Diagnostic Approaches in Myeloid Sarcoma
Abstract
1. Introduction
2. Historical Perspective
3. Epidemiology
4. Etiopathogenesis of EM AML
5. Clinical Presentation
6. Diagnosis of MS
7. Cytomorphology
8. Histopathology
9. Flow Cytometry
10. Genetics
11. Imaging Tests
12. Differential Diagnosis
13. Treatment of MS
14. Prognostic Implications
15. Conclusions
Author Contributions
Funding
Use of Artificial Intelligence
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2008. [Google Scholar]
- Deschler, B.; Lubbert, M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Tallman, M.S.; Douer, D.; Yahalom, J. How I treat extramedullary acute myeloid leukemia. Blood 2011, 118, 3785–3793. [Google Scholar] [CrossRef] [PubMed]
- Anekpuritanang, T.; Klairmont, M.M.; Gradowski, J.; Hagiwara, K.; Bailey, N.G.; Chandra, P.; Liu, Y.; Mulder, H.L.; Easton, J.; Zhang, J.; et al. In a multi-institutional cohort of myeloid sarcomas, NFE2 mutation prevalence is lower than previously reported. Blood Adv. 2021, 5, 5057–5059. [Google Scholar] [CrossRef]
- Jutzi, J.S.; Basu, T.; Pellmann, M.; Kaiser, S.; Steinemann, D.; Sanders, M.A.; Hinai, A.S.A.; Zeilemaker, A.; Bojtine Kovacs, S.; Koellerer, C.; et al. Altered NFE2 activity predisposes to leukemic transformation and myelosarcoma with AML-specific aberrations. Blood 2019, 133, 1766–1777. [Google Scholar] [CrossRef]
- Lazarevic, V.; Orsmark-Pietras, C.; Lilljebjorn, H.; Pettersson, L.; Rissler, M.; Lubking, A.; Ehinger, M.; Juliusson, G.; Fioretos, T. Isolated myelosarcoma is characterized by recurrent NFE2 mutations and concurrent preleukemic clones in the bone marrow. Blood 2018, 131, 577–581. [Google Scholar] [CrossRef]
- Avni, B.; Koren-Michowitz, M. Myeloid sarcoma: Current approach and therapeutic options. Ther. Adv. Hematol. 2011, 2, 309–316. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.I.; Ishimaru, T.; McGregor, D.H.; Okada, H.; Steer, A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, hiroshima-nagasaki 1949–1969. Cancer 1973, 31, 948–955. [Google Scholar] [CrossRef]
- Neiman, R.S.; Barcos, M.; Berard, C.; Bonner, H.; Mann, R.; Rydell, R.E.; Bennett, J.M. Granulocytic sarcoma: A clinicopathologic study of 61 biopsied cases. Cancer 1981, 48, 1426–1437. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Serpick, A.A. Granulocytic sarcoma (chloroma). Blood 1970, 35, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Untaaveesup, S.; Trithiphen, S.; Kulchutisin, K.; Rungjirajittranon, T.; Leelakanok, N.; Panyoy, S.; Kaokunakorn, T.; Owattanapanich, W. Genetic alterations in myeloid sarcoma among acute myeloid leukemia patients: Insights from 37 cohort studies and a meta-analysis. Front. Oncol. 2024, 14, 1325431. [Google Scholar] [CrossRef] [PubMed]
- Almond, L.M.; Charalampakis, M.; Ford, S.J.; Gourevitch, D.; Desai, A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin. Lymphoma Myeloma Leuk. 2017, 17, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Werstein, B.; Dunlap, J.; Cascio, M.J.; Ohgami, R.S.; Fan, G.; Press, R.; Raess, P.W. Molecular Discordance between Myeloid Sarcomas and Concurrent Bone Marrows Occurs in Actionable Genes and Is Associated with Worse Overall Survival. J. Mol. Diagn. 2020, 22, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.W.; Reinert, J.; Borchert, N.M.; Panagiota, V.; Gabdoulline, R.; Thol, F.; Heuser, M.; Fiedler, W. Newly diagnosed isolated myeloid sarcoma-paired NGS panel analysis of extramedullary tumor and bone marrow. Ann. Hematol. 2021, 100, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Greenland, N.Y.; Van Ziffle, J.A.; Liu, Y.C.; Qi, Z.; Prakash, S.; Wang, L. Genomic analysis in myeloid sarcoma and comparison with paired acute myeloid leukemia. Hum. Pathol. 2021, 108, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Stolzel, F.; Luer, T.; Lock, S.; Parmentier, S.; Kuithan, F.; Kramer, M.; Alakel, N.S.; Sockel, K.; Taube, F.; Middeke, J.M.; et al. The prevalence of extramedullary acute myeloid leukemia detected by (18)FDG-PET/CT: Final results from the prospective PETAML trial. Haematologica 2020, 105, 1552–1558. [Google Scholar] [CrossRef]
- Pileri, S.A.; Ascani, S.; Cox, M.C.; Campidelli, C.; Bacci, F.; Piccioli, M.; Piccaluga, P.P.; Agostinelli, C.; Asioli, S.; Novero, D.; et al. Myeloid sarcoma: Clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Miyoshi, H.; Yoshida, N.; Takizawa, J.; Sone, H.; Ohshima, K. Clinicopathological, Cytogenetic, and Prognostic Analysis of 131 Myeloid Sarcoma Patients. Am. J. Surg. Pathol. 2016, 40, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Claerhout, H.; Van Aelst, S.; Melis, C.; Tousseyn, T.; Gheysens, O.; Vandenberghe, P.; Dierickx, D.; Boeckx, N. Clinicopathological characteristics of de novo and secondary myeloid sarcoma: A monocentric retrospective study. Eur. J. Haematol. 2018, 100, 603–612. [Google Scholar] [CrossRef]
- Ullman, D.I.; Dorn, D.; Jones, J.A.; Fasciano, D.; Ping, Z.; Kanakis, C.; Koenig, R.G.; Salzman, D.; Peker, D. Clinicopathological and molecular characteristics of extramedullary acute myeloid leukaemia. Histopathology 2019, 75, 185–192. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Kantarjian, H.M.; Wen, S.; Keating, M.J.; O’Brien, S.; Brandt, M.; Pierce, S.; Freireich, E.J.; Medeiros, L.J.; Estey, E. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 2008, 113, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.Y.; Lin, D.T.; Tien, H.F.; Yang, R.S.; Chen, C.Y.; Wu, K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009, 122, 238–246. [Google Scholar] [CrossRef]
- Movassaghian, M.; Brunner, A.M.; Blonquist, T.M.; Sadrzadeh, H.; Bhatia, A.; Perry, A.M.; Attar, E.C.; Amrein, P.C.; Ballen, K.K.; Neuberg, D.S.; et al. Presentation and outcomes among patients with isolated myeloid sarcoma: A Surveillance, Epidemiology, and End Results database analysis. Leuk. Lymphoma 2015, 56, 1698–1703. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Dock, G.; Warthin, A.S. A new case of chloroma with leukemia. With a study of cases reported since 1893. Trans. Assoc. Am. Phys. 1904, 19, 64–115. [Google Scholar]
- Burns, A. Observations of Surgical Anatomy in Head and Neck; Thomas Royce: Edinburgh, UK, 1811; pp. 364–366. [Google Scholar]
- King, A. Case of Chloroma. Mon. J. Med. Sci. 1853, 8, 97–104. [Google Scholar]
- Piller, G. Leukaemia—A brief historical review from ancient times to 1950. Br. J. Haematol. 2001, 112, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Comings, D.E.; Fayen, A.W.; Carter, P. Myeloblastoma Preceding Blood and Marrow Evidence of Acute Leukemia. Cancer 1965, 18, 253–258. [Google Scholar] [CrossRef]
- Rappaport, H. Tumors of the Hematopoietic System; Armed Forces Institute of Pathology: Washington, DC, USA, 1966. [Google Scholar]
- Shallis, R.M.; Gale, R.P.; Lazarus, H.M.; Roberts, K.B.; Xu, M.L.; Seropian, S.E.; Gore, S.D.; Podoltsev, N.A. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: A tale of misnomers, controversy and the unresolved. Blood Rev. 2021, 47, 100773. [Google Scholar] [CrossRef]
- Nagamine, M.; Miyoshi, H.; Kawamoto, K.; Takeuchi, M.; Yamada, K.; Yanagida, E.; Kohno, K.; Ohshima, K. Clinicopathological analysis of myeloid sarcoma with megakaryocytic differentiation. Pathology 2022, 54, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Ponisch, W.; Schmidt, S.A.; Wienbeck, S.; Braulke, F.; Schramm, D.; Surov, A. Clinical and imaging features of myeloid sarcoma: A German multicenter study. BMC Cancer 2019, 19, 1150. [Google Scholar] [CrossRef]
- Ganzel, C.; Manola, J.; Douer, D.; Rowe, J.M.; Fernandez, H.F.; Paietta, E.M.; Litzow, M.R.; Lee, J.W.; Luger, S.M.; Lazarus, H.M.; et al. Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but Lacks Independent Significance: Analysis of Patients in ECOG-ACRIN Cancer Research Group Trials, 1980–2008. J. Clin. Oncol. 2016, 34, 3544–3553. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Bartley, A.C.; Patnaik, M.M.; Litzow, M.R.; Al-Kali, A.; Go, R.S. Clinical features and outcomes of extramedullary myeloid sarcoma in the United States: Analysis using a national data set. Blood Cancer J. 2017, 7, e592. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Kantarjian, H.M.; Estey, E.; Cortes, J.E.; Verstovsek, S.; Faderl, S.; Thomas, D.A.; Garcia-Manero, G.; Ferrajoli, A.; Manning, J.T.; et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 2003, 17, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Kitko, C.L.; Couriel, D.R.; Braun, T.M.; Choi, S.W.; Magenau, J.; Mineishi, S.; Pawarode, A.; Yanik, G.; Levine, J.E. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Haematologica 2013, 98, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Solh, M.; DeFor, T.E.; Weisdorf, D.J.; Kaufman, D.S. Extramedullary relapse of acute myelogenous leukemia after allogeneic hematopoietic stem cell transplantation: Better prognosis than systemic relapse. Biol. Blood Marrow Transplant. 2012, 18, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Yuda, S.; Fuji, S.; Onishi, A.; Tanaka, T.; Inamoto, Y.; Kurosawa, S.; Kim, S.W.; Fukuda, T. Extramedullary Relapse of Acute Myelogenous Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Szomor, A.; Passweg, J.R.; Tichelli, A.; Hoffmann, T.; Speck, B.; Gratwohl, A. Myeloid leukemia and myelodysplastic syndrome relapsing as granulocytic sarcoma (chloroma) after allogeneic bone marrow transplantation. Ann. Hematol. 1997, 75, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Bekassy, A.N.; Hermans, J.; Gorin, N.C.; Gratwohl, A. Granulocytic sarcoma after allogeneic bone marrow transplantation: A retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996, 17, 801–808. [Google Scholar] [PubMed]
- Byrd, J.C.; Edenfield, W.J.; Shields, D.J.; Dawson, N.A. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: A clinical review. J. Clin. Oncol. 1995, 13, 1800–1816. [Google Scholar] [CrossRef]
- Seymour, J.F.; Pierce, S.A.; Kantarjian, H.M.; Keating, M.J.; Estey, E.H. Investigation of karyotypic, morphologic and clinical features in patients with acute myeloid leukemia blast cells expressing the neural cell adhesion molecule (CD56). Leukemia 1994, 8, 823–826. [Google Scholar]
- Byrd, J.C.; Weiss, R.B. Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8; 21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer 1994, 73, 2107–2112. [Google Scholar] [CrossRef]
- Baer, M.R.; Stewart, C.C.; Lawrence, D.; Arthur, D.C.; Byrd, J.C.; Davey, F.R.; Schiffer, C.A.; Bloomfield, C.D. Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8; 21) (q22; q22). Blood 1997, 90, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, C.A.; Raimondi, S.C.; Head, D.; Krance, R.; Mirro, J., Jr.; Kalwinsky, D.K.; Ayers, G.D.; Behm, F.G. Distinctive immunophenotypic features of t(8; 21) (q22; q22) acute myeloblastic leukemia in children. Blood 1992, 80, 3182–3188. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Fellinger, E.; Huvos, A.; Beresford, H.; Melamed, M.; Triche, T.; Rettig, W. Immunohistochemical analysis of neural cell adhesion molecules. Differential expression in small round cell tumors of childhood and adolescence. Am. J. Pathol. 1991, 139, 275. [Google Scholar]
- Xavier, S.G.; Fagundes, E.M.; Hassan, R.o.; Bacchi, C.; Conchon, M.; Tabak, D.G.; Spector, N.; Zalcberg, I.R. Granulocytic sarcoma of the small intestine with CBFβ/MYH11 fusion gene: Report of an aleukaemic case and review of the literature. Leuk. Res. 2003, 27, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Kaiafa, G.D.; Perifanis, V.; Diamantidis, M.D.; Giouleme, O.; Voulgaridou, V.; Beretouli, E.; Kalogera-Fountzila, A.; Kaloutsi, V. Aberrant expression of myeloid and B cell markers in an aggressive multiple-site myeloid sarcoma. Ann. Hematol. 2012, 91, 1157–1159. [Google Scholar] [CrossRef]
- Cross, A.H.; Goorha, R.M.; Nuss, R.; Behm, F.G.; Murphy, S.B.; Kalwinsky, D.K.; Raimondi, S.; Kitchingman, G.R.; Mirro, J., Jr. Acute myeloid leukemia with T-lymphoid features: A distinct biologic and clinical entity. Blood 1988, 72, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, H.; Nagai, M.; Yamaoka, G.; Ohnishi, H.; Kawakami, K. Specific skin manifestations in CD56 positive acute myeloid leukemia. J. Cutan. Pathol. 1999, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.; Meijer, C.J.; Dalac, S.; Willemze, R.; Maynadié, M.; Machet, L.; Casasnovas, O.; Vergier, B.; Teitell, M.A. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. Am. J. Clin. Pathol. 2004, 122, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Cho-Vega, J.H.; Medeiros, L.J.; Prieto, V.G.; Vega, F. Leukemia cutis. Am. J. Clin. Pathol. 2008, 129, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Faaij, C.M.; Willemze, A.J.; Revesz, T.; Balzarolo, M.; Tensen, C.P.; Hoogeboom, M.; Vermeer, M.H.; van Wering, E.; Zwaan, C.M.; Kaspers, G.J.; et al. Chemokine/chemokine receptor interactions in extramedullary leukaemia of the skin in childhood AML: Differential roles for CCR2, CCR5, CXCR4 and CXCR7. Pediatr. Blood Cancer 2010, 55, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Burkle, A. The CXCR4 chemokine receptor in acute and chronic leukaemia: A marrow homing receptor and potential therapeutic target. Br. J. Haematol. 2007, 137, 288–296. [Google Scholar] [CrossRef]
- Sapienza, M.R.; Fuligni, F.; Melle, F.; Tabanelli, V.; Indio, V.; Laginestra, M.A.; Motta, G.; Mazzara, S.; Cerroni, L.; Pileri, A.; et al. MicroRNA profiling of blastic plasmacytoid dendritic cell neoplasm and myeloid sarcoma. Hematol. Oncol. 2020, 38, 831–833. [Google Scholar] [CrossRef]
- Yang, L.X.; Zhang, C.T.; Yang, M.Y.; Zhang, X.H.; Liu, H.C.; Luo, C.H.; Jiang, Y.; Wang, Z.M.; Yang, Z.Y.; Shi, Z.P.; et al. C1Q labels a highly aggressive macrophage-like leukemia population indicating extramedullary infiltration and relapse. Blood 2023, 141, 766–786. [Google Scholar] [CrossRef] [PubMed]
- Ottone, T.; Silvestrini, G.; Piazza, R.; Travaglini, S.; Gurnari, C.; Marchesi, F.; Nardozza, A.M.; Fabiani, E.; Attardi, E.; Guarnera, L.; et al. Expression profiling of extramedullary acute myeloid leukemia suggests involvement of epithelial-mesenchymal transition pathways. Leukemia 2023, 37, 2383–2394. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Liao, T.-T.; Yang, M.-H. Emerging roles of epithelial-mesenchymal transition in hematological malignancies. J. Biomed. Sci. 2018, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, F.; Chang, C.; Byon, J.; Papayannopoulou, T.; Deeg, H.J.; Marcondes, A.M. Transcriptional regulation of miR-10a/b by TWIST-1 in myelodysplastic syndromes. Haematologica 2013, 98, 414–419. [Google Scholar] [CrossRef]
- Emadi, A.B.; Maria, R. Acute Myeloid Leukemia in Adults, 15th ed.; Wintrobe’s Clinical Hematology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Kogut, N.; Tsai, N.C.; Thomas, S.H.; Palmer, J.; Paris, T.; Murata-Collins, J.; Forman, S.J. Extramedullary relapse following reduced intensity allogeneic hematopoietic cell transplant for adult acute myelogenous leukemia. Leuk. Lymphoma 2013, 54, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Mufti, G.; Salisbury, J.; Du Vivier, A.; Creamer, D. Spectrum of clinical presentation, treatment and prognosis in a series of eight patients with leukaemia cutis. Clin. Exp. Dermatol. 2006, 31, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Paydas, S.; Zorludemir, S. Leukaemia cutis and leukaemic vasculitis. Br. J. Dermatol. 2000, 143, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Park, H.J.; Oh, S.T.; Lee, J.Y.; Cho, B.K. A case of leukemia cutis at the site of a prior catheter insertion. Ann. Dermatol. 2009, 21, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, M.J.; KIM, H.O.; Park, Y.M. Leukemia cutis limited to the needle puncture sites. J. Dermatol. 2010, 37, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Lee, D.A. Leukemic gingival infiltration. N. Engl. J. Med. 2008, 358, 274. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, R.; Tyagi, A.; Chopra, A.; Kumar, A.; Vishnubhatla, S.; Bakhshi, S. Myeloid Sarcoma Predicts Superior Outcome in Pediatric AML; Can Cytogenetics Solve the Puzzle? Clin. Lymphoma Myeloma Leuk. 2018, 18, e249–e254. [Google Scholar] [CrossRef]
- Dalland, J.C.; Meyer, R.; Ketterling, R.P.; Reichard, K.K. Myeloid Sarcoma With CBFB-MYH11 Fusion (inv(16) or t(16;16)) Prevails in the Abdomen. Am. J. Clin. Pathol. 2020, 153, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.b.; Szczepaniak, A.; Barańska, M.; Kaźmierczak, M.; Paluszewska, M.; Jędrzejczak, W.W.; Guzicka-Kazimierczak, R.; Knopińska-Posłuszny, W.; Grzybowska-Izydorczyk, O.; Pluta, A.; et al. Primary and Secondary Central Nervous System Involvement in Acute Myeloid Leukemia. J. Leuk. 2019, 7, 257. [Google Scholar] [CrossRef]
- Stewart, D.J.; Keating, M.J.; McCredie, K.B.; Smith, T.L.; Youness, E.; Murphy, S.G.; Bodey, G.P.; Freireich, E.J. Natural history of central nervous system acute leukemia in adults. Cancer 1981, 47, 184–196. [Google Scholar] [CrossRef]
- Louis, E.D.; Mayer, S.A.; Noble, J.M. Merritt’s Neurology, 14th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022. [Google Scholar]
- Cervantes, G.M.; Cayci, Z. Intracranial CNS Manifestations of Myeloid Sarcoma in Patients with Acute Myeloid Leukemia: Review of the Literature and Three Case Reports from the Author’s Institution. J. Clin. Med. 2015, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Kanda, Y.; Yamashita, T.; Takahashi, S.; Mori, T.; Nakaseko, C.; Fujimaki, K.; Yokota, A.; Fujisawa, S.; Matsushima, T.; et al. Central nervous system relapse of leukemia after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2008, 14, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Bommer, M.; von Harsdorf, S.; Döhner, H.; Bunjes, D.; Ringhoffer, M. Neoplastic meningitis in patients with acute myeloid leukemia scheduled for allogeneic hematopoietic stem cell transplantation. Haematologica 2010, 95, 1969–1972. [Google Scholar] [CrossRef]
- Alhashim, N.; Aljurf, M.; Hassanein, M.; Chaudhri, N.; Hashmi, S.; El-Gohary, G.; Alsharif, F.; Alsermani, M.; Alhumaid, M.; Beihany, A.A.; et al. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia: Clinical characteristics, incidence, risk factors and outcomes. Bone Marrow Transplant. 2018, 53, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Frietsch, J.J.; Hunstig, F.; Wittke, C.; Junghanss, C.; Franiel, T.; Scholl, S.; Hochhaus, A.; Hilgendorf, I. Extra-medullary recurrence of myeloid leukemia as myeloid sarcoma after allogeneic stem cell transplantation: Impact of conditioning intensity. Bone Marrow Transplant. 2021, 56, 101–109. [Google Scholar] [CrossRef]
- Campidelli, C.; Agostinelli, C.; Stitson, R.; Pileri, S.A. Myeloid sarcoma: Extramedullary manifestation of myeloid disorders. Am. J. Clin. Pathol. 2009, 132, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.S.; Medeiros, L.J. Extramedullary Manifestations of Myeloid Neoplasms. Am. J. Clin. Pathol. 2015, 144, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.C. Diagnostic Approach to Tissue Examination and Testing. In Wintrobe’s Clinical Hematology, 15th ed.; Means, R.J., Jr., Rodgers, G., Glader, B., Arber, D.A., Appelbaum, F.R., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2024; Volume 2. [Google Scholar]
- Bakst, R.; Powers, A.; Yahalom, J. Diagnostic and Therapeutic Considerations for Extramedullary Leukemia. Curr. Oncol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Arber, D.A.; Borowitz, M.J.; Cessna, M.; Etzell, J.; Foucar, K.; Hasserjian, R.P.; Rizzo, J.D.; Theil, K.; Wang, S.A.; Smith, A.T.; et al. Initial Diagnostic Workup of Acute Leukemia: Guideline From the College of American Pathologists and the American Society of Hematology. Arch. Pathol. Lab. Med. 2017, 141, 1342–1393. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Brandwein, J.; Yi, Q.L.; Chun, K.; Patterson, B.; Brien, B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk. Res. 2004, 28, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Halahleh, K.; Alhalaseh, Y.; Al-Rimawi, D.; Da’na, W.; Alrabi, K.; Kamal, N.; Muradi, I.; Abdel-Razeq, H. Extramedullary acute myeloid leukemia (eAML): Retrospective single center cohort study on clinico-pathological, molecular analysis and survival outcomes. Ann. Med. Surg. 2021, 72, 102894. [Google Scholar] [CrossRef]
- Kudva, R.; Monappa, V.; Solanke, G.; Valiathan, M.; Rao, A.C.K.; Geetha, V. Myeloid sarcoma: A clinicopathological study with emphasis on diagnostic difficulties. J. Cancer Res. Ther. 2017, 13, 989–993. [Google Scholar] [PubMed]
- Kaur, V.; Swami, A.; Alapat, D.; Abdallah, A.O.; Motwani, P.; Hutchins, L.F.; Jethava, Y. Clinical characteristics, molecular profile and outcomes of myeloid sarcoma: A single institution experience over 13 years. Hematology 2018, 23, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Comerford, C.; Ni Mhaolcatha, S.; Hayes, B.; Mykytiv, V. The first case of acute myeloid leukaemia/myeloid sarcoma with cytokeratin expression on blasts diagnosed on urine specimen. Hematol. Oncol. Stem Cell Ther. 2021, 14, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Dayton, V.J.; Beckman, A.; Linden, M. Myeloid Sarcoma Expressing Keratins and Mimicking Carcinoma-Case Report and Literature Review. Lab. Med. 2022, 53, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Loscocco, G.G.; Vannucchi, A.M. Myeloid sarcoma: More and less than a distinct entity. Ann. Hematol. 2023, 102, 1973–1984. [Google Scholar] [CrossRef]
- Chang, C.C.; Eshoa, C.; Kampalath, B.; Shidham, V.B.; Perkins, S. Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Correlation with blast differentiation in bone marrow. Am. J. Clin. Pathol. 2000, 114, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Sangle, N.A.; Schmidt, R.L.; Patel, J.L.; Medeiros, L.J.; Agarwal, A.M.; Perkins, S.L.; Salama, M.E. Optimized immunohistochemical panel to differentiate myeloid sarcoma from blastic plasmacytoid dendritic cell neoplasm. Mod. Pathol. 2014, 27, 1137–1143. [Google Scholar] [CrossRef]
- Wang, H.Q.; Li, J. Clinicopathological features of myeloid sarcoma: Report of 39 cases and literature review. Pathol. Res. Pract. 2016, 212, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shu, Y.; Tang, Y.; Zhao, S.; Jia, Y.; Ji, J.; Ma, H.; Lin, T.; Zheng, K.; Xu, H.; et al. RNA sequencing of myeloid sarcoma, shed light on myeloid sarcoma stratification. Cancer Med. 2023, 12, 9156–9166. [Google Scholar] [CrossRef]
- Pastoret, C.; Houot, R.; Llamas-Gutierrez, F.; Boulland, M.L.; Marchand, T.; Tas, P.; Ly-Sunnaram, B.; Gandemer, V.; Lamy, T.; Roussel, M.; et al. Detection of clonal heterogeneity and targetable mutations in myeloid sarcoma by high-throughput sequencing. Leuk. Lymphoma 2017, 58, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Jeon, Y.K.; Sun, C.H.; Yun, H.S.; Hong, J.; Shin, D.Y.; Kim, I.; Yoon, S.S.; Koh, Y. RTK-RAS pathway mutation is enriched in myeloid sarcoma. Blood Cancer J. 2018, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.A.; Yang, C.F.; Tinawi-Aljundi, R.; Cooper, L.; Long, P.; Allan, R.H.; Borowitz, M.J.; Berg, K.D.; Murphy, K.M. FLT3 mutations in myeloid sarcoma. Br. J. Haematol. 2004, 126, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Kantarjian, H.M.; Tran, K.M.; Kazmi, S.M.; Kadia, T.M.; Borthakur, G.; Verstovsek, S.; O’Brien, S.; Garcia-Manero, G.; Estrov, Z.; et al. Cytogenetic and Molecular Characterization of Extramedullary Disease (EMD) in Patients (PTS) with Acute Myeloid Leukemia (AML). Blood 2012, 120, 2592. [Google Scholar] [CrossRef]
- Eckardt, J.N.; Stölzel, F.; Kunadt, D.; Röllig, C.; Stasik, S.; Wagenführ, L.; Jöhrens, K.; Kuithan, F.; Krämer, A.; Scholl, S.; et al. Molecular profiling and clinical implications of patients with acute myeloid leukemia and extramedullary manifestations. J. Hematol. Oncol. 2022, 15, 60. [Google Scholar] [CrossRef]
- Falini, B.; Lenze, D.; Hasserjian, R.; Coupland, S.; Jaehne, D.; Soupir, C.; Liso, A.; Martelli, M.P.; Bolli, N.; Bacci, F.; et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia 2007, 21, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Kashofer, K.; Gornicec, M.; Lind, K.; Caraffini, V.; Schauer, S.; Beham-Schmid, C.; Wolfler, A.; Hoefler, G.; Sill, H.; Zebisch, A. Detection of prognostically relevant mutations and translocations in myeloid sarcoma by next generation sequencing. Leuk. Lymphoma 2018, 59, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Willekens, C.; Renneville, A.; Broutin, S.; Saada, V.; Micol, J.B.; Delahousse, J.; Poinsignon, V.; Bories, C.; Berthon, C.; Itzykson, R.; et al. Mutational profiling of isolated myeloid sarcomas and utility of serum 2HG as biomarker of IDH1/2 mutations. Leukemia 2018, 32, 2008–2081. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Knepper, T.C.; Deutsch, Y.E.; Samra, W.; Watts, J.M.; Bradley, T.J.; Chan, O.; Hussaini, M.O.; Zhang, L.; Sweet, K.L.; et al. Molecular annotation of extramedullary acute myeloid leukemia identifies high prevalence of targetable mutations. Cancer 2022, 128, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bachanova, V.; Ketterling, R.P.; Begna, K.H.; Hanson, C.A.; Viswanatha, D.S. A case of nonleukemic myeloid sarcoma with FIP1L1-PDGFRA rearrangement: An unusual presentation of a rare disease. Am. J. Surg. Pathol. 2013, 37, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.; Barwad, A.; Pubbaraju, S.V. Complete response of monoblastic myeloid sarcoma with FIP1L1- PDGFRA rearrangement to imatinib monotherapy. Br. J. Haematol. 2014, 165, 583. [Google Scholar] [CrossRef]
- Mandelker, D.; Dal Cin, P.; Jacene, H.A.; Armand, P.; Stone, R.M.; Lindeman, N.I. Refractory myeloid sarcoma with a FIP1L1-PDGFRA rearrangement detected by clinical high throughput somatic sequencing. Exp. Hematol. Oncol. 2015, 4, 30. [Google Scholar] [CrossRef][Green Version]
- Matrai, Z.; Andrikovics, H.; Csomor, J.; Timár, B.; Kozma, A.; Tordai, A.; Masszi, T. Extramedullary Myeloid Sarcoma With Eosinophilia and FIP1L1-Pdgfra Rearrangement: Complete Cytogenetic Response To Imatinib Therapy. Blood 2013, 122, 5033. [Google Scholar] [CrossRef]
- Ramia de Cap, M.; Wu, L.P.; Hirt, C.; Pihan, G.A.; Patel, S.S.; Tam, W.; Bueso-Ramos, C.E.; Kanagal-Shamanna, R.; Raess, P.W.; Siddon, A.; et al. Myeloid sarcoma with NPM1 mutation may be clinically and genetically distinct from AML with NPM1 mutation: A study from the Bone Marrow Pathology Group. Leuk. Lymphoma 2023, 64, 972–980. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Z.; Wan, D.; Cao, W.; Xing, H.; Liu, Z.; Fan, J.; Wang, H.; Lu, R.; Zhang, Y.; et al. Clinical characteristics, treatment, and prognosis of 118 cases of myeloid sarcoma. Sci. Rep. 2022, 12, 6752. [Google Scholar] [CrossRef] [PubMed]
- Pui, M.H.; Fletcher, B.D.; Langston, J.W. Granulocytic sarcoma in childhood leukemia: Imaging features. Radiology 1994, 190, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.; Lech-Marańda, E.; Warzocha, K. Involvement of the central nervous system in adult patients with acute myeloid leukemia. Hematol. Clin. Pract. 2013, 4, 218–226. [Google Scholar]
- Fritz, J.; Vogel, W.; Bares, R.; Horger, M. Radiologic spectrum of extramedullary relapse of myelogenous leukemia in adults. AJR Am. J. Roentgenol. 2007, 189, 209–218. [Google Scholar] [CrossRef]
- Guermazi, A.; Feger, C.; Rousselot, P.; Merad, M.; Benchaib, N.; Bourrier, P.; Mariette, X.; Frija, J.; de Kerviler, E. Granulocytic sarcoma (chloroma): Imaging findings in adults and children. AJR Am. J. Roentgenol. 2002, 178, 319–325. [Google Scholar] [CrossRef]
- Ooi, G.C.; Chim, C.S.; Khong, P.L.; Au, W.Y.; Lie, A.K.; Tsang, K.W.; Kwong, Y.L. Radiologic manifestations of granulocytic sarcoma in adult leukemia. AJR Am. J. Roentgenol. 2001, 176, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Labopin, M.; Cornelissen, J.; Socie, G.; Rocha, V.; Mohty, M.; EBMT, A.o. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: A report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haematologica 2011, 96, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.; Szczepaniak, A.; Baranska, M.; Kazmierczak, M.; Paluszewska, M.; Jedrzejczak, W.; Guzicka Kazmierczak, R.; Knopinska Posluszny, W.; Grzybowska-Izydorczyk, O.; Pluta, A.; et al. A Comparison of Clinical Characteristics and Treatment Outcome in Myeloid Sarcoma Versus Acute Myeloid Leukemia Patients without Extramedullary Involvement—Case Control Study of the Polish Adult Leukemia Group (PALG). Blood 2017, 130, 1311. [Google Scholar]
- Stolzel, F.; Rollig, C.; Radke, J.; Mohr, B.; Platzbecker, U.; Bornhauser, M.; Paulus, T.; Ehninger, G.; Zophel, K.; Schaich, M. (1)(8)F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica 2011, 96, 1552–1556. [Google Scholar] [CrossRef]
- Bakst, R.; Yahalom, J. Radiation therapy for leukemia cutis. Pract. Radiat. Oncol. 2011, 1, 182–187. [Google Scholar] [CrossRef]
- Yamauchi, K.; Yasuda, M. Comparison in treatments of nonleukemic granulocytic sarcoma: Report of two cases and a review of 72 cases in the literature. Cancer 2002, 94, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.P.; Bulkeley, W., 3rd; Zhang, L.; Menes, M.; Bui, M.M. A practical approach to diagnose soft tissue myeloid sarcoma preceding or coinciding with acute myeloid leukemia. Ann. Diagn. Pathol. 2014, 18, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.J.; Medeiros, L.J.; Elenitoba-Johnson, K.; Kuchnio, M.; Jaffe, E.S.; Stetler-Stevenson, M. Extramedullary myeloid cell tumors. An immunohistochemical study of 29 cases using routinely fixed and processed paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 1995, 119, 790–798. [Google Scholar] [PubMed]
- Menasce, L.P.; Banerjee, S.S.; Beckett, E.; Harris, M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: A study of 26 cases. Histopathology 1999, 34, 391–398. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.P. Benign extramedullary myeloid proliferations. Mod. Pathol. 2007, 20, 405–415. [Google Scholar] [CrossRef]
- Marvin-Peek, J.; Gilbert, J.S.; Pollyea, D.A.; DiNardo, C.D. Frontline therapy of acute myeloid leukemia with lower intensity regimens: Where are we now and where can we go? Am. J. Hematol. 2024, 99, 1790–1801. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, Z.; Chen, Z.; Fu, J.; Hong, P.; Feng, W. Gene Mutations and Targeted Therapies of Myeloid Sarcoma. Curr. Treat. Options Oncol. 2023, 24, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Otoukesh, S.; Zhang, J.; Nakamura, R.; Stein, A.S.; Forman, S.J.; Marcucci, G.; Pullarkat, V.; Aldoss, I. The efficacy of venetoclax and hypomethylating agents in acute myeloid leukemia with extramedullary involvement. Leuk. Lymphoma 2020, 61, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Sanber, K.; Ye, K.; Tsai, H.L.; Newman, M.; Webster, J.A.; Gojo, I.; Ghiaur, G.; Prince, G.T.; Gondek, L.P.; Smith, B.D.; et al. Venetoclax in combination with hypomethylating agent for the treatment of advanced myeloproliferative neoplasms and acute myeloid leukemia with extramedullary disease. Leuk. Lymphoma 2023, 64, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Ofran, Y.; Boissel, N.; Brunet Mauri, S.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.C.; Wei, J.; Xu, Y.J.; Zhang, Y.; Li, Y.H.; Wu, A.Q.; Fan, L.; Zhu, Y.; Liu, F.Q.; et al. Outcomes of allogeneic hematopoietic stem cell transplantation versus intensive chemotherapy in patients with myeloid sarcoma: A nationwide representative multicenter study. Bone Marrow Transplant. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Dabaja, B.S.; Specht, L.K.; Yahalom, J. Use of Radiation in Extramedullary Leukemia/Chloroma: Guidelines From the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M.; Abdel Karim, N.; Eldessouki, I.; Gaber, O.; Rahouma, M.; Ghareeb, M. Myeloid Sarcoma. Oncol. Res. Treat. 2019, 42, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shahin, O.A.; Ravandi, F. Myeloid sarcoma. Curr. Opin. Hematol. 2020, 27, 88–94. [Google Scholar] [CrossRef]
 Standard option,
Standard option,  Alternative option.
Alternative option.
 Standard option,
Standard option,  Alternative option.
Alternative option.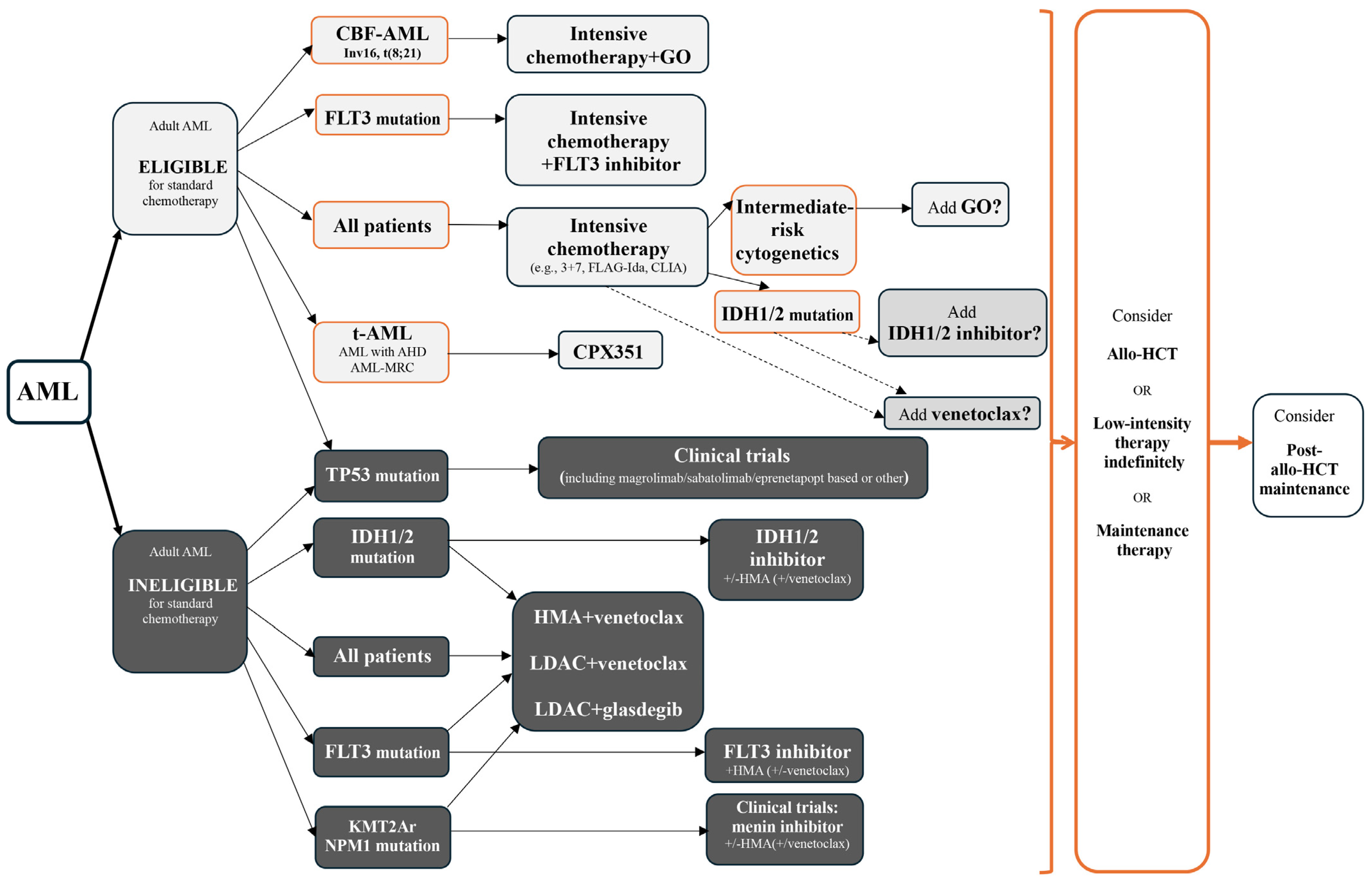

| Etiopathogenesis of Myeloid Sarcoma | ||
|---|---|---|
| General factors predisposing to AML | Occupational exposures | Workers exposed to rubber, paint, embalming fluids, pesticides, ethylene oxide, petroleum, poultry, munitions, automobiles, nuclear power, plastics, and electrical wiring, as well as gasoline station attendants, beauticians, barbers, and cosmetologists. |
| Environmental factors | Ionising radiation | |
| Lifestyle-related factors | Smoking, obesity | |
| Heritable genetic factors | CEBPA, DDX41, RUNX1, ANKRD26, ETV6, GATA2, ELANE, HAX1, G6PC3, MPL, RBM8A, SBDS, SRP72, trisomy 21, monosomy 7 | |
| Clonal haematopoiesis | DNMT3A, TET2, JAK2, ASXL1, TP53, GNAS, PPM1D, BCORL1, SF3B1, EZH2 | |
| Therapy-related AML | Cytotoxic therapy: alkylating agents, topoisomerase II inhibitors, nucleoside analogues, anti-tubulins Radiation therapy | |
| Factors predisposing to MS | Genetics | t(8;21), inv(16); KMT2Ar, CBFB, monosomy7, del(5q), del(9q), loss of X, loss of Y, trisomy 4, trisomy 8 |
| Molecular abnormalities | (1) RAS pathway: NRAS, KRAS, PTPN11, CBL, NF1, BRAF (2) Activated signalling: FLT3, BCR::ABL1, JAK2, KIT, SH2B3, CBL, CSF3R (3) DNA methylation: DNMT3A, KMT2A, TET2, IDH1, IDH2 (4) Cohesin complex: STAG2, RAD21, SMC3 (5) RNA splicing: SRSF2, SF3B1, U2AF1 (6) Transcription factors: GATA2, RUNX1, CEBPA, ETV6, BCOR, BCORL1, STAT3 (7) Chromatin modification: ASXL1, EZH2, SETD2 (8) Tumour suppressors: TP53, WT1, NF1, PHF6 (9) Others: NPM1, SETBP1 Most patients harbour multiple mutations (>1), with the most frequently observed being: NPM1, NRAS, DNMT3A | |
| Cells morphology | Monocytic component | |
| Aberrant antigen expression | Aberrant expression of myeloid cell, B-cell: CD19, T-cell: CD4, CD7, NK-cell: CD56 antigens | |
| Pathogenic pathways | BONE MARROW AND EXTRAMEDULLARY NICHE Regulatory abnormalities: (1) Loss of RAF kinase inhibitor protein and activation of RAS-MAPK/ERK pathway (2) Deregulation in the EMT pathway, altered ECM–receptor interactions and local adhesion pathways, formation of a CsC-supportive niche Contributors to EMT and invasiveness: (1) ECM proteins, the upregulation of six collagen isoforms: FN1, TNC, THBS2, and LAMA/LAMB1, the promotion of EMT and drug resistance (2) The role of transcription factor TWIST1 in EMT and apoptosis: the interactions with adhesion molecules, the disruption of the epithelial–mesenchymal balance, the enhancement of leukemic cell invasion, the promotion of EMT, and the increase in overall invasiveness STEM CELL MIGRATION (1) Altered expression and dysregulation of membrane adhesion molecules, chemokine receptors/ligands, and integrins (2) Impaired adhesion of LSCs to BM niches, the mobilisation and migration of LSCs into PB, spleen, and EM sites (3) Overexpression of CXCR4, CCR5, CX3CR1, CXCR7, CCR2 (4) Interactions between CLA and E-selectin on skin endothelial cells; ICAM-1 with LFA-1; CCL3 with CCR5 on blasts (5) Expression of PD-L1 (6) Activation of the RAS-MAPK/ERK pathway (7) Pathogenic NEF2 mutations (8) Presence of macrophage-like leukaemia subset with complement C1Q positivity | |
| Epigenetic/microRNA profiles | Some cases shared features between MS and BPDCN | |
| Surgical (Excisional) Tissue Biopsy | Fine Needle Biopsy | Core Biopsy | Body Fluids (e.g., Cerebrospinal Fluid, Pleural Effusion) | |
|---|---|---|---|---|
| Tissue architecture | Undisturbed, broad heterogeneity | Disrupted | Locally undisturbed, reduced heterogeneity | Not applicable |
| Morphology | Low-power architecture and high-power cytomorphology | Detailed cellular morphology | Low-power architecture limited by biopsy size | Detailed cellular morphology |
| Procedure invasiveness | Surgical excision procedure The most invasive procedure | The least invasive procedure The lowest risk of complications Image guidance may be necessary | A minimally invasive procedure The possibility of performing percutaneously or endoscopically. Image guidance may be necessary. | Usually less invasive than direct tissue biopsy |
| Limitations | Accessibility may be challenging depending on the anatomical location (e.g., brain, retroperitoneum, mediastinum). | Inappropriate for cases with rare malignant cells or structural characteristics that limit aspiration (e.g., fibrosis, necrosis). | Inappropriate for cases requiring analysis of extensive heterogeneity or scarce malignant cells. | Often relatively low in cellularity, mainly when obtained from small-volume or relatively sterile sites. Limited effectiveness of specific techniques, e.g., cell block preparation. |
| Most appropriate for diagnostics | Cell block preparation. Scarce malignant cells and/or diagnostic features depending on low-power architectural distortion. | Homogeneous populations of readily identifiable (immunophenotypically aberrant) cells | Aberrant cell populations homogeneous or mixed, or displaying distinct architectural patterns. Biopsy of deep or relatively inaccessible lesions that are not readily amenable to surgical resection. | Assessment of cells in cerebrospinal fluid, pleural, pericardial, peritoneal effusions, bronchoalveolar lavage, and vitreous fluid. |
| Morphologic examination methods | Cells collected into a tissue block and embedded in paraffin and immunophenotyping via IHC. | Cells smeared on glass slides and stained or collected into a tissue block and embedded in paraffin and immunophenotyping via IHC | Cells smeared on glass slides and stained or collected into a tissue block and embedded in paraffin and immunophenotyping via IHC | The workup used techniques similar to those used in FNA samples: stained smears for cytomorphology and collected tissue fragments for embedding into a cell block. |
| Type of obtained cells | Fixed cells | Viable and intact, disaggregated cells | A reasonable number of viable cells suitable for morphology assessment. | Viable cells |
| Diagnostics methods | Immunophenotyping by IHC, FISH, NGS | Immunophenotyping by flow cytometry, conventional karyotyping, FISH and a wide range of molecular genetic studies Cultures | Immunophenotyping by IHC and flow cytometry, conventional karyotyping, FISH and a wide range of molecular genetic studies Cultures | Immunophenotyping by flow cytometry, conventional karyotyping, FISH and a wide range of molecular genetic studies Cultures |
| Characteristics | The highest potential diagnostic yield. | The intermediate potential diagnostic yield. | The intermediate potential diagnostic yield. It could be combined with FNA, similar to the combination of bone marrow aspirate and core biopsy. | The intermediate potential diagnostic yield. Depending on the site, e.g., cerebrospinal fluid, pleural or peritoneal effusions, or bronchoalveolar lavage. |
| Flow Cytometry | Immunohistochemistry | |
|---|---|---|
| Markers number analysed | Many (typically 8–12 in the majority of clinical laboratories) | One (occasionally two) |
| Spectrum of measurable intensity | Broad | Limited |
| Category of information collected | Quantitative | Qualitative |
| Processing duration | Fast (under 1 h) | More time-consuming (several hours) |
| Sample | Undamaged, disaggregated cells | Cryopreserved tissue or formalin-fixed, paraffin-embedded tissue samples |
| Correlation with cell morphology | Indirect | Direct |
| Most appropriate for | Samples of non-cohesive live cells require analysis of multiple markers per cell—no critical correlation with morphology. | Paraffin-embedded samples or populations not suited to disaggregation. Limited markers per cell analysed—uncommon populations of cells with morphological abnormalities. |
| Characteristics | Counting cells and immunophenotyping with cell differentiation | The effacement of tissue architecture. Pleomorphic infiltrate of early-stage myelopoietic cells with maturation arrest at the blast phase. Variable sizes of cells. |
| Antigens expressed | Myeloblasts: CD34, CD117, CD13, CD15, CD33, HLA-DR, CD45, MPO Mature and immature monocytic cells: CD64, CD14, CD4, CD36, CD13, CD15, CD11b, CD11c, CD56 Megakaryoblastic or erythroid differentiation: CD41, CD61 or CD71, CD105, and CD235a | Frequently: MPO, CD33, CD13, CD68, and CD45 An immature granulocytic profile: CD34 and KIT (CD117) Monoblasts and immature monocytic cells: CD11b, CD11c, CD15, CD64, CD117 and lysozyme Less frequently: CD14 or CD34 More mature monocytic cell: CD14, CD68, CD163 Megakaryoblastic and erythroid differentiation: CD61 and glycophorin A. Exceptionally aberrantly expressed: markers of B-cells: CD19, T-cells: CD4, CD7 and NK-cells: CD56 Weak or only in a subset of cells: CD123, CD99, and TdT Rarely: aberrant cytokeratin expression as AE1/AE3 and CK8/18 Non-specific but highly sensitive for MS: lysozyme, CD68, CD43 |
| ELN 2022 Risk Category by Genetics at AML Diagnosis | Mutated Genes and Genetic Alterations | Rates (%) in MS Patients |
|---|---|---|
| Favourable | RUNX1::RUNX1T1 | 2–23 |
| CBFB::MYH11 | 9–17 | |
| NPM1 | 15–54 | |
| Intermediate | FLT3-ITD | 6–15 |
| MLL rearrangement | 7–11 | |
| Adverse | TP53 | 8–22 |
| RUNX1 | 7–11 | |
| Monosomy 7 | 8–11 | |
| Del(5q) | 5–8 | |
| Cytogenetic and/or molecular abnormalities not classified by ELN as favourable or adverse | NRAS | 11–31 |
| KRAS | 11–15 | |
| IDH1 | 15 | |
| IDH2 | 11–31 | |
| DNMT3A | 8–28 | |
| TET2 | 17–22 | |
| FLT3-TKD | 17 | |
| PTPN11 | 11–15 | |
| KIT | 14–15 | |
| CBL | 11 | |
| Trisomy 8 | 11–15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patkowska, E.; Krzywdzinska, A.; Solarska, I.; Wojtas, M.; Prochorec-Sobieszek, M. Diagnostic Approaches in Myeloid Sarcoma. Curr. Issues Mol. Biol. 2025, 47, 111. https://doi.org/10.3390/cimb47020111
Patkowska E, Krzywdzinska A, Solarska I, Wojtas M, Prochorec-Sobieszek M. Diagnostic Approaches in Myeloid Sarcoma. Current Issues in Molecular Biology. 2025; 47(2):111. https://doi.org/10.3390/cimb47020111
Chicago/Turabian StylePatkowska, Elzbieta, Agnieszka Krzywdzinska, Iwona Solarska, Magdalena Wojtas, and Monika Prochorec-Sobieszek. 2025. "Diagnostic Approaches in Myeloid Sarcoma" Current Issues in Molecular Biology 47, no. 2: 111. https://doi.org/10.3390/cimb47020111
APA StylePatkowska, E., Krzywdzinska, A., Solarska, I., Wojtas, M., & Prochorec-Sobieszek, M. (2025). Diagnostic Approaches in Myeloid Sarcoma. Current Issues in Molecular Biology, 47(2), 111. https://doi.org/10.3390/cimb47020111






