Genetic and Epigenetic Aberrations of SOX7 in Newly Diagnosed and Relapsed Multiple Myeloma as Well as Related Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. Chemicals and Reagents
2.3. FACS Sorting of Bone Marrow Tumor Cells
2.4. Isolation of DNA and RNA from MM Bone Marrow Tumor Cells
2.5. Quality Control of the MM Bone Marrow Tumor Cell DNA and RNA
2.6. Tonsil Samples from Control Cases
2.7. Tonsil PC Separations Through FACS
2.8. SOX7 Copy Number Analyses of Publicly Available SNP Array Data of Diagnostic MM Cases
2.9. SOX7 Deletion Analyses in Diagnostic and Relapsed MM Cases with qPCR
2.10. Promoter Methylation Analyses of SOX7 Using Publicly Available Methylation Array Data of MM and PCL
2.11. Integrated Analyses of Copy Number, Promoter Methylation, and Transcript Expression of the Candidate Tumor Suppressors Located in del8p23.1 in Diagnostic and Relapsed MM Cases
2.12. Evaluation of SOX7 mRNA Expression Levels Through Reanalyses of the Publicly Available GEP Data of Plasma Cell Neoplasms and Normal Plasma Cells
2.13. Evaluation of SOX7 mRNA Levels in Diagnostic and Relapsed MM Tumor Samples with qRT-PCR
2.14. Mutation Data of MM Samples
2.15. Evaluation of SOX7 mRNA Expression Levels in MM Cell Lines
2.16. Generation of Graphs and Statistical Analyses
3. Results
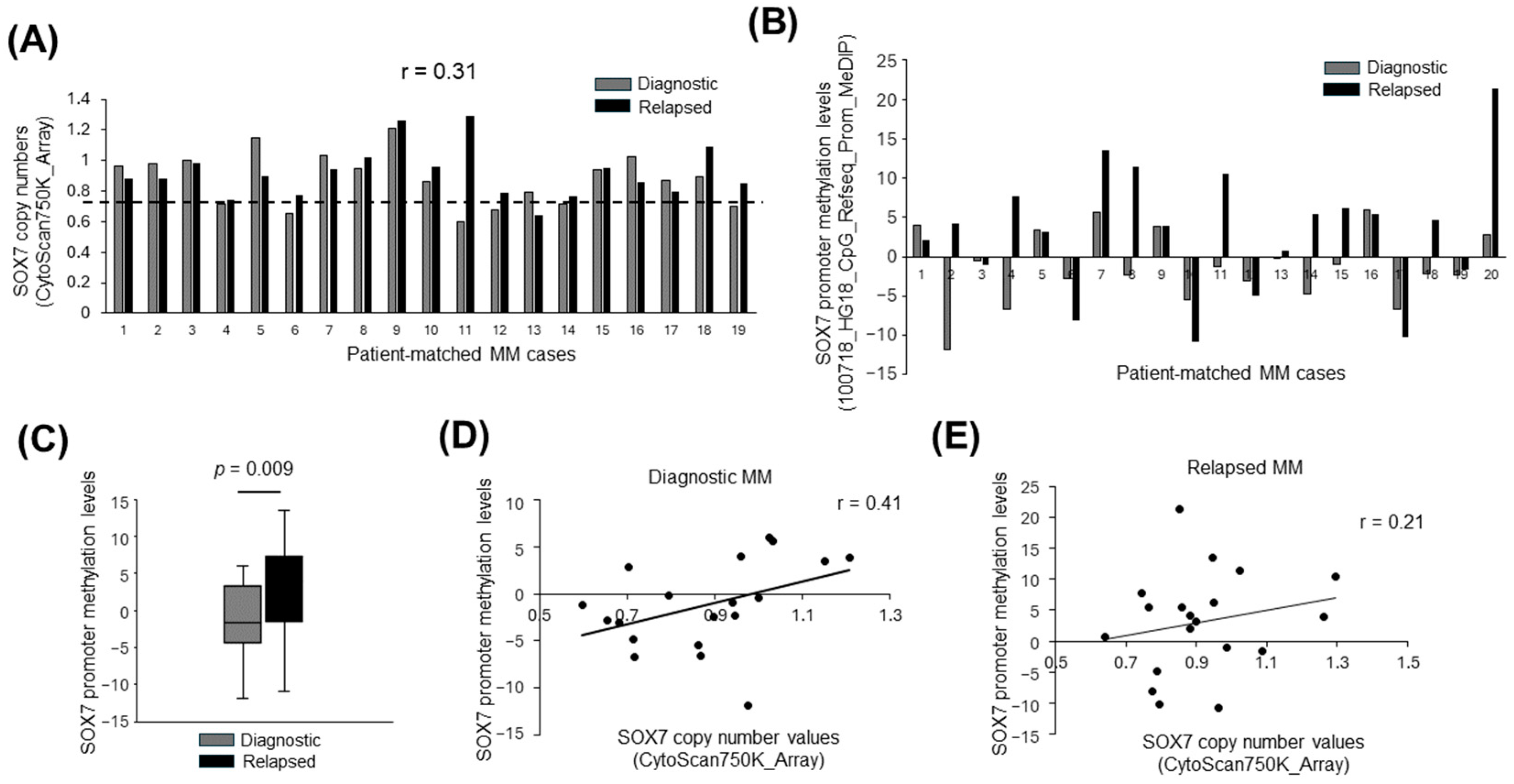
3.1. SOX7 Deletions Are Frequent in Diagnostic and Relapsed MM Samples
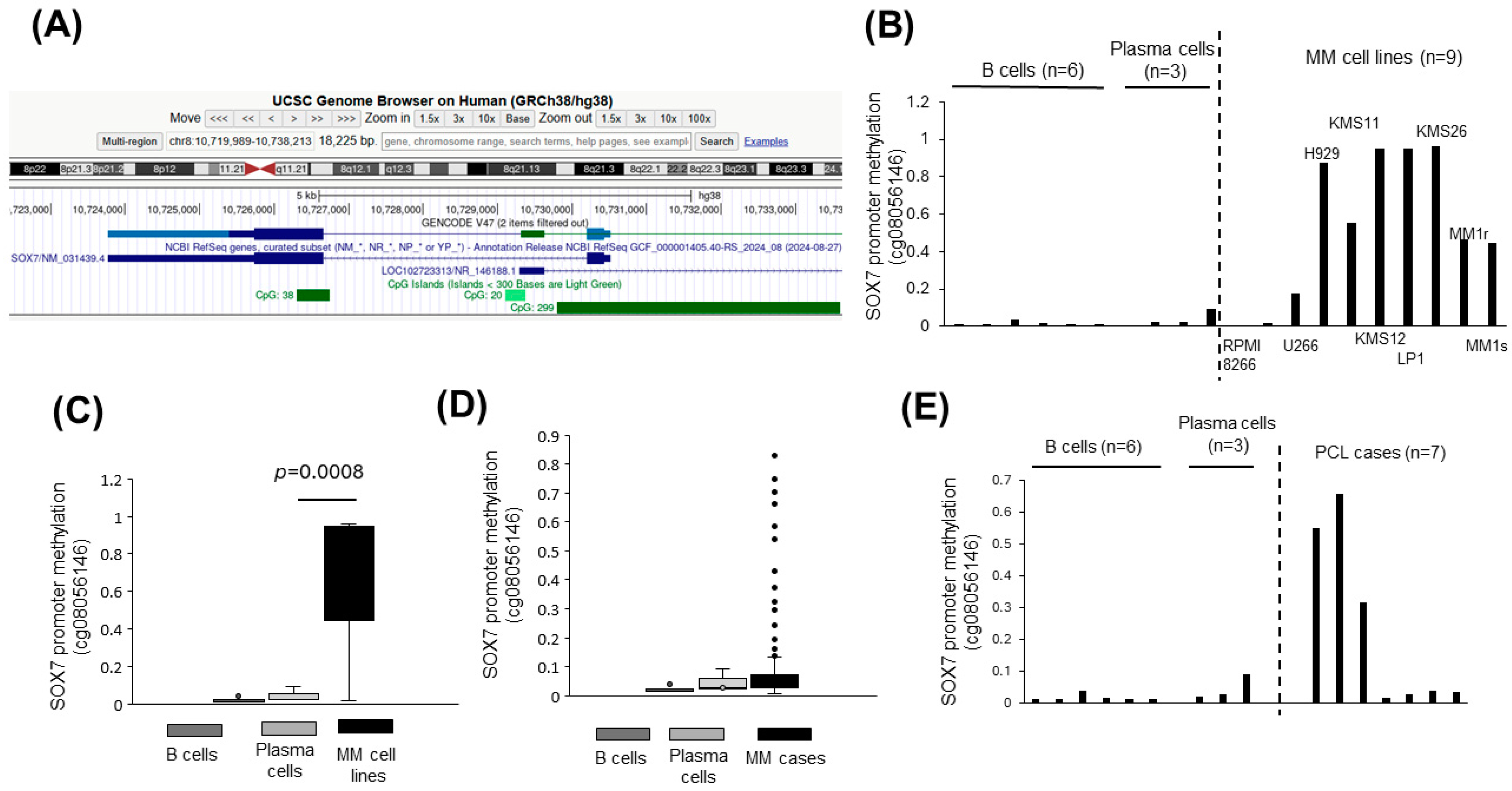
3.2. SOX7 Promoter Is Hypermethylated in Plasma Cell Malignancies
3.3. Deletion and Promoter Methylation of SOX7 Tend to Occur in Different MM Cases
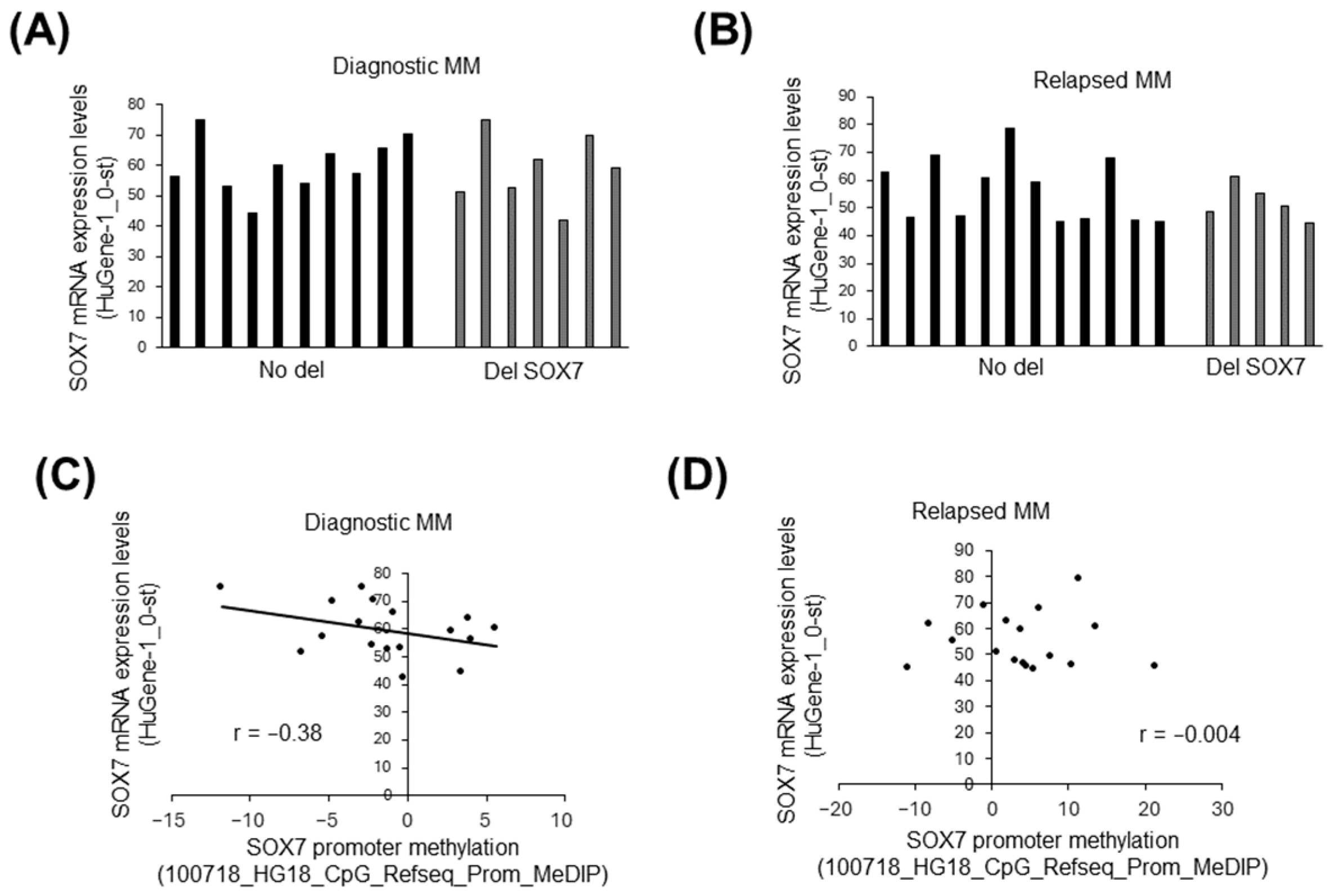
3.4. The Influence of Deletion and Methylation on Expression of Candidate Tumor Suppressors in 8p23.1
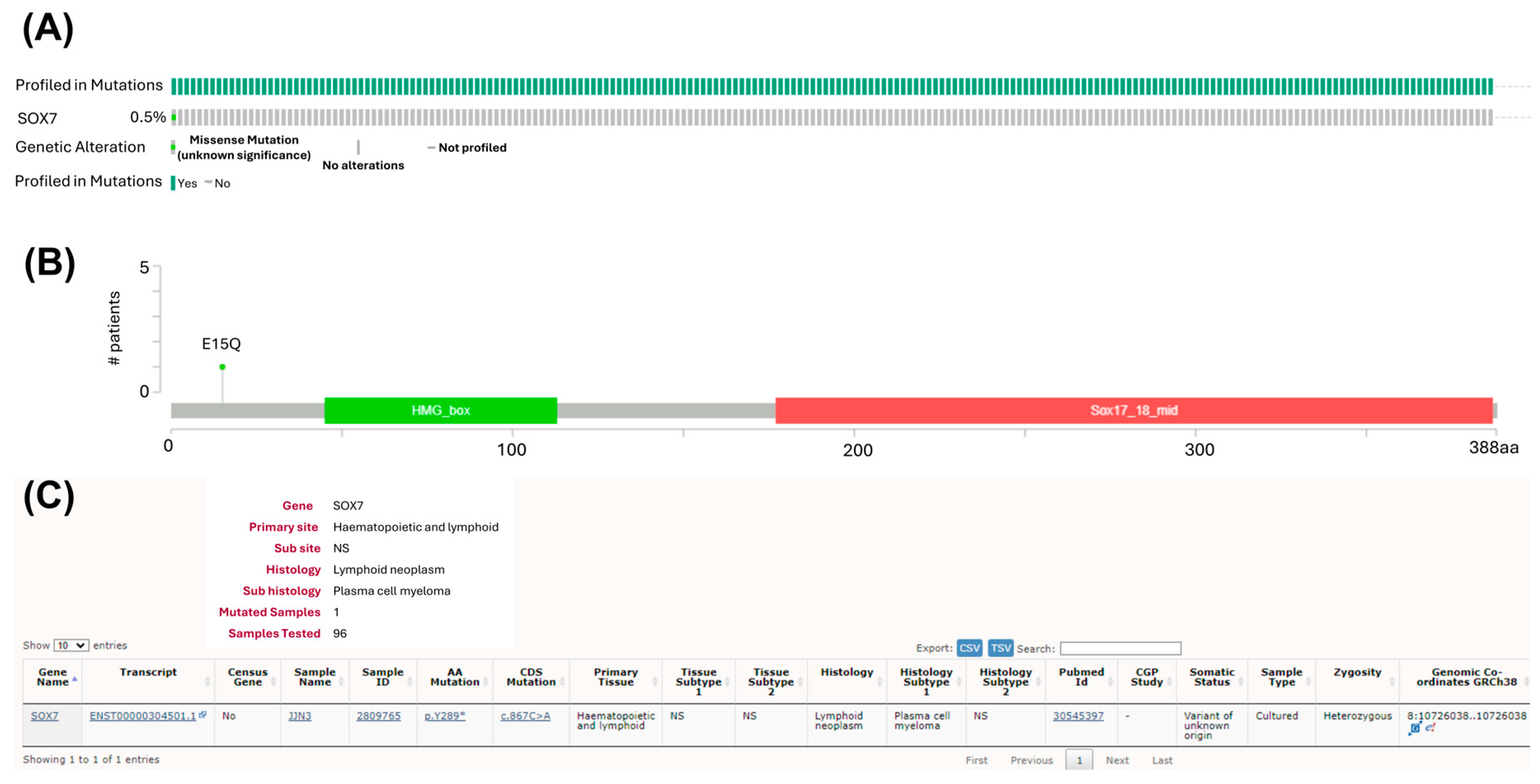
3.5. Mutations of SOX7 Are Infrequent in MM Tumor Samples
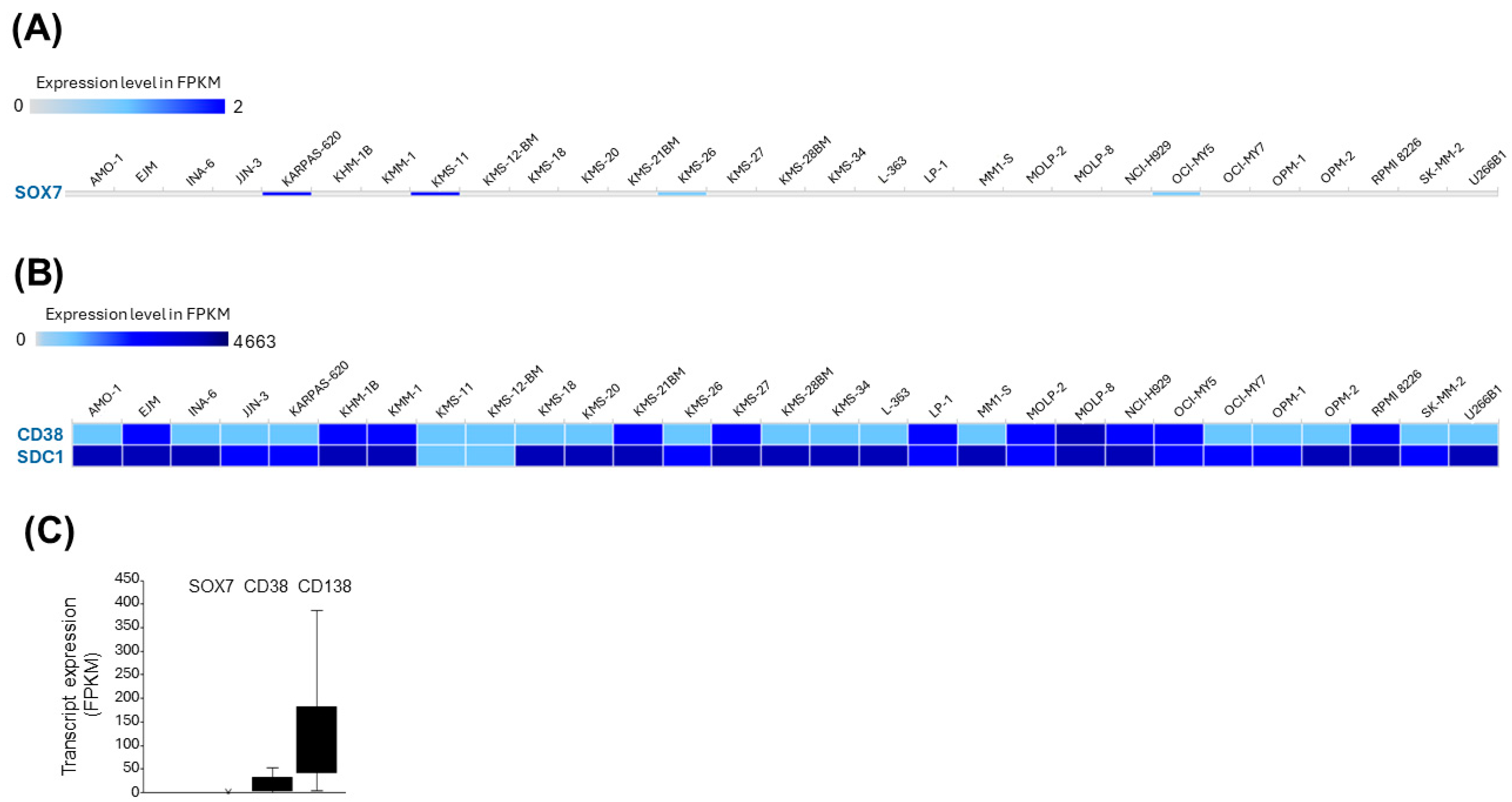
3.6. SOX7 Is Transcriptionally Silent in MM Cell Lines
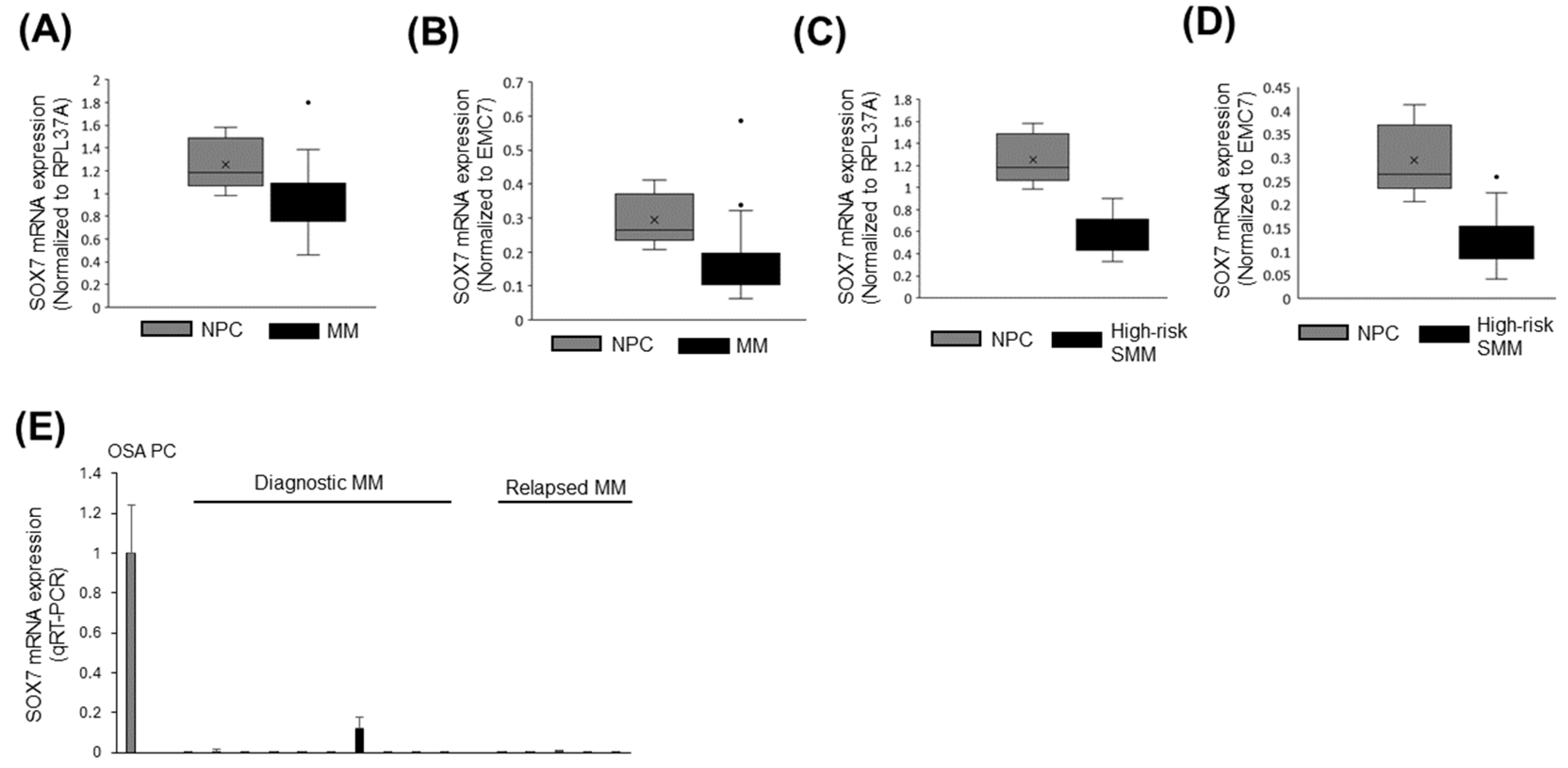
3.7. SOX7 Was Underexpressed in MM and High-Risk SMM Compared to Normal PCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| SMM | Smoldering multiple myeloma |
| PCL | Plasma cell leukemia |
| CRC | Colorectal cancer |
| QC | Quality check |
| ACK | Ammonium–chloride–potassium |
| MACS | Magnetic-activated cell sorting |
| GEP | Gene expression profiling |
| OSA | Obstructive sleep apnea |
| HDAC | Histone deacetylase |
| NGS | Next-generation sequencing |
References
- Raab, M.S.; Podar, K.; Breitkreutz, I.; Richardson, P.G.; Anderson, K.C. Multiple Myeloma. Lancet 2009, 374, 324–339. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; García-Sanz, R.; et al. Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef]
- Kuehl, W.M.; Bergsagel, P.L. Multiple Myeloma: Evolving Genetic Events and Host Interactions. Nat. Rev. Cancer 2002, 2, 175–187. [Google Scholar] [CrossRef]
- Ho, M.; Patel, A.; Goh, C.Y.; Moscvin, M.; Zhang, L.; Bianchi, G. Changing Paradigms in Diagnosis and Treatment of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (SMM). Leukemia 2020, 34, 3111–3125. [Google Scholar] [CrossRef]
- Albarracin, F.; Fonseca, R. Plasma Cell Leukemia. Blood Rev. 2011, 25, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or High-Dose Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Tricot, G.; Anaissie, E.; Shaughnessy, J.; Rasmussen, E.; van Rhee, F.; Fassas, A.; Zangari, M.; Hollmig, K.; Pineda-Roman, M.; et al. Thalidomide and Hematopoietic-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2006, 354, 1021–1030. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically Improved Survival in Multiple Myeloma Patients in the Recent Decade: Results from a Swedish Population-Based Study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef]
- Siegel, D.S.; Dimopoulos, M.A.; Ludwig, H.; Facon, T.; Goldschmidt, H.; Jakubowiak, A.; San-Miguel, J.; Obreja, M.; Blaedel, J.; Stewart, A.K. Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Therneau, T.M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Rajkumar, S.V.; Fonseca, R.; Witzig, T.E.; Lust, J.A.; Larson, D.R.; et al. Clinical Course of Patients with Relapsed Multiple Myeloma. Mayo Clin. Proc. 2004, 79, 867–874. [Google Scholar] [CrossRef]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug Resistance in Multiple Myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Esmeray, E.; Küçük, C. Genetic Alterations in b Cell Lymphoma Subtypes as Potential Biomarkers for Noninvasive Diagnosis, Prognosis, Therapy, and Disease Monitoring. Turk. J. Biol. 2020, 44, 1–14. [Google Scholar] [PubMed]
- Cao, Q.; Tian, Y.; Deng, Z.; Yang, F.; Chen, E. Epigenetic Alteration in Colorectal Cancer: Potential Diagnostic and Prognostic Implications. Int. J. Mol. Sci. 2024, 25, 3358. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Todd, N.W.; Qiu, Q.; Fan, T.; Zhao, R.Y.; Rodgers, W.H.; Fang, H.-B.; Katz, R.L.; Stass, S.A.; Jiang, F. Genetic Deletions in Sputum as Diagnostic Markers for Early Detection of Stage I Non-Small Cell Lung Cancer. Clin. Cancer Res. 2007, 13, 482–487. [Google Scholar] [CrossRef]
- Müller, D.; Győrffy, B. DNA Methylation-Based Diagnostic, Prognostic, and Predictive Biomarkers in Colorectal Cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188722. [Google Scholar] [CrossRef]
- Wei, B.; Wu, F.; Xing, W.; Sun, H.; Yan, C.; Zhao, C.; Wang, D.; Chen, X.; Chen, Y.; Li, M.; et al. A Panel of DNA Methylation Biomarkers for Detection and Improving Diagnostic Efficiency of Lung Cancer. Sci. Rep. 2021, 11, 16782. [Google Scholar] [CrossRef]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread Genetic Heterogeneity in Multiple Myeloma: Implications for Targeted Therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef]
- Lei, K.-F.; Wang, Y.-F.; Zhu, X.-Q.; Lu, P.-C.; Sun, B.-S.; Jia, H.-L.; Ren, N.; Ye, Q.-H.; Sun, H.-C.; Wang, L.; et al. Identification of MSRA Gene on Chromosome 8p as a Candidate Metastasis Suppressor for Human Hepatitis B Virus-Positive Hepatocellular Carcinoma. BMC Cancer 2007, 7, 172. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Huang, P.; Shi, R.; Lee, T.H.; Lu, G.; Zhang, Z.; Bronson, R.; Lu, K.P. The Telomerase Inhibitor PinX1 Is a Major Haploinsufficient Tumor Suppressor Essential for Chromosome Stability in Mice. J. Clin. Investig. 2011, 121, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Dong, W.; Li, L.; Feng, Y.; Pan, L.; Han, Z.; Wang, X.; Ren, G.; Su, D.; et al. SOX7, down-Regulated in Colorectal Cancer, Induces Apoptosis and Inhibits Proliferation of Colorectal Cancer Cells. Cancer Lett. 2009, 277, 29–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Stovall, D.B.; Wan, M.; Zhang, Q.; Chou, J.W.; Li, D.; Sui, G. SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. Int. J. Mol. Sci. 2018, 19, 1451. [Google Scholar] [CrossRef] [PubMed]
- Hayano, T.; Garg, M.; Yin, D.; Sudo, M.; Kawamata, N.; Shi, S.; Chien, W.; Ding, L.-W.; Leong, G.; Mori, S.; et al. SOX7 Is Down-Regulated in Lung Cancer. J. Exp. Clin. Cancer Res. 2013, 32, 17. [Google Scholar] [CrossRef]
- Li, B.; Ge, Z.; Song, S.; Zhang, S.; Yan, H.; Huang, B.; Zhang, Y. Decreased Expression of SOX7 Is Correlated with Poor Prognosis in Lung Adenocarcinoma Patients. Pathol. Oncol. Res. 2012, 18, 1039–1045. [Google Scholar] [CrossRef]
- Cui, J.; Xi, H.; Cai, A.; Bian, S.; Wei, B.; Chen, L. Decreased Expression of SOX7 Correlates with the Upregulation of the Wnt/β-Catenin Signaling Pathway and the Poor Survival of Gastric Cancer Patients. Int. J. Mol. Med. 2014, 34, 197–204. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Z.-Q.; Li, B.; Yin, S.-Y.; Sun, Q.; Kou, J.-J.; Ye, D.; Ferns, K.; Liu, H.-Y.; Liu, S.-L. Reduced Expression of SOX7 in Ovarian Cancer: A Novel Tumor Suppressor through the Wnt/β-Catenin Signaling Pathway. J. Ovarian Res. 2014, 7, 87. [Google Scholar] [CrossRef]
- Sun, Q.-Y.; Ding, L.-W.; Johnson, K.; Zhou, S.; Tyner, J.W.; Yang, H.; Doan, N.B.; Said, J.W.; Xiao, J.-F.; Loh, X.-Y.; et al. SOX7 Regulates MAPK/ERK-BIM Mediated Apoptosis in Cancer Cells. Oncogene 2019, 38, 6196–6210. [Google Scholar] [CrossRef] [PubMed]
- Seyhanli, A.; Hatipoglu, T.; Akman, B.; Hu, X.; Ozsan, G.H.; Kucuk, C. Genetic and Epigenetic Analyses Reveal Transcriptional Silencing of SOX7 Due to Deletion in a Multiple Myeloma Case with Double Relapse. UHOD—Uluslar. Hematol.-Onkol. Derg. 2023, 33, 54–56. [Google Scholar]
- Kim, D.; Park, C.Y.; Medeiros, B.C.; Weissman, I.L. CD19-CD45 Low/- CD38 High/CD138+ Plasma Cells Enrich for Human Tumorigenic Myeloma Cells. Leukemia 2012, 26, 2530–2537. [Google Scholar] [CrossRef]
- Hu, X.; Baytak, E.; Li, J.; Akman, B.; Okay, K.; Hu, G.; Scuto, A.; Zhang, W.; Küçük, C. The Relationship of REL Proto-Oncogene to Pathobiology and Chemoresistance in Follicular and Transformed Follicular Lymphoma. Leuk. Res. 2017, 54, 30–38. [Google Scholar] [CrossRef]
- Zhang, J.; Jima, D.D.; Jacobs, C.; Fischer, R.; Gottwein, E.; Huang, G.; Lugar, P.L.; Lagoo, A.S.; Rizzieri, D.A.; Friedman, D.R.; et al. Patterns of MicroRNA Expression Characterize Stages of Human B-Cell Differentiation. Blood 2009, 113, 4586–4594. [Google Scholar] [CrossRef]
- Medina, F.; Segundo, C.; Brieva, J.A. Purification of Human Tonsil Plasma Cells: Pre-Enrichment Step by Immunomagnetic Selection of CD31(+) Cells. Cytometry 2000, 39, 231–234. [Google Scholar] [CrossRef]
- Kjeldsen, E. Identification of Prognostically Relevant Chromosomal Abnormalities in Routine Diagnostics of Multiple Myeloma Using Genomic Profiling. Cancer Genom. Proteom. 2016, 13, 91–127. [Google Scholar]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant Global Methylation Patterns Affect the Molecular Pathogenesis and Prognosis of Multiple Myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; Corchete, L.A.; García, J.L.; López-Corral, L.; Fermiñán, E.; García, E.M.; Martín, A.A.; Hernández-Rivas, J.M.; García-Sanz, R.; San Miguel, J.F.; et al. Integrative Analysis of DNA Copy Number, DNA Methylation and Gene Expression in Multiple Myeloma Reveals Alterations Related to Relapse. Oncotarget 2016, 7, 80664–80679. [Google Scholar] [CrossRef] [PubMed]
- López-Corral, L.; Corchete, L.A.; Sarasquete, M.E.; Mateos, M.V.; García-Sanz, R.; Fermiñán, E.; Lahuerta, J.-J.; Bladé, J.; Oriol, A.; Teruel, A.I.; et al. Transcriptome Analysis Reveals Molecular Profiles Associated with Evolving Steps of Monoclonal Gammopathies. Haematologica 2014, 99, 1365–1372. [Google Scholar] [CrossRef]
- Tessoulin, B.; Moreau-Aubry, A.; Descamps, G.; Gomez-Bougie, P.; Maïga, S.; Gaignard, A.; Chiron, D.; Ménoret, E.; Le Gouill, S.; Moreau, P.; et al. Whole-Exon Sequencing of Human Myeloma Cell Lines Shows Mutations Related to Myeloma Patients at Relapse with Major Hits in the DNA Regulation and Repair Pathways. J. Hematol. Oncol. 2018, 11, 137. [Google Scholar] [CrossRef]
- Dong, J.T. Chromosomal Deletions and Tumor Suppressor Genes in Prostate Cancer. Cancer Metastasis Rev. 2001, 20, 173–193. [Google Scholar] [CrossRef]
- Iqbal, J.; Kucuk, C.; Deleeuw, R.J.; Srivastava, G.; Tam, W.; Geng, H.; Klinkebiel, D.; Christman, J.K.; Patel, K.; Cao, K.; et al. Genomic Analyses Reveal Global Functional Alterations That Promote Tumor Growth and Novel Tumor Suppressor Genes in Natural Killer-Cell Malignancies. Leukemia 2009, 23, 1139–1151. [Google Scholar] [CrossRef]
- Küçük, C.; Iqbal, J.; Hu, X.; Gaulard, P.; De Leval, L.; Srivastava, G.; Au, W.Y.; McKeithan, T.W.; Chan, W.C. PRDM1 Is a Tumor Suppressor Gene in Natural Killer Cell Malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 20119–20124. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, Y.; Lee, L.; Liu, X.; Shi, Y.; Liu, X.; Bouska, A.; Gong, Q.; Kong, L.; Wang, J.; et al. Genetic Manipulation of Primary Human Natural Killer Cells to Investigate the Functional and Oncogenic Roles of PRDM1. Haematologica 2021, 106, 2427–2438. [Google Scholar] [CrossRef]
- Bouska, A.; McKeithan, T.W.; Deffenbacher, K.E.; Lachel, C.; Wright, G.W.; Iqbal, J.; Smith, L.M.; Zhang, W.; Kucuk, C.; Rinaldi, A.; et al. Genome-Wide Copy-Number Analyses Reveal Genomic Abnormalities Involved in Transformation of Follicular Lymphoma. Blood 2014, 123, 1681–1690. [Google Scholar] [CrossRef]
- Küçük, C.; Hu, X.; Iqbal, J.; Gaulard, P.; Klinkebiel, D.; Cornish, A.; Dave, B.J.; Chan, W.C. HACE1 Is a Tumor Suppressor Gene Candidate in Natural Killer Cell Neoplasms. Am. J. Pathol. 2013, 182, 49–55. [Google Scholar] [CrossRef]
- Schinke, C.; Rasche, L.; Raab, M.S.; Weinhold, N. Impact of Clonal Heterogeneity in Multiple Myeloma. Hematol. Oncol. Clin. North Am. 2024, 38, 461–476. [Google Scholar] [CrossRef]
- Schmid, C.A.; Robinson, M.D.; Scheifinger, N.A.; Müller, S.; Cogliatti, S.; Tzankov, A.; Müller, A. DUSP4 Deficiency Caused by Promoter Hypermethylation Drives JNK Signaling and Tumor Cell Survival in Diffuse Large B Cell Lymphoma. J. Exp. Med. 2015, 212, 775–792. [Google Scholar] [CrossRef]
- Küçük, C.; Hu, X.; Jiang, B.; Klinkebiel, D.; Geng, H.; Gong, Q.; Bouska, A.; Iqbal, J.; Gaulard, P.; McKeithan, T.W.; et al. Global Promoter Methylation Analysis Reveals Novel Candidate Tumor Suppressor Genes in Natural Killer Cell Lymphoma. Clin. Cancer Res. 2015, 21, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Leshchenko, V.V.; Kuo, P.Y.; Shaknovich, R.; Yang, D.T.; Gellen, T.; Petrich, A.; Yu, Y.; Remache, Y.; Weniger, M.A.; Rafiq, S.; et al. Genomewide DNA Methylation Analysis Reveals Novel Targets for Drug Development in Mantle Cell Lymphoma. Blood 2010, 116, 1025–1034. [Google Scholar] [PubMed]
- Ballestar, E.; Wolffe, A.P. Methyl-CpG-Binding Proteins. Targeting Specific Gene Repression. Eur. J. Biochem. 2001, 268, 1–6. [Google Scholar] [CrossRef]
- Leighton, G.; Williams, D.C. The Methyl-CpG-Binding Domain 2 and 3 Proteins and Formation of the Nucleosome Remodeling and Deacetylase Complex. J. Mol. Biol. 2020, 432, 1624–1639. [Google Scholar] [CrossRef]
- O’Neill, H.; Lee, H.; Gupta, I.; Rodger, E.J.; Chatterjee, A. Single-Cell DNA Methylation Analysis in Cancer. Cancers 2022, 14, 6171. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic Regulation in Human Cancer: The Potential Role of Epi-Drug in Cancer Therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küçük, C.; Akman, B.; Hu, X.; Hatipoğlu, T.; Şeyhanlı, A.; Ceylan, A.; Yılmaz, B.; Öztürk, O.C.; Erdağ, T.K.; Özsan, G.H. Genetic and Epigenetic Aberrations of SOX7 in Newly Diagnosed and Relapsed Multiple Myeloma as Well as Related Neoplasms. Curr. Issues Mol. Biol. 2025, 47, 244. https://doi.org/10.3390/cimb47040244
Küçük C, Akman B, Hu X, Hatipoğlu T, Şeyhanlı A, Ceylan A, Yılmaz B, Öztürk OC, Erdağ TK, Özsan GH. Genetic and Epigenetic Aberrations of SOX7 in Newly Diagnosed and Relapsed Multiple Myeloma as Well as Related Neoplasms. Current Issues in Molecular Biology. 2025; 47(4):244. https://doi.org/10.3390/cimb47040244
Chicago/Turabian StyleKüçük, Can, Burcu Akman, Xiaozhou Hu, Tevfik Hatipoğlu, Ahmet Şeyhanlı, Arda Ceylan, Bircan Yılmaz, Osman Can Öztürk, Taner Kemal Erdağ, and Güner Hayri Özsan. 2025. "Genetic and Epigenetic Aberrations of SOX7 in Newly Diagnosed and Relapsed Multiple Myeloma as Well as Related Neoplasms" Current Issues in Molecular Biology 47, no. 4: 244. https://doi.org/10.3390/cimb47040244
APA StyleKüçük, C., Akman, B., Hu, X., Hatipoğlu, T., Şeyhanlı, A., Ceylan, A., Yılmaz, B., Öztürk, O. C., Erdağ, T. K., & Özsan, G. H. (2025). Genetic and Epigenetic Aberrations of SOX7 in Newly Diagnosed and Relapsed Multiple Myeloma as Well as Related Neoplasms. Current Issues in Molecular Biology, 47(4), 244. https://doi.org/10.3390/cimb47040244





