Senescence Modulation: An Applied Science Review of Strategies in Anti-Aging, Regenerative Aesthetics, and Oncology Therapy
Abstract
1. Introduction: Bridging Anti-Aging, Regeneration, and Oncology Through Senescence
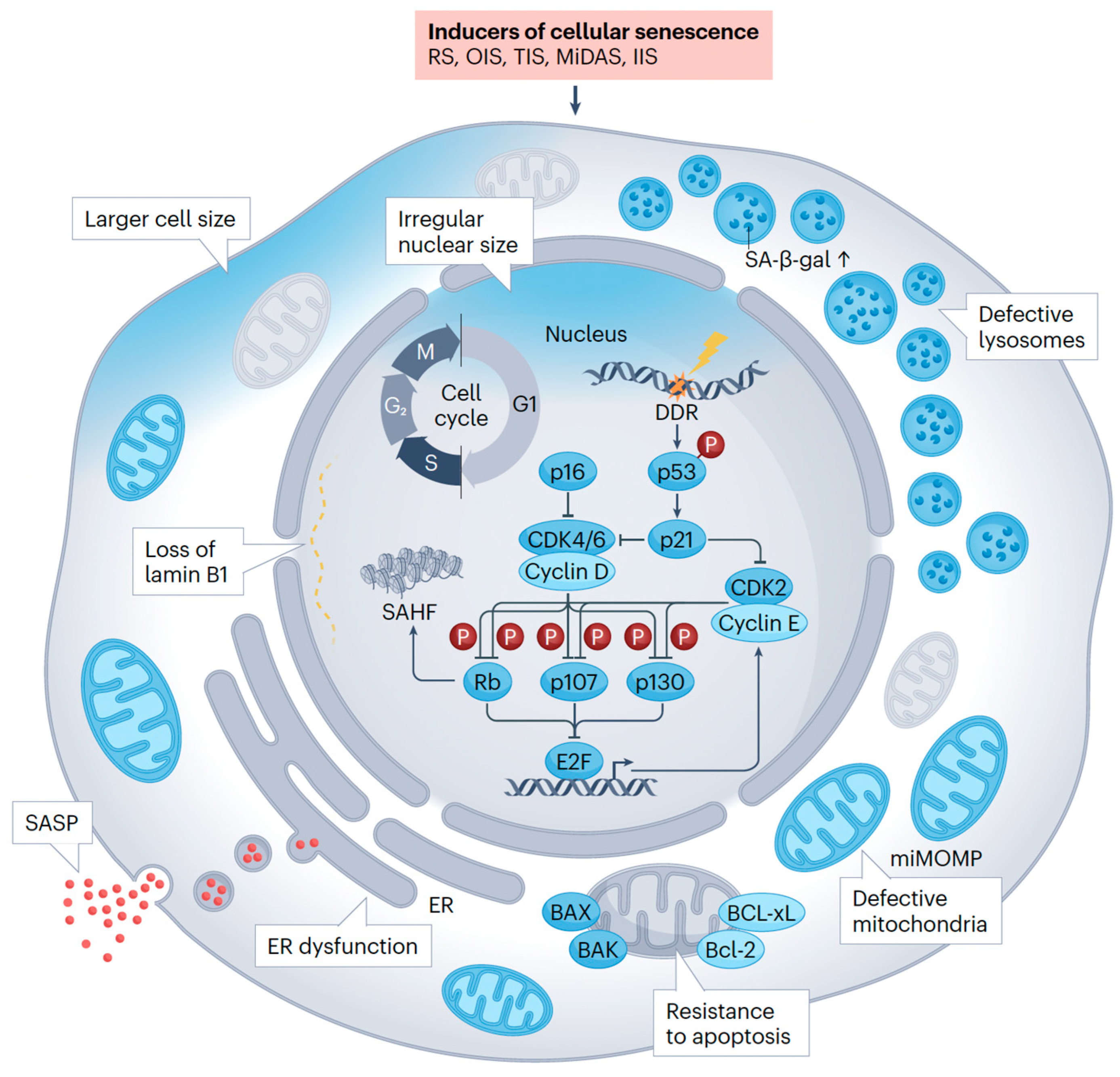
2. The Fundamental Role of Senescence: A Shared Biological Mechanism
3. Senescence Modulation in Dermatology, Anti-Aging and Regenerative Aesthetics: A Different Approach to Rejuvenation
4. Senescence Modulation in Oncology: A Multi-Level Therapeutic Target
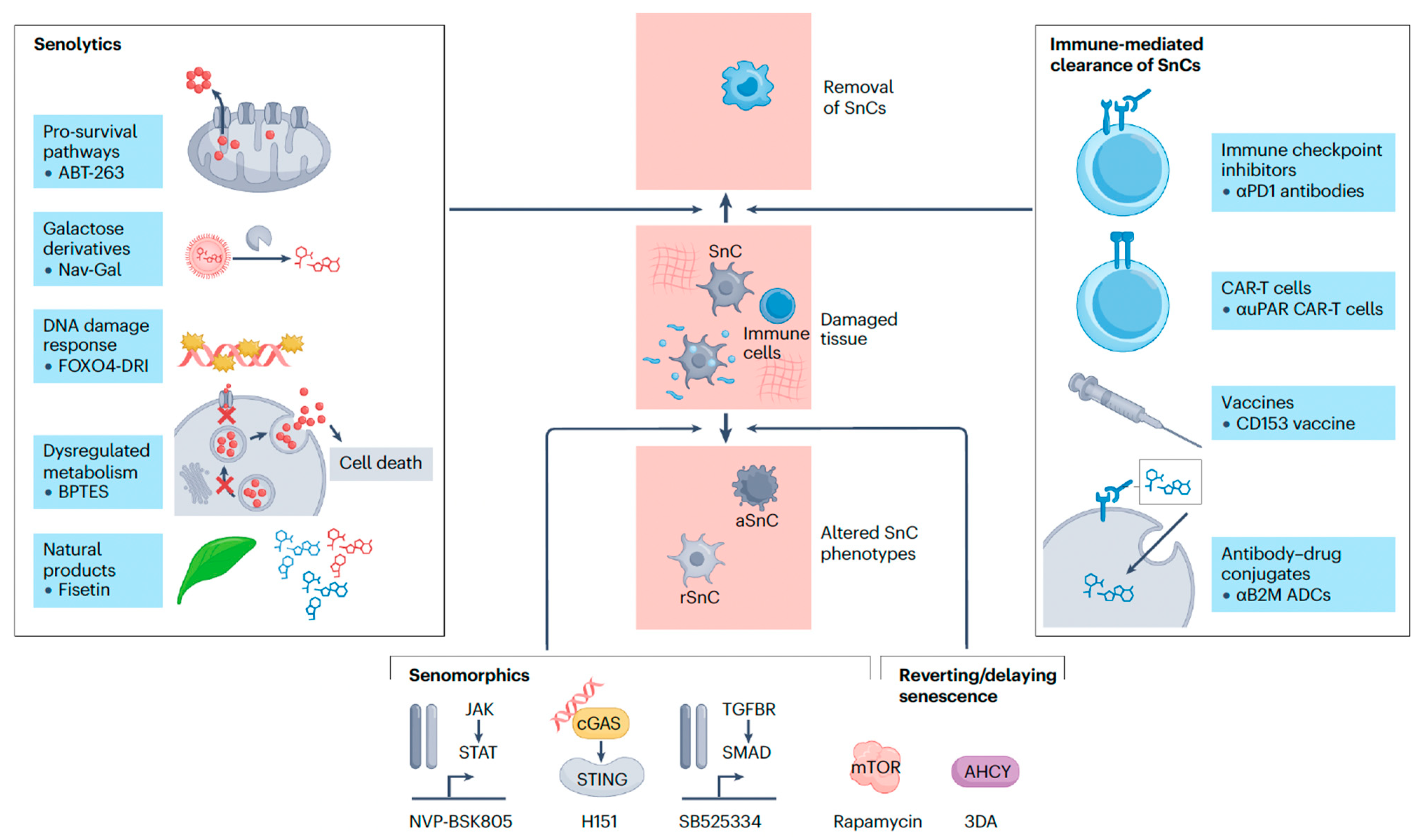
5. Targeting Senescence: Therapeutic Agents and Approaches
5.1. Mechanistic Classification of Senolytics (Targeted Elimination)
5.2. Mechanistic Classification of Senomorphics (SASP Suppression/Inhibition)
6. Discussion
6.1. The Challenge of SnC Heterogeneity and Predictive Biomarker Panels
6.2. Current Consensus
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DA | 3-deazaadenosine |
| ADCs | Antibody-drug conjugates |
| AHCY | S-adenosylhomocysteine hydrolase |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| aSnc | Activated senescent cell |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| BCL-xL | B-cell lymphoma-extra large |
| CAR | Chimeric antigen receptor |
| CD153 vaccine | Cluster of Differentiation 153 vaccine |
| CDK | Cyclin-dependent kinase |
| cGAS | Cyclic GMP-AMP synthase |
| CML | Chronic myeloid leukemia |
| D + Q | Dasatinib, a tyrosine kinase inhibitor, with quercetin, a naturally occurring flavonoid |
| DDR | DNA damage response |
| DNA | Deoxyribonucleic acid |
| E2F | E2 promoter-binding factor |
| ER | Endoplasmic reticulum |
| FAP-alpha | Fibroblast Activation Protein-alpha |
| HNSCC | Head and neck squamous cell carcinoma |
| IIS | Inflammation-induced Senescence |
| ICI | Immune checkpoint inhibitors |
| JAKs | Janus kinases |
| mAbs | Monoclonal antibodies |
| MCL | Mantle cell lymphoma |
| MIDAS | Mitochondria-induced Senescence |
| miMOMP | Mitochondrial outer membrane permeabilization |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| MSCs | Mesenchymal stem cells |
| mTOR | Mechanistic target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| OIS | Oncogene-induced Senescence |
| PDRN | Polydeoxyribonucleotide |
| PN | Polynucleotide |
| Rb | Retinoblastoma protein |
| RS | Replicative senescence |
| rSnC | Resident senescent cell |
| SA-β-gal | Senescence-Associated β-galactosidase |
| SAHF | Senescence-associated heterochromatin foci |
| SAPS | Senescence-associated pro-survival pathways |
| SASP | Senescence-associated secretory phenotype |
| siRNA | Small interfering RNA |
| SMAD | Mothers against decapentaplegic homolog |
| SnC | Senescent cell |
| STAT | Signal Transducer and Activator of Transcription |
| STING | Stimulator of Interferon Genes |
| TGFBR | Transforming growth factor beta receptor |
| TIS | Therapy-induced Senescence |
| TISnt | Therapy-induced Senescent Cells |
| uPAR | Urokinase plasminogen activator receptor |
| αB2M ADCs | Anti-beta-2-microglobulin Antibody-Drug Conjugates |
| αPD1 antibodies | Anti-Programmed Death-1 antibodies |
| αuPAR CAR-T cells | Urokinase plasminogen activator receptor Chimeric Antigen Receptor T-cells |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Nozawa, K.; Toma, S.; Shimizu, C. Distress and impacts on daily life from appearance changes due to cancer treatment: A survey of 1,034 patients in Japan. Glob. Health Med. 2023, 5, 54–61. [Google Scholar] [CrossRef]
- Proietti, I.; Skroza, N.; Mambrin, A.; Marraffa, F.; Tolino, E.; Bernardini, N.; Marchesiello, A.; Rossi, G.; Volpe, S.; Potenza, C. Aesthetic Treatments in Cancer Patients. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1831–1837. [Google Scholar] [CrossRef]
- Thompson, A.R.; Sewards, I.; Baker, S.R. Cancer and changes in facial appearance: A meta-ethnography of qualitative studies. Br. J. Health Psychol. 2020, 25, 129–151. [Google Scholar] [CrossRef]
- Papagni, M.; Renga, M.; Mogavero, S.; Veronesi, P.; Cavallini, M. The Esthetic Use of Botulinum Toxins in Cancer Patients: Providing a Foundation for Future Indications. Toxins 2025, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.; Jaśniak, A.; Wesołowska, W.; Pietrzak, J. Aesthetic Medicine Procedures in Cancer Survivors—A Literature Review. J. Educ. Health Sport 2025, 81, 65415. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef]
- Liao, Z.; Yeo, H.L.; Wong, S.W.; Zhao, Y. Cellular senescence: Mechanisms and therapeutic potential. Biomedicines 2021, 9, 1769. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes. Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Ahmed Khan, Z.; Nawaz, A.; Khan, I. Leadership Theories and Styles: A Literature Review. J. Resour. Dev. Manag. 2016, 16, 1–7. [Google Scholar]
- Wang, H.; Yu, Y.; Li, R.; Zhang, H.; Chen, Z.-S.; Sun, C.; Zhuang, J. Immunoregulatory mechanisms in the aging microenvironment: Targeting the senescence-associated secretory phenotype for cancer immunotherapy. Acta Pharm. Sin. B 2025, 15, 4476–4496. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Antonangeli, F.; Sozzani, S.; Santoni, A.; Cippitelli, M.; Soriani, A. The senescence journey in cancer immunoediting. Mol. Cancer 2024, 23, 68. [Google Scholar] [CrossRef]
- Deng, G.; Xu, C.; Mo, D. Identification and mechanistic insights of cell senescence-related genes in psoriasis. PeerJ 2025, 13, e18818. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell Biol. 2016, 2016, 9259646. [Google Scholar] [CrossRef]
- Thau, H.; Gerjol, B.P.; Hahn, K.; von Gudenberg, R.W.; Knoedler, L.; Stallcup, K.; Emmert, M.Y.; Buhl, T.; Wyles, S.P.; Tchkonia, T.; et al. Senescence as a molecular target in skin aging and disease. Ageing Res. Rev. 2025, 105, 102686. [Google Scholar] [CrossRef]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, J.; Feng, J.; Cheng, H. Cellular Senescence and Anti-Aging Strategies in Aesthetic Medicine: A Bibliometric Analysis and Brief Review. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2243–2259. [Google Scholar] [CrossRef]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Galimberti, D.; Candore, G. Senotherapeutics to Counteract Senescent Cells Are Prominent Topics in the Context of Anti-Ageing Strategies. Int. J. Mol. Sci. 2024, 25, 1792. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. npj Aging 2024, 10, 53. [Google Scholar] [CrossRef]
- Thompson, E.L.; Pitcher, L.E.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Development of new approaches for skin care. Plast. Reconstr. Surg. 2022, 150, 12s–19s. [Google Scholar] [CrossRef]
- Lueangarun, S.; Visutjindaporn, P.; Parcharoen, Y.; Jamparuang, P.; Tempark, T. A systematic review and meta-analysis of randomized controlled trials of United States Food and Drug Administration-approved, home-use, low-level light/laser therapy devices for pattern hair loss: Device design and technology. J. Clin. Aesthet. Dermatol. 2021, 14, E64–E75. [Google Scholar]
- Heidari Beigvand, H.; Razzaghi, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Safari, S.; Rezaei-Tavirani, M.; Mansouri, V.; Heidari, M.H. Assessment of laser effects on skin rejuvenation. J. Lasers Med. Sci. 2020, 11, 212–219. [Google Scholar] [CrossRef]
- Akinbiyi, T.; Othman, S.; Familusi, O.; Calvert, C.; Card, E.B.; Percec, I. Better results in facial rejuvenation with fillers. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2763. [Google Scholar] [CrossRef]
- Lampridou, S.; Bassett, S.; Cavallini, M.; Christopoulos, G. The effectiveness of polynucleotides in esthetic medicine: A systematic review. J. Cosmet. Dermatol. 2025, 24, e16721. [Google Scholar] [CrossRef]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yu, Y.; Wang, L.; Zhao, M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics. Cell Prolif. 2024, 57, e13586. [Google Scholar] [CrossRef]
- Alencar-Silva, T.; Barcelos, S.M.; Silva-Carvalho, A.; Sousa, M.; Rezende, T.M.B.; Pogue, R.; Saldanha-Araújo, F.; Franco, O.L.; Boroni, M.; Zonari, A.; et al. Senotherapeutic Peptide 14 Suppresses Th1 and M1 Human T Cell and Monocyte Subsets In Vitro. Cells 2024, 13, 813. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Czajkowski, K.; Herbet, M.; Murias, M.; Piątkowska-Chmiel, I. Senolytics: Charting a new course or enhancing existing anti-tumor therapies? Cell. Oncol. 2025, 48, 351–371. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Mongelli, A.; Atlante, S.; Barbi, V.; Bachetti, T.; Martelli, F.; Farsetti, A.; Gaetano, C. Treating Senescence like Cancer: Novel Perspectives in Senotherapy of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7984. [Google Scholar] [CrossRef]
- Luo, J.; Sun, T.; Liu, Z.; Liu, Y.; Liu, J.; Wang, S.; Shi, X.; Zhou, H. Persistent accumulation of therapy-induced senescent cells: An obstacle to long-term cancer treatment efficacy. Int. J. Oral. Sci. 2025, 17, 59. [Google Scholar] [CrossRef]
- Piskorz, W.M.; Cechowska-Pasko, M. Senescence of Tumor Cells in Anticancer Therapy-Beneficial and Detrimental Effects. Int. J. Mol. Sci. 2022, 23, 11082. [Google Scholar] [CrossRef]
- Imawari, Y.; Nakanishi, M. Senescence and senolysis in cancer: The latest findings. Cancer Sci. 2024, 115, 2107–2116. [Google Scholar] [CrossRef]
- Battram, A.M.; Bachiller, M.; Martín-Antonio, B. Senescence in the Development and Response to Cancer with Immunotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2020, 21, 4346. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Dal Bo, M.; Gambirasi, M.; Vruzhaj, I.; Cecchin, E.; Pishdadian, A.; Toffoli, G.; Safa, A. Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine. Int. J. Mol. Sci. 2025, 26, 4982. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Byrd, J.C.; Waselenko, J.K.; Maneatis, T.J.; Murphy, T.; Ward, F.T.; Monahan, B.P.; Sipe, M.A.; Donegan, S.; White, C.A. Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: Association with increased infusion-related side effects and rapid blood tumor clearance. J. Clin. Oncol. 1999, 17, 791–795. [Google Scholar] [CrossRef]
- Grothey, A.; Blay, J.Y.; Pavlakis, N.; Yoshino, T.; Bruix, J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat. Rev. 2020, 86, 101993. [Google Scholar] [CrossRef]
- Ali, A.; DiPersio, J.F. ReCARving the future: Bridging CAR T-cell therapy gaps with synthetic biology, engineering, and economic insights. Front. Immunol. 2024, 15, 1432799. [Google Scholar] [CrossRef]
- Meguro, S.; Makabe, S.; Yaginuma, K.; Onagi, A.; Tanji, R.; Matsuoka, K.; Hoshi, S.; Koguchi, T.; Kayama, E.; Hata, J.; et al. Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer. Curr. Oncol. 2025, 32, 467. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Jacobs, W.; Khalifeh, M.; Koot, M.; Palacio-Castañeda, V.; van Oostrum, J.; Ansems, M.; Verdurmen, W.P.R.; Brock, R. RNA-based logic for selective protein expression in senescent cells. Int. J. Biochem. Cell Biol. 2024, 174, 106636. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, Y.; Huang, H.C.; Mitchison, T.J. Navitoclax (ABT-263) accelerates apoptosis during drug-induced mitotic arrest by antagonizing Bcl-xL. Cancer Res. 2011, 71, 4518–4526. [Google Scholar] [CrossRef]
- Sun, W.; Gao, Y.; Wu, Y.; Wu, W.; Wang, C.; Chen, J.; Luan, C.; Hua, M.; Liu, W.; Gong, W.; et al. Targeted apoptosis of senescent cells by valproic acid alleviates therapy-induced cellular senescence and lung aging. Phytomedicine 2024, 135, 156131. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, H.; Wu, H. Cellular senescence in health, disease, and lens aging. Pharmaceuticals 2025, 18, 244. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent. Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Ostojic, A.; Vrhovac, R.; Verstovsek, S. Ruxolitinib: A new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosis. Future Oncol. 2011, 7, 1035–1043. [Google Scholar] [CrossRef]
- Lamming, D.W. Inhibition of the mechanistic target of rapamycin (mTOR)-rapamycin and beyond. Cold Spring Harb. Perspect. Med. 2016, 6, a025924. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin extends life- and health span because it slows aging. Aging 2013, 5, 592–598. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Revilla-Nuin, B.; Ramírez, P.; Pons, J.A. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J. Transplant. 2016, 6, 183–192. [Google Scholar] [CrossRef]
- Fu, T.E.; Zhou, Z. Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J. Transl. Int. Med. 2025, 13, 33–47. [Google Scholar] [CrossRef]
- McHugh, D.; Duran, I.; Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug Discov. 2025, 24, 57–71. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Carlos Acosta, J.; Adams, P.D.; d’Adda di Fagagna, F.; Baker, D.J.; Bishop, C.L.; Chandra, T.; Collado, M.; Gil, J.; Gorgoulis, V.; et al. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 2024, 187, 4150–4175. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B.; et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024, 25, 1001–1023. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Fernández-Maestre, I.; Chowdhury, S.; Ho, Y.-J.; Nadella, S.; Graham, C.; Carrasco, S.E.; Nnuji-John, E.; Feucht, J.; Hinterleitner, C.; et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 2024, 4, 336–349. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusmanto, S.J. Senescence Modulation: An Applied Science Review of Strategies in Anti-Aging, Regenerative Aesthetics, and Oncology Therapy. Curr. Issues Mol. Biol. 2025, 47, 989. https://doi.org/10.3390/cimb47120989
Kusmanto SJ. Senescence Modulation: An Applied Science Review of Strategies in Anti-Aging, Regenerative Aesthetics, and Oncology Therapy. Current Issues in Molecular Biology. 2025; 47(12):989. https://doi.org/10.3390/cimb47120989
Chicago/Turabian StyleKusmanto, Steven Januar. 2025. "Senescence Modulation: An Applied Science Review of Strategies in Anti-Aging, Regenerative Aesthetics, and Oncology Therapy" Current Issues in Molecular Biology 47, no. 12: 989. https://doi.org/10.3390/cimb47120989
APA StyleKusmanto, S. J. (2025). Senescence Modulation: An Applied Science Review of Strategies in Anti-Aging, Regenerative Aesthetics, and Oncology Therapy. Current Issues in Molecular Biology, 47(12), 989. https://doi.org/10.3390/cimb47120989





