Exploratory Urinary Proteomic Profiling in Pregnancies with Fetal Aneuploidies: Molecular Insights into Maternal–Fetal Metabolic Communication
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Demographic Data and Eligibility Criteria
2.2. Urine Sample Collection
2.3. Sample Preparation for MS-Based Proteomics
2.4. Label Free Nano-LC-IMS-MS and Data Analysis
3. Results
3.1. Clinical and Demographic Profile
3.2. Protein Identification and Functional Annotation
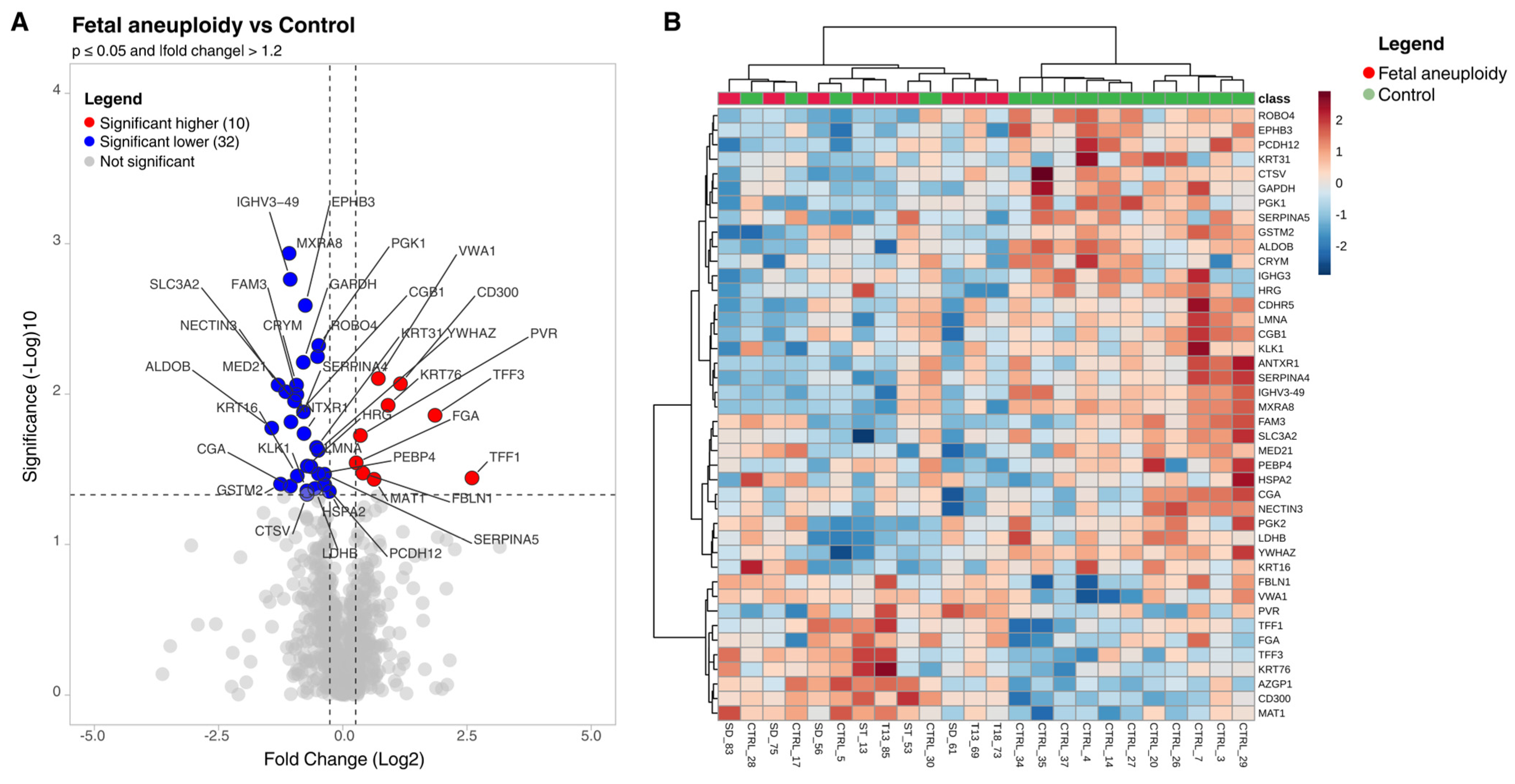
3.3. Differentially Abundant Proteins (Aneuploidy vs. Control)
3.4. Contextual Comparison with the Reference Urinary Proteome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurca, R.L.; Pralea, I.E.; Iacobescu, M.; Rus, I.; Iuga, C.A.; Stamatian, F. Non-Invasive Prenatal Screening for Down Syndrome: A Review of Mass-Spectrometry-Based Approaches. Life 2025, 15, 695. [Google Scholar] [CrossRef]
- Corry, E.; Mone, F.; Segurado, R.; Downey, P.; McParland, P.; McAuliffe, F.M.; Mooney, E.E. Placental Disease and Abnormal Umbilical Artery Doppler Waveforms in Trisomy 21 Pregnancy: A Case-Control Study. Placenta 2016, 47, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hatt, L.; Aagaard, M.M.; Bach, C.; Graakjaer, J.; Sommer, S.; Agerholm, I.E.; Kølvraa, S.; Bojesen, A. Microarray-Based Analysis of Methylation of 1st Trimester Trisomic Placentas from Down Syndrome, Edwards Syndrome and Patau Syndrome. PLoS ONE 2016, 11, e0160319. [Google Scholar] [CrossRef]
- Guibourdenche, J.; Leguy, M.C.; Pidoux, G.; Hebert-Schuster, M.; Laguillier, C.; Anselem, O.; Grangé, G.; Bonnet, F.; Tsatsaris, V. Biochemical Screening for Fetal Trisomy 21: Pathophysiology of Maternal Serum Markers and Involvement of the Placenta. Int. J. Mol. Sci. 2023, 24, 7669. [Google Scholar] [CrossRef]
- Millington, D.S. How Mass Spectrometry Revolutionized Newborn Screening. J. Mass Spectrom. Adv. Clin. Lab 2024, 32, 1–10. [Google Scholar] [CrossRef]
- Law, K.P.; Han, T.L.; Tong, C.; Baker, P.N. Mass Spectrometry-Based Proteomics for Pre-Eclampsia and Preterm Birth. Int. J. Mol. Sci. 2015, 16, 10952–10985. [Google Scholar] [CrossRef] [PubMed]
- Elkahlout, R.; Mohammed, S.G.A.A.; Najjar, A.; Farrell, T.; Rifai, H.A.; Al-Dewik, N.; Qoronfleh, M.W. Application of Proteomics in Maternal and Neonatal Health: Advancements and Future Directions. Proteom. Clin. Appl. 2025, 19, e70004. [Google Scholar] [CrossRef] [PubMed]
- Joenväärä, S.; Holm, M.; Saraswat, M.; Agarwal, R.; Tohmola, T.; Kajantie, E.; Räikkönen, K.; Laivuori, H.; Villa, P.M.; Hämäläinen, E.; et al. Quantitative Urine Proteomics in Pregnant Women for the Identification of Predictive Biomarkers for Preeclampsia. Transl. Med. Commun. 2022, 7, 1. [Google Scholar] [CrossRef]
- Bujold, E.; Fillion, A.; Roux-Dalvai, F.; Scott-Boyer, M.P.; Giguère, Y.; Forest, J.C.; Gotti, C.; Laforest, G.; Guerby, P.; Droit, A. Proteomic Analysis of Maternal Urine for the Early Detection of Preeclampsia and Fetal Growth Restriction. J. Clin. Med. 2021, 10, 4679. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, L.; Wang, J.; Jin, Q. Urinary Proteomic and Non-Prefractionation Quantitative Phosphoproteomic Analysis during Pregnancy and Non-Pregnancy. BMC Genom. 2013, 14, 777. [Google Scholar] [CrossRef]
- Guo, H.X.; Zhu, Y.B.; Wu, C.P.; Zhong, M.; Hu, S.W. Potential Urine Biomarkers for Gestational Hypertension and Preeclampsia. Mol. Med. Rep. 2019, 19, 2463–2470. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.; Ran, X.; Jia, X.; Xing, Y.; Dai, T.; Song, W.; Wu, Z.; Sun, W.; Shan, D. Urinary Proteomics for Noninvasive Prenatal Screening of Trisomy 21: New Biomarker Candidates. OMICS 2021, 25, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Wang, H.; Khatri, P.; Niu, Y.; Song, W.; Zhao, S.; Jiang, Y.; Ma, Q.; Liu, X.; Zhang, R.; et al. The Urinary Peptidome as a Noninvasive Biomarker Development Strategy for Prenatal Screening of Down’s Syndrome. OMICS A J. Integr. Biol. 2019, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kolialexi, A.; Anagnostopoulos, A.K.; Mavrou, A.; Tsangaris, G.T. Application of Proteomics for Diagnosis of Fetal Aneuploidies and Pregnancy Complications. J. Proteom. 2009, 72, 731–739. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Iles, R.K. Shotgun Metabolomic Profiles in Maternal Urine Identify Potential Mass Spectral Markers of Abnormal Fetal Biochemistry—Dihydrouracil and Progesterone in the Metabolism of Down Syndrome. Biomed. Chromatogr. 2015, 29, 1173–1183. [Google Scholar] [CrossRef]
- Soporan, M.A.; Pralea, I.E.; Iacobescu, M.; Moldovan, R.C.; Alkhzouz, C.; Miclea, D.; Iuga, C.A. Salivary Proteome Insights: Evaluation of Saliva Preparation Methods in Mucopolysaccharidoses Research. Biomedicines 2025, 13, 662. [Google Scholar] [CrossRef]
- Zhao, M.; Li, M.; Yang, Y.; Guo, Z.; Sun, Y.; Shao, C.; Li, M.; Sun, W.; Gao, Y. A Comprehensive Analysis and Annotation of Human Normal Urinary Proteome. Sci. Rep. 2017, 7, 3024. [Google Scholar] [CrossRef]
- Ito, T.; Takahashi, H.; Horie, K.; Nagayama, S.; Ogoyama, M.; Fujiwara, H. Confined Placental Mosaicism with Trisomy 13 Complicated by Severe Preeclampsia: A Case Report and Literature Review. J. Obstet. Gynaecol. Res. 2024, 50, 1737–1741. [Google Scholar] [CrossRef]
- Hessellund Samson, M.; Vestergaard, E.M.; Milman, N.; Seier Poulsen, S.; Nexo, E. Circulating Serum Trefoil Factors Increase Dramatically during Pregnancy. Scand. J. Clin. Lab. Investig. 2008, 68, 369–374. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-Evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.K.J.; Smith, C.R.; Diamandis, E.P. Amniotic Fluid Proteome Analysis from down Syndrome Pregnancies for Biomarker Discovery. J. Proteome Res. 2010, 9, 3574–3582. [Google Scholar] [CrossRef]
- Ahenkorah, J.; Hottor, B.; Byrne, S.; Bosio, P.; Ockleford, C.D. Immunofluorescence Confocal Laser Scanning Microscopy and Immuno-Electron Microscopic Identification of Keratins in Human Materno-Foetal Interaction Zone. J. Cell Mol. Med. 2009, 13, 735–748. [Google Scholar] [CrossRef] [PubMed]
- DiTommaso, T.; Cottle, D.L.; Pearson, H.B.; Schlüter, H.; Kaur, P.; Humbert, P.O.; Smyth, I.M. Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier. PLoS Genet. 2014, 10, e1004706. [Google Scholar] [CrossRef]
- Vijayaraj, P.; Kroeger, C.; Reuter, U.; Hartmann, D.; Magin, T.M. Keratins Regulate Yolk Sac Hematopoiesis and Vasculogenesis through Reduced BMP-4 Signaling. Eur. J. Cell Biol. 2010, 89, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kröger, C.; Vijayaraj, P.; Reuter, U.; Windoffer, R.; Simmons, D.; Heukamp, L.; Leube, R.; Magin, T.M. Placental Vasculogenesis Is Regulated by Keratin-Mediated Hyperoxia in Murine Decidual Tissues. Am. J. Pathol. 2011, 178, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Franz, T.; Tamai, Y.; Taketo, M.M.; Magin, T.M. Targeted Deletion of Keratins 18 and 19 Leads to Trophoblast Fragility and Early Embryonic Lethality. EMBO J. 2000, 19, 5060–5070. [Google Scholar] [CrossRef]
- Lim, H.Y.G.; Alvarez, Y.D.; Gasnier, M.; Wang, Y.; Tetlak, P.; Bissiere, S.; Wang, H.; Biro, M.; Plachta, N. Keratins Are Asymmetrically Inherited Fate Determinants in the Mammalian Embryo. Nature 2020, 585, 404–409. [Google Scholar] [CrossRef]
- Watson, E.D. 2005 Trophoblast Research Award Lecture: Defects in the Keratin Cytoskeleton Disrupt Normal Murine Placental Development and Trophoblast Cell Function. Placenta 2007, 28, S111–S115. [Google Scholar] [CrossRef]
- Gauster, M.; Blaschitz, A.; Siwetz, M.; Huppertz, B. Keratins in the Human Trophoblast. Histol. Histopathol. 2013, 28, 817–825. [Google Scholar] [CrossRef]
- Bouillot, S.; Tillet, E.; Carmona, G.; Prandini, M.H.; Gauchez, A.S.; Hoffmann, P.; Alfaidy, N.; Cand, F.; Huber, P. Protocadherin-12 Cleavage Is a Regulated Process Mediated by ADAM10 Protein: Evidence of Shedding up-Regulation in Pre-Eclampsia. J. Biol. Chem. 2011, 286, 15195–15204. [Google Scholar] [CrossRef]
- Rampon, C.; Bouillot, S.; Climescu-Haulica, A.; Prandini, M.H.; Cand, F.; Vandenbrouck, Y.; Huber, P. Protocadherin 12 Deficiency Alters Morphogenesis and Transcriptional Profile of the Placenta. Physiol. Genom. 2008, 34, 193–204. [Google Scholar] [CrossRef]
- Kokkinos, M.I.; Murthi, P.; Wafai, R.; Thompson, E.W.; Newgreen, D.F. Cadherins in the Human Placenta—Epithelial-Mesenchymal Transition (EMT) and Placental Development. Placenta 2010, 31, 747–755. [Google Scholar] [CrossRef]
- Parameshwar, P.K.; Sagrillo-Fagundes, L.; Fournier, C.; Girard, S.; Vaillancourt, C.; Moraes, C. Disease-Specific Extracellular Matrix Composition Regulates Placental Trophoblast Fusion Efficiency. Biomater. Sci. 2021, 9, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Luppi, S.; Fejza, A.; Giolo, E.; Ricci, G.; Andreuzzi, E. Extracellular Matrix and Pregnancy: Functions and Opportunities Caught in the Net. Reprod. Biol. Endocrinol. 2025, 23, 24. [Google Scholar] [CrossRef]
- Kolla, V.; Jenö, P.; Moes, S.; Tercanli, S.; Lapaire, O.; Choolani, M.; Hahn, S. Quantitative Proteomics Analysis of Maternal Plasma in down Syndrome Pregnancies Using Isobaric Tagging Reagent (ITRAQ). J. Biomed. Biotechnol. 2010, 2010, 952047. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Kancharla, S.; Kolli, P.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Fibulins in Embryonic Stage Development and Their Involvement in Various Diseases. Biomolecules 2021, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.A.; Kern, C.B.; Fresco, V.M.; Wessels, A.; Thompson, R.P.; McQuinn, T.C.; Twal, W.O.; Mjaatvedt, C.H.; Drake, C.J.; Argraves, W.S. Fibulin-1 Is Required for Morphogenesis of Neural Crest-Derived Structures. Dev. Biol. 2008, 319, 336–345. [Google Scholar] [CrossRef]
- Orvik, A.B.; Andersen, M.R.; Pedersen, L.; Ritz, C.; Stender, S.; Szecsi, P.B. Plasma Fibulin-1 Levels during Pregnancy and Delivery: A Longitudinal Observational Study. BMC Pregnancy Childbirth 2021, 21, 629. [Google Scholar] [CrossRef]
- Neiman, M.; Hedberg, J.J.; Dönnes, P.R.; Schuppe-Koistinen, I.; Hanschke, S.; Schindler, R.; Uhlén, M.; Schwenk, J.M.; Nilsson, P. Plasma Profiling Reveals Human Fibulin-1 as Candidate Marker for Renal Impairment. J. Proteome Res. 2011, 10, 4925–4934. [Google Scholar] [CrossRef]
- Flitcroft, J.G.; Verheyen, J.; Vemulkar, T.; Welbourne, E.N.; Rossi, S.H.; Welsh, S.J.; Cowburn, R.P.; Stewart, G.D. Early Detection of Kidney Cancer Using Urinary Proteins: A Truly Non-Invasive Strategy. BJU Int. 2022, 129, 290–303. [Google Scholar] [CrossRef]
- Di Meo, A.; Batruch, I.; Brown, M.D.; Yang, C.; Finelli, A.; Jewett, M.A.; Diamandis, E.P.; Yousef, G.M. Searching for Prognostic Biomarkers for Small Renal Masses in the Urinary Proteome. Int. J. Cancer 2020, 146, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Sandoval-Cooper, M.J.; Paiva, M.; Kobayashi, T.; Ploplis, V.A.; Castellino, F.J. Fibrinogen Stabilizes Placental-Maternal Attachment during Embryonic Development in the Mouse. Am. J. Pathol. 2002, 160, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.; Hamburger, J.; Batt, D.; Zahn, J.; Beilin, Y. Point-of-Care Fibrinogen Testing in Pregnancy. Anesth. Analg. 2019, 129, E86–E88. [Google Scholar] [CrossRef]
- Vasani, A.; Kumar, M.S. Advances in the Proteomics of Amniotic Fluid to Detect Biomarkers for Chromosomal Abnormalities and Fetomaternal Complications during Pregnancy. Expert Rev. Proteom. 2019, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Jirka, M.; Blanický, P.; Šrajer, J.; Zwinger, A.; Jirásek, J.E. Human Serum Zn-A2-Glycoprotein in Amniotic Fluid. Clin. Chim. Acta 1978, 85, 107–110. [Google Scholar] [CrossRef]
- Mudd, S.H.; Tangerman, A.; Stabler, S.P.; Allen, R.H.; Wagner, C.; Zeisel, S.H.; Levy, H.L. Maternal Methionine Adenosyltransferase I/III Deficiency: Reproductive Outcomes in a Woman with Four Pregnancies. J. Inherit. Metab. Dis. 2003, 26, 443–458. [Google Scholar] [CrossRef]
- Chien, Y.H.; Abdenur, J.E.; Baronio, F.; Bannick, A.A.; Corrales, F.; Couce, M.; Donner, M.G.; Ficicioglu, C.; Freehauf, C.; Frithiof, D.; et al. Mudd’s Disease (MAT I/III Deficiency): A Survey of Data for MAT1A Homozygotes and Compound Heterozygotes. Orphanet J. Rare Dis. 2015, 10, 99. [Google Scholar] [CrossRef]
- Xu, Y.; Tarquini, F.; Romero, R.; Kim, C.J.; Tarca, A.L.; Bhatti, G.; Lee, J.; Sundell, I.B.; Mittal, P.; Kusanovic, J.P.; et al. Peripheral CD300a+CD8+ T Lymphocytes with a Distinct Cytotoxic Molecular Signature Increase in Pregnant Women with Chronic Chorioamnionitis. Am. J. Reprod. Immunol. 2012, 67, 184–197. [Google Scholar] [CrossRef]
- Szereday, L.; Nagy, D.U.; Vastag, F.; Mezosi, L.; Meggyes, M. Immunological Profiling of CD8+ and CD8− NK Cell Subpopulations and Immune Checkpoint Alterations in Early-Onset Preeclampsia and Healthy Pregnancy. Int. J. Mol. Sci. 2024, 25, 8378. [Google Scholar] [CrossRef]
- Huang, J.; Qi, Y.; Zeng, X.; Huang, W.; Chen, D. Simultaneous Quantification of Plasma Immunoglobulin Subclasses for Assessment of Maternal and Fetal Immune Response during Pregnancy. J. Chromatogr. A 2022, 1673, 463096. [Google Scholar] [CrossRef]
- Dechavanne, C.; Guillonneau, F.; Chiappetta, G.; Sago, L.; Lévy, P.; Salnot, V.; Guitard, E.; Ehrenmann, F.; Broussard, C.; Chafey, P.; et al. Mass Spectrometry Detection of G3m and IGHG3 Alleles and Follow-Up of Differential Mother and Neonate IgG3. PLoS ONE 2012, 7, e46097. [Google Scholar] [CrossRef]
- Einarsdottir, H.; Ji, Y.; Visser, R.; Mo, C.; Luo, G.; Scherjon, S.; Van Der Schoot, C.E.; Vidarsson, G. H435-Containing Immunoglobulin G3 Allotypes Are Transported Efficiently across the Human Placenta: Implications for Alloantibody-Mediated Diseases of the Newborn. Transfusion 2014, 54, 665–671. [Google Scholar] [CrossRef]
- Long, Y.; Zeng, S.; Gao, F.; Liu, F.; Zhang, Y.; Zhou, C.; Zhu, C.; Zhao, X.; Han, M.; Gan, Q.; et al. SERPINA5 May Promote the Development of Preeclampsia by Disruption of the UPA/UPAR Pathway. Transl. Res. 2023, 251, 14–26. [Google Scholar] [CrossRef]
- Güralp, O.; Tüten, N.; Gök, K.; Hamzaoglu, K.; Bulut, H.; Schild-Suhren, M.; Malik, E.; Tüten, A. Serum Kallistatin Level Is Decreased in Women with Preeclampsia. J. Perinat. Med. 2021, 49, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Chao, A.S.; Chen, J.K.; Chao, A.; Chang, Y.L.; Cheng, P.J.; Chang, S.D.; Wang, H.S. Network Analyses of Differentially Expressed Proteins in Amniotic Fluid Supernatant Associated with Abnormal Human Karyotypes. Fertil. Steril. 2009, 92, 96–107. [Google Scholar] [CrossRef]
- Khedun, S.M.; Naicker, T.; Moodley, J.; Naidoo, S.; Bhoola, K.D. Changes in Urinary Tissue Kallikrein Excretion in Black African Women with Hypertensive Disorders of Pregnancy. Immunopharmacology 1997, 36, 243–247. [Google Scholar] [CrossRef]
- Kovatz, S.; Arber, I.; Korzets, Z.; Rathaus, M.; Ben Aderet, N.; Bernheim, J. Urinary Kallikrein in Normal Pregnancy, Pregnancy with Hypertension, and Toxemia. Nephron 1985, 40, 48–51. [Google Scholar] [CrossRef]
- Valdes, G.; Kaufmann, P.; Corthorn, J.; Erices, R.; Brosnihan, K.B.; Joyner-Grantham, J.N. Vasodilator Factors in the Systemic and Local Adaptations to Pregnancy. Reprod. Biol. Endocrinol. 2009, 7, 79. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Fedorov, I.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V.V.; Volochaeva, M.V.; Starodubtseva, N.L.; Frankevich, V.E.; Nikolaev, E.N.; Shmakov, R.G.; et al. Mirnas and Their Gene Targets—A Clue to Differentiate Pregnancies with Small for Gestational Age Newborns, Intrauterine Growth Restriction, and Preeclampsia. Diagnostics 2021, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Lin, L.; Xie, H.; Zhang, W.; Zhang, H.; Wang, G. Analysis of Differentially Expressed Proteome in Urine from Non-Small Cell Lung Cancer Patients. Chin. J. Lung Cancer 2015, 18, 138–145. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Zheng, Y.; Liu, X.; Luo, M.; Liu, W.; Zhao, Y.; Zou, L. EPHB4 Regulates Human Trophoblast Cell Line HTR-8/SVneo Function: Implications for the Role of EPHB4 in Preeclampsia. Biol. Reprod. 2016, 95, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Sumigama, S.; Kotani, T.; Wang, J.; Miki, R.; Moriyama, Y.; Nakano, T.; Mano, Y.; Tsuda, H.; Tamakoshi, K.; et al. Possible Association between Cathepsin v and the Development of Placenta Accreta Spectrum Disorders. Gynecol. Obs. Invest. 2019, 84, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Anderle, P.; Hostettler, L.; Baumann, M.U.; Surbek, D.V.; Ontsouka, E.C.; Albrecht, C. Identification of Placental Nutrient Transporters Associated with Intrauterine Growth Restriction and Pre-Eclampsia. BMC Genom. 2018, 19, 173. [Google Scholar] [CrossRef]
- Ajmeriya, S.; Kashyap, N.; Gul, A.; Ahirwar, A.; Singh, S.; Tripathi, S.; Dhar, R.; Nayak, N.R.; Karmakar, S. Aberrant Expression of Solute Carrier Family Transporters in Placentas Associated with Pregnancy Complications. Placenta 2025, 159, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, J.; Li, J.; Cai, S.; Liu, S.; Hong, L.; Yin, T.; Zhang, Y.; Diao, L. Metabolic Reprogramming of Immune Cells at the Maternal-Fetal Interface and the Development of Techniques for Immunometabolism. Front. Immunol. 2021, 12, 717014. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Deng, Z.M.; Dai, F.F.; Liu, H.; Cheng, Y.X. The Impact of Early Pregnancy Metabolic Disorders on Pregnancy Outcome and the Specific Mechanism. Eur. J. Med. Res. 2023, 28, 197. [Google Scholar] [CrossRef]
- Kolialexi, A.; Tounta, G.; Mavrou, A.; Tsangaris, G.T. Proteomic Analysis of Amniotic Fluid for the Diagnosis of Fetal Aneuploidies. Expert Rev. Proteom. 2011, 8, 175–185. [Google Scholar] [CrossRef]
- Sultana, Z.; Qiao, Y.; Maiti, K.; Smith, R. Involvement of Oxidative Stress in Placental Dysfunction, the Pathophysiology of Fetal Death and Pregnancy Disorders. Reproduction 2023, 166, R25–R38. [Google Scholar] [CrossRef]
- Tsangaris, G.T.; Karamessinis, P.; Kolialexi, A.; Garbis, S.D.; Antsaklis, A.; Mavrou, A.; Fountoulakis, M. Proteomic Analysis of Amniotic Fluid in Pregnancies with Down Syndrome. Proteomics 2006, 6, 4410–4419. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.J.; Yan, L.Y.; Wang, W.; Yu, S.; Wang, X.; Zhang, W.Y. Proteomic Analysis of the Alteration of Protein Expression in the Placenta of Down Syndrome. Chin. Med. J. 2011, 124, 3738–3745. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Case Group (n1 = 9) * | Control Group (n2 = 15) * | p-Value |
|---|---|---|---|
| Maternal age (years) | 38.6 (31.3–40.3) {26.4–43.8} | 29.4 (26.5–31.9) {15.8–37.6} | 0.007 |
| Gestational age (weeks) | 16.7 (16.3–17.1) {15.6–17.6} | 12.4 (12.1–12.6) {12–13.1} | <0.001 |
| Marital status Married | 5 (55.6) | 12(80.0) | 0.356 |
| Place of origin Rural | 3 (33.3) | 4 (26.7) | 1.0 |
| Educational Level Low | 7 (70) | 8 (53.3) | 0.678 |
| Smoking Status Smoker | 4 (44.4) | 1 (6.7) | 0.047 |
| Dysmenorrhea | 1 (10) | - | 0.40 |
| Threatened miscarriage | 1 (10) | 2 (13.3) | 1.0 |
| Folic acid supplementation | 8 (88.8) | 15 (100) | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurca, R.L.; Soporan, M.-A.; Pralea, I.-E.; Gheorghiu, I.; Rus, I.; Stamatian, F.; Iuga, C.-A. Exploratory Urinary Proteomic Profiling in Pregnancies with Fetal Aneuploidies: Molecular Insights into Maternal–Fetal Metabolic Communication. Curr. Issues Mol. Biol. 2025, 47, 973. https://doi.org/10.3390/cimb47120973
Jurca RL, Soporan M-A, Pralea I-E, Gheorghiu I, Rus I, Stamatian F, Iuga C-A. Exploratory Urinary Proteomic Profiling in Pregnancies with Fetal Aneuploidies: Molecular Insights into Maternal–Fetal Metabolic Communication. Current Issues in Molecular Biology. 2025; 47(12):973. https://doi.org/10.3390/cimb47120973
Chicago/Turabian StyleJurca, Răzvan Lucian, Maria-Andreea Soporan, Ioana-Ecaterina Pralea, Ioana Gheorghiu, Iulia Rus, Florin Stamatian, and Cristina-Adela Iuga. 2025. "Exploratory Urinary Proteomic Profiling in Pregnancies with Fetal Aneuploidies: Molecular Insights into Maternal–Fetal Metabolic Communication" Current Issues in Molecular Biology 47, no. 12: 973. https://doi.org/10.3390/cimb47120973
APA StyleJurca, R. L., Soporan, M.-A., Pralea, I.-E., Gheorghiu, I., Rus, I., Stamatian, F., & Iuga, C.-A. (2025). Exploratory Urinary Proteomic Profiling in Pregnancies with Fetal Aneuploidies: Molecular Insights into Maternal–Fetal Metabolic Communication. Current Issues in Molecular Biology, 47(12), 973. https://doi.org/10.3390/cimb47120973









