seed Priming Induced Growth and Biochemical Changes in Wheat Under Water Deficit Conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Hu, F.; Jiang, S.; Wang, Z.; Hu, K.; Xie, Y.; Zhou, L.; Zhu, J.; Xing, D.; Du, B. Seed priming with selenium: Effects on germination, seedling growth, biochemical attributes, and grain yield in rice growing under flooding conditions. Plant Direct 2022, 6, e378. [Google Scholar] [CrossRef]
- Gholami, S.; Dehaghi, M.A.; Rezazadeh, A.; Naji, A.M. Seed germination and physiological responses of quinoa to selenium priming under drought stress. Bragantia 2022, 81, e0722. [Google Scholar] [CrossRef]
- Cai, L.; Abbey, L.; MacDonald, M. Changes in Endogenous Carotenoids, Flavonoids, and Phenolics of Drought-Stressed Broccoli Seedlings After Ascorbic Acid Preconditioning. Plants 2024, 13, 3513. [Google Scholar] [CrossRef]
- Baltazar, M.; Reis, S.; Carvalho, A.; Lima-Brito, J. Cytological and yield-related analyses in offspring of primed bread wheat (Triticum aestivum L.) seeds. Genet. Resour. Crop Evol. 2021, 68, 359–370. [Google Scholar] [CrossRef]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed priming can enhance and retain stress tolerance in ensuing generations by inducing epigenetic changes and trans-generational memory. Physiol. Plant. 2023, 175, e13881. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Ozbingol, N.; Corbineau, F.; Groot, S.P.C.; Bino, R.J.; Come, D. Activation of the Cell Cycle in Tomato (Lycopersicon esculentum Mill.) Seeds during Osmoconditioning as Related to Temperature and Oxygen. Ann. Bot. 1999, 84, 245–251. [Google Scholar] [CrossRef]
- de Castro, R.D.; van Lammeren, A.A.M.; Groot, S.P.C.; Bino, R.J.; Hilhorst, H.W.M. Cell Division and Subsequent Radicle Protrusion in Tomato Seeds Are Inhibited by Osmotic Stress But DNA Synthesis and Formation of Microtubular Cytoskeleton Are Not1. Plant Physiol. 2000, 122, 327–336. [Google Scholar] [CrossRef]
- Sharma, S.N.; Maheshwari, A. Expression patterns of DNA repair genes associated with priming small and large chickpea (Cicer arietinum) seeds. Seed Sci. Tech. 2015, 43, 250–261. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Wang, X.; Wu, S.; Wang, X. Ascorbate–glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J. Plant Physiol. 2011, 168, 226–232. [Google Scholar] [CrossRef]
- Chojnowski, M.; Corbineau, F.; Côme, D. Physiological and biochemical changes induced in sunflower seeds by osmopriming and subsequent drying, storage and aging. Seed Sci. Res. 1997, 7, 323–332. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of seed quality: From present to future. Seed Sci. Res. 2012, 22, S61–S68. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Waqas Mazhar, M.; Ishtiaq, M.; Maqbool, M.; Mahmoud, E.A.; Ullah, F.; Elansary, H.O. Optimizing bitter gourd (Momordica charantia L.) performance: Exploring the impact of varied seed priming durations and zinc oxide nanoparticle concentrations on germination, growth, phytochemical attributes, and agronomic outcomes. Cogent Food Agric. 2024, 10, 2313052. [Google Scholar] [CrossRef]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Kazemeini, S.A.; Alinia, M.; Mastinu, A. The effect of seed priming with melatonin on improving the tolerance of Zea mays L. var saccharata to paraquat-induced oxidative stress through photosynthetic systems and enzymatic antioxidant activities. Physiol. Mol. Plant Pathol. 2023, 124, 101967. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Torun, H.; Dosedělová, V.; Tarkowski, P.; Ayaz, F.A. Exogenous melatonin ameliorates ionizing radiation-induced damage by modulating growth, osmotic adjustment and photosynthetic capacity in wheat seedlings. Plant Physiol. Biochem. 2022, 187, 67–76. [Google Scholar] [CrossRef]
- Anwar, A.; Yu, X.; Li, Y. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Hafeez, A.; Adrees, M.; ur Rehman, M.Z.; Rizwan, M.; Ali, S. Effect of different seed priming agents on chromium accumulation, oxidative defense, glyoxalase system and mineral nutrition in canola (Brassica napus L.) cultivars. Environ. Pollut. 2022, 309, 119769. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Moulick, D.; Santra, S.C.; Ghosh, D. Rice seed priming with Se: A novel approach to mitigate As induced adverse consequences on growth, yield and As load in brown rice. J. Hazard. Mater. 2018, 355, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zogli, P.; Pingault, L.; Grover, S.; Louis, J. Ento(o)mics: The intersection of ‘omic’ approaches to decipher plant defense against sap-sucking insect pests. Biot. Interact. AGRI 2019 2020, 56, 153–161. [Google Scholar] [CrossRef]
- Sazegari, S.; Zinati, Z.; Tahmasebi, A. Dynamic transcriptomic analysis uncovers key genes and mechanisms involved in seed priming-induced tolerance to drought in barley. Gene Rep. 2020, 21, 100941. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Jing, J.; Li, Z.; Ahmed, A.; Shi, Y.; Yang, Y.; Huang, J.; Zhang, W. Transcriptomic Analyses Reveal Carbon Dots-Based Seed priming in the Regulation of Root Growth in Rice. J. Plant Growth Regul. 2023, 42, 7614–7623. [Google Scholar] [CrossRef]
- Hussain, S.; Yin, H.; Peng, S.; Khan, F.A.; Khan, F.; Sameeullah, M.; Hussain, H.A.; Huang, J.; Cui, K.; Nie, L. Comparative Transcriptional Profiling of Primed and Non-primed Rice Seedlings under Submergence Stress. Front. Plant Sci. 2016, 7, 1125. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Zannino, L.; Pagano, P.; Doria, E.; Dondi, D.; Macovei, A.; Biggiogera, M.; Araújo, S.d.S.; Balestrazzi, A. Changes in genotoxic stress response, ribogenesis and PAP (3’-phosphoadenosine 5’-phosphate) levels are associated with loss of desiccation tolerance in overprimed Medicago truncatula seeds. Plant Cell Environ. 2022, 45, 1457–1473. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M. Lost in Translation: Physiological Roles of Stored mRNAs in Seed Germination. Plants 2020, 9, 347. [Google Scholar] [CrossRef]
- Catusse, J.; Meinhard, J.; Job, C.; Strub, J.; Fischer, U.; Pestsova, E.; Westhoff, P.; Dorsselaer, A.; Job, D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics 2011, 11, 1569–1580. [Google Scholar] [CrossRef]
- Lokdarshi, A.; Papdi, C.; Pettkó-Szandtner, A.; Dorokhov, S.; Scheres, B.; Magyar, Z.; Von Arnim, A.G.; Bögre, L.; Horváth, B.M. ErbB-3 Binding Protein 1 Regulates Translation and Counteracts Reinoblastoma Related to Maintain the Root Meristem. Plant Physiol. 2020, 182, 919–932. [Google Scholar] [CrossRef]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic Proteomics Emphasizes the Importance of Selective mRNA Translation and Protein Turnover during Arabidopsis Seed Germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Yan, H.; Mao, P. Comparative Time-Course Physiological Responses and Proteomic Analysis of Melatonin Priming on Promoting Germination in Aged Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci. 2021, 22, 811. [Google Scholar] [CrossRef] [PubMed]
- Fercha, A.; Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Samperi, R.; Stampachiacchiere, S.; Laganà, A. Comparative analysis of metabolic proteome variation in ascorbate-primed and unprimed wheat seeds during germination under salt stress. J. Proteom. 2014, 108, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Li, N.; Li, S.; Guo, J.; Li, X. Parental salt priming improves the low temperature tolerance in wheat offspring via modulating the seed proteome. Plant Sci. 2022, 324, 111428. [Google Scholar] [CrossRef] [PubMed]
- İşeri, Ö.D.; Sahin, F.I.; Haberal, M. Sodium Chloride Priming Improves Salinity Response of Tomato at Seedling Stage. J. Plant Nutr. 2014, 37, 374–392. [Google Scholar] [CrossRef]
- Vanitha, C.; Kathiravan, M.; Umarani, R.; Sathiya, K.; Menaka, C.; Yuvaraj, M.; Cyriac, J. Seed Priming with Nano Silica Alleviates Drought Stress through Regulating Antioxidant Defense System and Osmotic Adjustment in Soybean (Glycine max L.). Silicon 2024, 16, 2157–2170. [Google Scholar] [CrossRef]
- Jatana, B.S.; Grover, S.; Ram, H.; Baath, G.S. Seed Priming: Molecular and Physiological Mechanisms Underlying Biotic and Abiotic Stress Tolerance. Agronomy 2024, 14, 2901. [Google Scholar] [CrossRef]
- Hussain, S.; Zheng, M.; Khan, F.; Khaliq, A.; Fahad, S.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci. Rep. 2015, 5, 8101. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Dong, Q.; An, J.; Song, W.; Guan, Y.; He, F.; Huang, Y.; Hu, J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017, 83, 27–41. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Alcantara, B.K.; Rizzi, V.; Gaziola, S.A.; Azevedo, R.A. Soluble amino acid profile, mineral nutrient and carbohydrate content of maize kernels harvested from plants submitted to ascorbic acid seed priming. Ann. Braz. Acad. Sci. 2016, 89, 695–704. [Google Scholar] [CrossRef]
- Rice-Evans, C. Screening of Phenolics and Flavonoids for Antioxidant Activity. In Antioxidant Food Supplements in Human Health; Packer, L., Hiramatsu, M., Yoshikawa, T., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 239–253. ISBN 978-0-12-543590-1. [Google Scholar]
- Marcos, F.C.C.; Silveira, N.M.; Mokochinski, J.B.; Sawaya, A.C.H.F.; Marchiori, P.E.R.; Machado, E.C.; Souza, G.M.; Landell, M.G.A.; Ribeiro, R.V. Drought tolerance of sugarcane is improved by previous exposure to water deficit. J. Plant Physiol. 2018, 223, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Virlouvet, L.; Avenson, T.J.; Du, Q.; Zhang, C.; Liu, N.; Fromm, M.; Avramova, Z.; Russo, S.E. Dehydration Stress Memory: Gene Networks Linked to Physiological Responses During Repeated Stresses of Zea mays. Front. Plant Sci. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Zhang, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Salicylic acid and cold priming induce late-spring freezing tolerance by maintaining cellular redox homeostasis and protecting photosynthetic apparatus in wheat. Plant Growth Regul. 2020, 90, 109–121. [Google Scholar] [CrossRef]
- Bäurle, I.; Trindade, I. Chromatin regulation of somatic abiotic stress memory. J. Exp. Bot. 2020, 71, 5269–5279. [Google Scholar] [CrossRef]
- Manoharlal, R.; Saiprasad, G.V.S.; Kovařík, A. Gene-specific DNA demethylation changes associates with ethylene induced germination of soybean [Glycine max (L.) Merrill]. Plant Physiol. Rep. 2019, 24, 272–277. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Kim, M.; Ralph, P.J.; Marín-Guirao, L.; Pernice, M.; Procaccini, G. Stress Memory in Seagrasses: First Insight Into the Effects of Thermal Priming and the Role of Epigenetic Modifications. Front. Plant Sci. 2020, 11, 494. [Google Scholar] [CrossRef]
- Manoharlal, R.; Saiprasad, G.V.S. Global histone H3 hyperacetylation-associated epigenetic changes induced in ethephon-primed sprouts of soybean [Glycine max (L.) Merrill]. Acta Physiol. Plant. 2020, 42, 26. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are There Unidentified Factors Involved in the Germination of Nanoprimed Seeds? Front. Plant Sci. 2020, 11, 832. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed Priming with Zinc Oxide Nanoparticles to Enhance Crop Tolerance to Environmental Stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef] [PubMed]

| Common Primer (% of Studies) | Primer Class | Efficacy (%) | Plants Studied |
|---|---|---|---|
| 1. Salicylic acid (18%) | Hormone | 100 | Peppers, chia, sesame, barley, peas, cotton, rice, wheat, barley, cucumber, lentils, mung bean, corn, canola, cantaloupe, wild sunflower, kidney bean, zucchini, eggplant, cumin, hargel |
| 2. Zn (16%) | Nutri | 100 | Millet, gourd, corn, mung bean, peas, rice, spinach, chick peas, wheat, sesame, quinoa, corn, rice, peppers, eggplant, safflower, lettuce |
| 3. Gibberellic acid (13%) | Hormone | 90 | Carrot, tomato, sunflower, rice, legumes, alhagi, okra, cotton, barley, hargel, cucumber, tomato, wheat, cowpea, chia, lettuce |
| 4. KNO3 (12%) | Halo | 68 | Carrot, lettuce, sunflower, rice, wheat, mustard, cotton, tomato, cucumber, chick pea, wild sunflower, corn, cowpea, pepper, chia |
| 5. Se (9%) | Nutri | 100 | Quinoa, tomato, jalapeno, turnip, bok choy, wheat, sorghum, mustard, rice, canola |
| 6. CaCl2 (9%) | Halo | 86 | Lettuce, barley, mustard, wheat, peas, rice, allium, canola |
| 7. PEG (8%) | Osmo | 92 | Tomato, wheat, Chinese skullcap, onion, cauliflower, peppers, allium, tomato |
| 8. Chitosan (7%) | Other | 100 | Ashwagandha, corn, lettuce, mung bean, wheat, clover, cumin |
| 9. Melatonin (5%) | PGR | 100 | Zinnia, peanuts, halophytes, wheat, corn, rice, triticale |
| 10. Ascorbic acid (5%) | PGR | 71 | Rice, wheat, molinga, broccoli, stevia |
| Seed Priming Agent | Crop | Modes of Action |
|---|---|---|
| Selenium | Rice, Wheat | Activates antioxidant enzymes (SOD, POD, CAT), increases chlorophyll content, and enhances stress tolerance [122,125]. |
| Ascorbic Acid | Broccoli | Increases carotenoid content, protects chloroplasts, maintains photosynthetic machinery, and modulates ABA. |
| Zn Oxide Nanoparticles | Bitter Gourd | Enhances phenolic and flavonoid content, and improves phytochemical profile [137]. |
| Sodium Nitroprusside | Wheat | Activates antioxidant defense, enhances phenolic content, and improves photosynthesis and transpiration [138]. |
| Melatonin | Maize | Reduces membrane permeability, enhances photosynthetic efficiency, and protects chlorophyll [139,140]. |
| Gibberellic Acid | Cucumber, Rapeseed | Increases photosynthesis, transpiration rates, and chlorophyll content [141,142]. |
| Salicylic Acid | Zucchini | Enhances photosynthesis, antioxidant capacity, and chlorophyll content [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, M.T.; Mohan, V.R. Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Curr. Issues Mol. Biol. 2025, 47, 177. https://doi.org/10.3390/cimb47030177
MacDonald MT, Mohan VR. Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Current Issues in Molecular Biology. 2025; 47(3):177. https://doi.org/10.3390/cimb47030177
Chicago/Turabian StyleMacDonald, Mason T., and Vijaya R. Mohan. 2025. "Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance" Current Issues in Molecular Biology 47, no. 3: 177. https://doi.org/10.3390/cimb47030177
APA StyleMacDonald, M. T., & Mohan, V. R. (2025). Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Current Issues in Molecular Biology, 47(3), 177. https://doi.org/10.3390/cimb47030177







