Mechanistic Insights into the Wound Healing Activity of Plant Species in Diabetic Ulcers
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Results of Literature Search
3.2. Origin of the Studied Plant Species
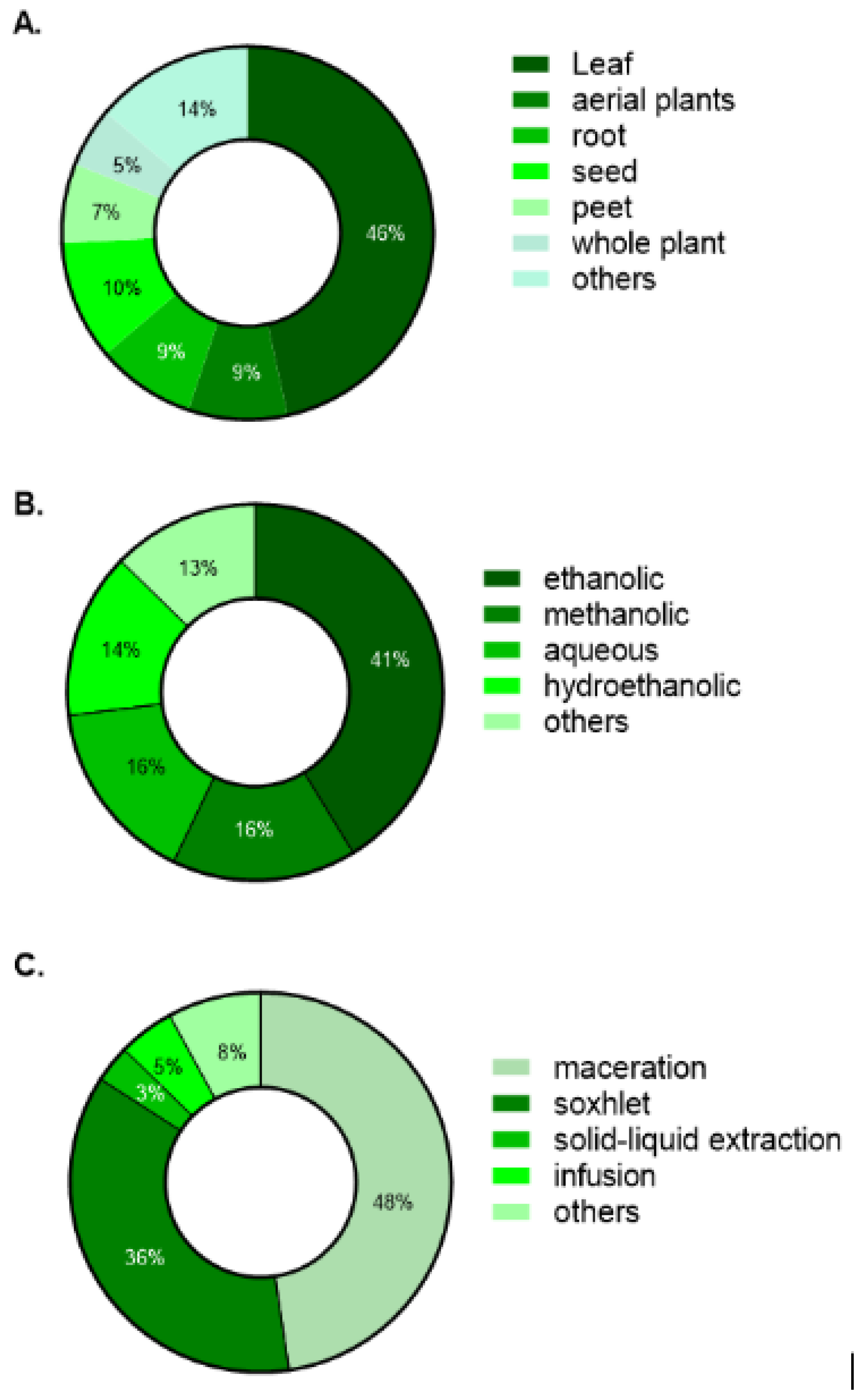
3.3. Plant Parts, Solvents, and Extraction Techniques
3.4. Experimental Model
4. Discussion
4.1. Geographic and Methodological Overview
4.2. Plant Species Modulating the Phases of Diabetic Wound Healing
4.2.1. Species Influencing the Inflammatory Phase
4.2.2. Species Influencing the Proliferative Phase
4.2.3. Species Influencing the Remodeling Phase
4.3. Recommendations for Translational Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End Products |
| bFGF | basic Fibroblast Growth Factor |
| CAPES | Coordination for the Improvement of Higher Education Personnel |
| CAT | Catalase |
| CD31 | Cluster of Differentiation 31 |
| CD34 | Cluster of Differentiation 34 |
| CD68 | Cluster of Differentiation 68 |
| CFU/g | Colony Forming Units per gram |
| CNPq | Brazilian National Council for Scientific and Technological Development |
| COX-2 | Cyclooxygenase-2 |
| DUF | Diabetic Foot Ulcers |
| DNA | Deoxyribonucleic Acid |
| DPLP | Data and Project Leadership |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| eNOS | endothelial Nitric Oxide Synthase |
| ERK1/2 | Extracellular signal-Regulated Kinase ½ |
| FAPEMA | Maranhão Research Foundation |
| FGF-2 | Fibroblast Growth Factor 2 |
| FINEP | Studies and Projects Funding |
| GLUT-1 | Glucose Transporter 1 |
| GSH | Glutathione |
| H2O2 | Hydrogen Peroxide |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| HO-1 | Heme Oxygenase-1 |
| HPX | Hydroxyproline |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IFN-γ | Interferon-gamma |
| IGF-1 | insulin-like Growth Factor 1 |
| IL-12 | Interleukin-12 |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MDA | Malondialdehyde |
| MMP | Matrix Metalloproteinases |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMP-9 | Matrix Metalloproteinase-9 |
| mRNA | messenger Ribonucleic Acid |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| n.i | not informed |
| n.r | not reported |
| NF-κB | Nuclear Factor kappa B |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PCC | Population, Concept, Context |
| PDGF | Platelet-Derived Growth Factor |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B |
| PPGCS | Graduate Program in Health Sciences |
| PPGST | Graduate Program in Health and Technology |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QCRI | Qatar Computing Research Institute |
| RAGE | Receptor for Advanced Glycation End Products |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| STZ | Streptozotocin |
| TAC | Total Antioxidant Capacity |
| T-AOC | Total Antioxidant Capacity |
| TGF-β | Transforming Growth Factor-beta |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TIMP-2 | Tissue Inhibitor of Metalloproteinases-2 |
| TNF-α | Tumor Necrosis Factor-alpha |
| tTG | tissue Transglutaminase |
| VEGF | Vascular Endothelial Growth Factor |
| WBC | White Blood Cells |
References
- Yang, Y.; Zhong, S.; Meng, F.; Cui, X. Multi-Functional hydrogels to promote diabetic wound Healing: A review. Chem. Eng. J. 2024, 497, 154855. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.-M. Intelligent biobased hydrogels for diabetic wound healing: A review. Chem. Eng. J. 2024, 484, 149493. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Elafros, M.A.; Viswanathan, V.; Jensen, T.S.; Bennett, D.L.; Feldman, E.L. The global and regional burden of diabetic peripheral neuropathy. Nat. Rev. Neurol. 2025, 21, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Perveen, W.; Ahsan, H.; Shahzad, R.; Fayyaz, S.; Zaif, A.; Paracha, M.A.; Nuhmani, S.; Khan, M.; Alghadir, A.H. Prevalence of peripheral neuropathy, amputation, and quality of life in patients with diabetes mellitus. Sci. Rep. 2024, 14, 14430. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kamolz, L.P.; Kotzbeck, P. Modeling Wound Chronicity In Vivo: The Translational Challenge to Capture the Complexity of Chronic Wounds. J. Investig. Dermatol. 2024, 144, 1454–1470. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhang, D.; Deng, J.; Liu, Y.; Li, W.; Nie, X. Preparation and Safety Evaluation of Centella asiatica Total Glycosides Nitric Oxide Gel and Its Therapeutic Effect on Diabetic Cutaneous Ulcers. Evid. Based Complement. Altern. Med. 2022, 25, 1419146. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Choi, Y.; Lim, K.M.; Jeong, Y.; Dayem, A.A.; Lee, Y.; An, J.; Song, K.; Jang, S.B.; et al. Improved Wound Healing and Skin Regeneration Ability of 3,2′-Dihydroxyflavone-Treated Mesenchymal Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 6964. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A Comprehensive Review on Biology, Genetic Improvement, Agro and Process Technology of German Chamomile (Matricaria chamomilla L.). Plants 2021, 11, 29. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rangra, N.K.; Samanta, S.; Pradhan, K.K. Evaluation of Acacia auriculiformis benth. Leaves for wound healing activity in type 2 diabetic rats. Pharmacogn. Mag. 2021, 17, 129–142. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M.; Vargas, S.R. Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia 2006, 77, 286–289. [Google Scholar] [CrossRef]
- Patel, N.S.; Viren, N.N.; Hirapara, H.N.; Hemangini, R.A.; Seema, N.B.; Chandrab-hanu, T. Effect of alcoholic extract of Adhatoda vasica L. leaves and allium cepa L. buld in diabetic wound healing in wistar albino rats. Pol. Pharm. Soc. 2018, 75, 89–95. [Google Scholar]
- Gharaboghaz, M.N.Z.; Farahpour, M.R.; Saghaie, S. Topical co-administration of Teucrium polium hydroethanolic extract and Aloe vera gel triggered wound healing by accelerating cell proliferation in diabetic mouse model. Biomed. Pharmacother. 2020, 127, 110189. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 25, 555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Ma, Z.J.; Wang, Y.; Sun, B.; Guo, X.; Pan, C.Q.; Chen, L.M. Angelica Dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS ONE 2017, 12, e0177862. [Google Scholar] [CrossRef]
- Ponrasu, T.; Suguna, L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int. Wound J. 2012, 9, 613–623. [Google Scholar] [CrossRef]
- Alanazi, A.Z.; Al-Rejaie, S.S.; Ahmed, M.M.; Alhazzani, K.; Alhosaini, K.; As Sobeai, H.M.; Alsanea, S.; Alam, P.; Almarfadi, O.M.; Alqahtani, A.S.; et al. Papel protetor do extrato de Dodonaea viscosa contra hepatotoxicidade e nefrotoxicidade induzidas por estreptozotocina em ratos. Saudi. Pharm. J. 2023, 31, 101669. [Google Scholar] [CrossRef]
- Hyun, S.W.; Kim, J.; Jo, K.; Kim, J.S.; Kim, C.S. Aster koraiensis extract improves impaired skin wound healing during hyperglycemia. Integr. Med. Res. 2018, 7, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ayubi-Rad, M.; Yosefi, S.; Hajizadeh, M.; Jafari-Naveh, H.; Hassanipour, M.; Alamchi, F.; Fadai, A.; Khorasani, F.; Hosseini, F.; Bemani, M.; et al. Effects of Astragalus fasciculifolius gum on wound healing in streptozotocin-induced diabetic rats. J. Herbmed. Pharmacol. 2020, 9, 328–332. [Google Scholar] [CrossRef]
- Zlabiene, U.; Baranauskaite, J.; Kopustinskiene, D.M.; Bernatoniene, J. In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L. Pharmaceutics 2021, 13, 732. [Google Scholar] [CrossRef]
- Getahun, A.; Kifle, Z.D.; Ambikar, D.; Atnafie, S.A. In vivo evaluation of 80% methanolic leaves crude extract and solvent fractions of buddleja polystachya fresen (buddlejaceae) for wound healing activity in normal and diabetic mice. Metabol. Open 2021, 11, 100110. [Google Scholar] [CrossRef]
- Jagadeep Chandra, S.; Mahadevan, S.; Ramesh, R. Evaluation of the wound healing activity of Caesalpinia bonducella and Cyclea peltata extracts in experimentally induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2017, 9, 211–217. [Google Scholar] [CrossRef]
- Machado de Freitas Coelho, N.P.; Nogueira, V.C.; Cardoso, M.A.C.; Lopes, L.S.; Nascimento, P.P.; Rocha, E.S.; da Silva, C.L.P.; Arisawa, E.A.L. Cenostigma macrophyllum Tul. on the healing of skin wounds in rats with diabetes mellitus. Acta Cir. Bras. 2013, 28, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.S.; Pemmineti, S. Evaluation of Centella asiatica leaf extract for wound healing in streptozotocin-induced diabetic rats. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1082. [Google Scholar]
- Maurya, H.; Semwal, M.; Dubey, S.K. Pharmacological Evaluation of Chrozophora tinctoria as Wound Healing Potential in Diabetic Rat’s Model. Biomed. Res. Int. 2016, 2016, 7475124. [Google Scholar] [CrossRef]
- Aksoy, H.; Sen, A.; Sancar, M.; Sekerler, T.; Akakin, D.; Bitis, L.; Uras, F.; Kultur, S.; Izzettin, F.V. Ethanol extract of Cotinus coggygria leaves accelerates wound healing process in diabetic rats. Pharm. Biol. 2016, 54, 2732–2736. [Google Scholar] [CrossRef]
- Zadeh, M.A.A.; Ebrahimi, M.; Salarian, A.A.; Abtahi, S.R.; Jahandideh, A. Evaluation of Beneficial Influence of Local Application of Crocus pallasii subsp. Haussknechtii Boiss. Extract on Healing of Full Thickness Excisional Infected Wounds in Diabetic Rats. Bull. Emerg. Trauma 2020, 8, 169–178. [Google Scholar]
- Shaikh, S.S.; Murthy, K.; Shete, R.V.; Solunke, R.S. Comparative evaluation of wound healing activity of tilvadi ghrita and durva ghrita on diabetic wound model in rats. Int. J. Res. Pharm. Sci. 2019, 10, 3376–3384. [Google Scholar] [CrossRef]
- Pawar, S.S.; Mahajan, H.B. Evaluation of wound healing activity of leaf extract of Dodonaea viscosa Jacq in alloxan-induced diabetic Wistar albino rats. Int. J. Pharm. Sci. Res. 2013, 4, 2359–2363. [Google Scholar] [CrossRef]
- Sunarjo, L.; Oedijani; Suharti.; Susanto, H.S.; Fatmasari, D. Impact of mangosteen rind on TNF-α level of diabetic wound healing. J. Int. Dent. Med. Res. 2021, 14, 591–594. [Google Scholar] [CrossRef]
- Bardaa, S.; Makni, K.; Boudaouara, O.; Bardaa, T.; Ktari, N.; Hachicha, S.; Ben Salah, R.; Kallel, R.; Sahnoun, Z.; Boufi, S. Development and Evaluation of the Wound Healing Effect of a Novel Topical Cream Formula Based on Ginkgo biloba Extract on Wounds in Diabetic Rats. Biomed. Res. Int. 2021, 2021, 6474706. [Google Scholar] [CrossRef]
- Lee, G.S.; Choi, J.Y.; Choi, Y.J.; Yim, D.S.; Kang, T.J.; Cheong, J.H. The Wound Healing Effect of Hydnocarpi Semen Extract on Ulcer in Diabetic Mice. Biomol. Ther. 2010, 18, 329–335. [Google Scholar] [CrossRef]
- Hirapara, H.; Ghori, V.; Anovadiya, A.; Baxi, S.; Tripathi, C. Effects of ethanolic extract of Jasminum grandiflorum Linn. flowers on wound healing in diabetic Wistar albino rats. Avicenna J. Phytomed. 2017, 7, 401–408. [Google Scholar] [PubMed]
- Mekala, S.; Kumar Naresh, M.; Das, L.; Shetty, N.; Amuthan, A.; Vulli, V.; Bhogireddy, N. Evaluation of wound healing activity of ethanolic extract of Lantana camara in streptozotocin induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 631–633. [Google Scholar]
- Bramara, B.V.B.; Vasavi, H.S.; Sudeep, H.V.; Prasad, K.S. Hydroalcoholic extract from Lepidium meyenii (black maca) root exerts wound healing activity in streptozotocin-induced diabetic rats. Wound Med. 2017, 19, 75–81. [Google Scholar] [CrossRef]
- Al-Ahmad, B.E.M.; Kashmoola, M.A.; Jabbar, O.A.; Mokhtar, K.I.; Mohamad, N.; Rahim, R.A.; Shaban, M.N. Histopathological Changes of the Flaxseed Extract on Skin Wound Healing in Diabetic Rabbits. J. Med. Sci. 2020, 8, 881–892. [Google Scholar] [CrossRef]
- Naji, S.; Zarei, L.; Pourjabali, M.; Mohammadi, R. The Extract of Lycium depressum Stocks Enhances Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. J. Low Extrem. Wounds 2017, 16, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Marchianti, A.C.N.; Ulfa, E.U.; Sakinah, E.N. The dose dependence analysis of the water fraction of Merremia mammosa (Lour.) extract on diabetic wound healing enhancement. Hiroshima J. Med. Sci. 2018, 67, 29–34. [Google Scholar]
- Sakinah, E.N.; Ulfa, E.U.; Marchianti, A.C.N. The effectiveness of Merremia mammosa (Lour.) extract fractions as diabetic wound healers on diabetic rat model. Hiroshima J. Med. Sci. 2018, 67, 70–77. [Google Scholar]
- Sumantri, I.B.; Ismayadi; Mustanti, L.F. The Potency of Wound Healing of Nanogel-containing Mikania micrantha Leaves Extract in Hyperglycemic Rats. Pharm. Nanotechnol. 2021, 9, 339–346. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; Mehta, P.; Vyas, S.P.; Shukla, S.; Bajpai, V.K. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem. Toxicol. 2010, 48, 61–64. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Arulselvan, P.; Cheah, P.S.; Abas, F.; Fakurazi, S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Devel. Ther. 2016, 10, 1715–3170. [Google Scholar] [CrossRef]
- Nourbar, E.; Mirazi, N.; Yari, S.; Rafieian-Kopaei, M.; Nasri, H. Effect of Hydroethanolic Extract of Nigella sativa L. on Skin Wound Healing Process in Diabetic Male Rats. Int. J. Prev. Med. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.S. Effect of Ocimum sanctum Linn. Leaf Extract On Wound Healing in Streptozotocin Induced Diabetic Rats. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 845. [Google Scholar]
- Al-basher, G.; Al-otibi, F. Biological Activity of Olive Leaf Extract and Regulation of Tissue Transglutaminase Expression in Diabetic Wound Healing. Int. J. Pharmacol. 2018, 14, 963–972. [Google Scholar] [CrossRef]
- Mohammadi, N.; Bahrami, G.; Ghiasvand, N.; Miraghaei, S.; Madani, S.H.; Karimi, I.; Shokoohinia, Y. The wound healing effect of various extracts from Onosma microcarpum root in a diabetic animal model. J. Rep. Pharm. Sci. 2017, 6, 59–67. [Google Scholar] [CrossRef]
- Naga, K.; Saradhi, P.; Rao, N.; Mohan, R.; Balaji, M.; La, S.; Raman, V. Evaluation of wound healing activity of Phragmites vallatoria leaf ethanol extract in STZ-induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2017, 4, 393–395. [Google Scholar]
- Liao, T.T.; Sukpat, S.; Chansriniyom, C.; Patumraj, S. Topical combined Phyllanthus emblica Linn. and simvastatin improves wound healing in diabetic mice by enhancing angiogenesis and reducing neutrophil infiltration. Biomed. Rep. 2023, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Pan-Yue, Q.; Ya-Jing, X.; Xiang-Duo, Z.; Jun-Hua, D.; Bin, Q.; Xue-Fang, L.; Jing-Ping, L.; Jie, Y. Effect and mechanisms of Polygonatum kingianum (polygonati rhizome) on wound healing in diabetic rats. J. Ethnopharmacol. 2022, 298, 115612. [Google Scholar] [CrossRef] [PubMed]
- Tayade, P.M.; Borde, S.N.; Chandrasekar, N.; Jagtap, S.A.; Joshi, A.S. Evaluation of wound healing properties of Psoralea corolifolia Linn in diabetic rats. Pharmacol. Online 2011, 1, 282–288. [Google Scholar]
- Karim, S.; Alkreathy, H.; Ahmad, A.; Khan, M. Effects of methanolic extract based-gel from Saudi pomegranate peels with enhanced healing potential on excision wounds in diabetic rats. Front. Pharmacol. 2021, 12, 704503. [Google Scholar] [CrossRef] [PubMed]
- Dardmah, F.; Farahpour, M.R. Quercus infectoria gall extract aids wound healing in a streptozocin-induced diabetic mouse model. J. Wound Care 2021, 30, 618–625. [Google Scholar] [CrossRef]
- Lau, T.W.; Lam, F.F.; Lau, K.M.; Chan, Y.W.; Lee, K.M.; Sahota, D.S.; Ho, Y.Y.; Fung, K.P.; Leung, P.C.; Lau, C.B. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J. Ethnopharmacol. 2009, 123, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Abu-Al-Basal, M.A. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J. Ethnopharmacol. 2010, 131, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.S.; Kumar, S.; Topo, F.A.; Lakshmi, P.K.; Suryavanshi, P. Sida cordifolia Linn. accelerates wound healing process in type 2 diabetic rats. J. Acute Med. 2016, 9, 32–38. [Google Scholar] [CrossRef]
- Pandian, C.; Srinivasan, A.; Pelapolu, I.C. Evaluation of wound healing activity of hydroalcoholic extract of leaves of Stachytarpheta jamaicensis in streptozotocin induced diabetic rats. Der Pharm. Lett. 2013, 5, 193–200. [Google Scholar]
- de Aguiar, D.S.; Correa, A.; Antunes, F.; Ferrez, A.F.; Vencato, S.; Amado, G.; Wiiland, E.; Correa, D.; Grivicich, I.; Souza, A. Benefits of Stryphnodendron adstringens when associated with hydrogel on wound healing in diabetic rats. Clin. Phytoscience 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Pinto, S.C.; Bueno, F.G.; Panizzon, G.P.; Morais, G.; Dos Santos, P.V.; Baesso, M.L.; Leite-Mello, E.V.; de Mello, J.C. Stryphnodendron adstringens: Clarifying wound healing in streptozotocin-induced diabetic rats. Planta Med. 2015, 81, 934–941. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H.; Parimelazhagan, T.; Duraib, G. Syzygium mundagam bark methanol extract restores skin to normal in diabetic wounded rats. Biomed. Pharmacother. 2017, 94, 781–786. [Google Scholar] [CrossRef]
- Shrivastava, A.; Mishra, A.K.; Abida, M.; Ahmad, A.; Fabuzinadah, M.; Khan, N.A. Extracts of Tridax procumbens Linn. leaves causes wound healing in diabetic and non-diabetic laboratory animals. Wound Med. 2015, 29, 100185. [Google Scholar] [CrossRef]
- Roy, S.K.; Mishra, P.K.; Nandy, S.; Datta, R.; Chakraborty, B. Potential wound healing activity of the different extract of Typhonium trilobatum in albino rats. Asian Pac. J. Trop. Biomed. 2012, 2, 1477–1486. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, A. An ethno-pharmacological study of wound healing medicinal plants used by traditional healers in Dhamtari, Chhattisgarh, India. Int. J. Exp. Res. Rev. 2024, 38, 194–207. [Google Scholar] [CrossRef]
- Gao, R.R.; Hu, Y.T.; Dan, Y.; Hao, L.J.; Liu, X.; Song, J.Y. Chinese herbal medicine resources: Where we stand. Chin. Herb. Med. 2019, 12, 3–13. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharmaceut. Sci. 2011, 1, 98–106. [Google Scholar]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015, 4, 196. [Google Scholar] [CrossRef]
- Heinrich, M.; Edwards, S.; Moerman, D.E.; Leonti, M. Ethnopharmacological field studies: A critical assessment of their conceptual basis and methods. J. Ethnopharmacol. 2009, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91 (Suppl. S3), e20190105. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, M.; Fan, Y. Estresse oxidativo e morte celular programada em feridas diabéticas: Uma revisão abrangente. Sci. Prog. 2025, 108. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Lan, C.C.; Wu, C.S.; Huang, S.M.; Wu, I.H.; Chen, G.S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Node, K.; Inoue, T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc. Diabetol. 2009, 29, 8–23. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, G.; Cao, X.; Dong, J.; Song, F.; Niu, Y. Blocking AGE-RAGE Signaling Improved Functional Disorders of Macrophages in Diabetic Wound. J. Diabetes Res. 2017, 2017, 1428537. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Y.; Wu, J.; Zhang, J.; Xiong, X.; Mao, J.; Wei, Y.; Miao, C.; Zhang, H. Dysregulation of neutrophil in sepsis: Recent insights and advances. Cell Commun. Signal. 2025, 23, 87. [Google Scholar] [CrossRef]
- Krajnc, M.; Pečovnik Balon, B.; Krajnc, I. Non-traditional risk factors for coronary calcification and its progression in patients with type 2 diabetes: The impact of postprandial glycemia and fetuin-A. J. Int. Med. Res. 2019, 47, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Yadav, P.S.; Singh, M.; Vinayagam, R.; Shukla, P. Therapies and delivery systems for diabetic wound care: Current insights and future directions. Front. Pharmacol. 2025, 16, 1628252. [Google Scholar] [CrossRef]
- Barakat, M.; DiPietro, L.A.; Chen, L. Limited Treatment Options for Diabetic Wounds: Barriers to Clinical Translation Despite Therapeutic Success in Murine Models. Adv. Wound Care 2021, 10, 436–460. [Google Scholar] [CrossRef] [PubMed]
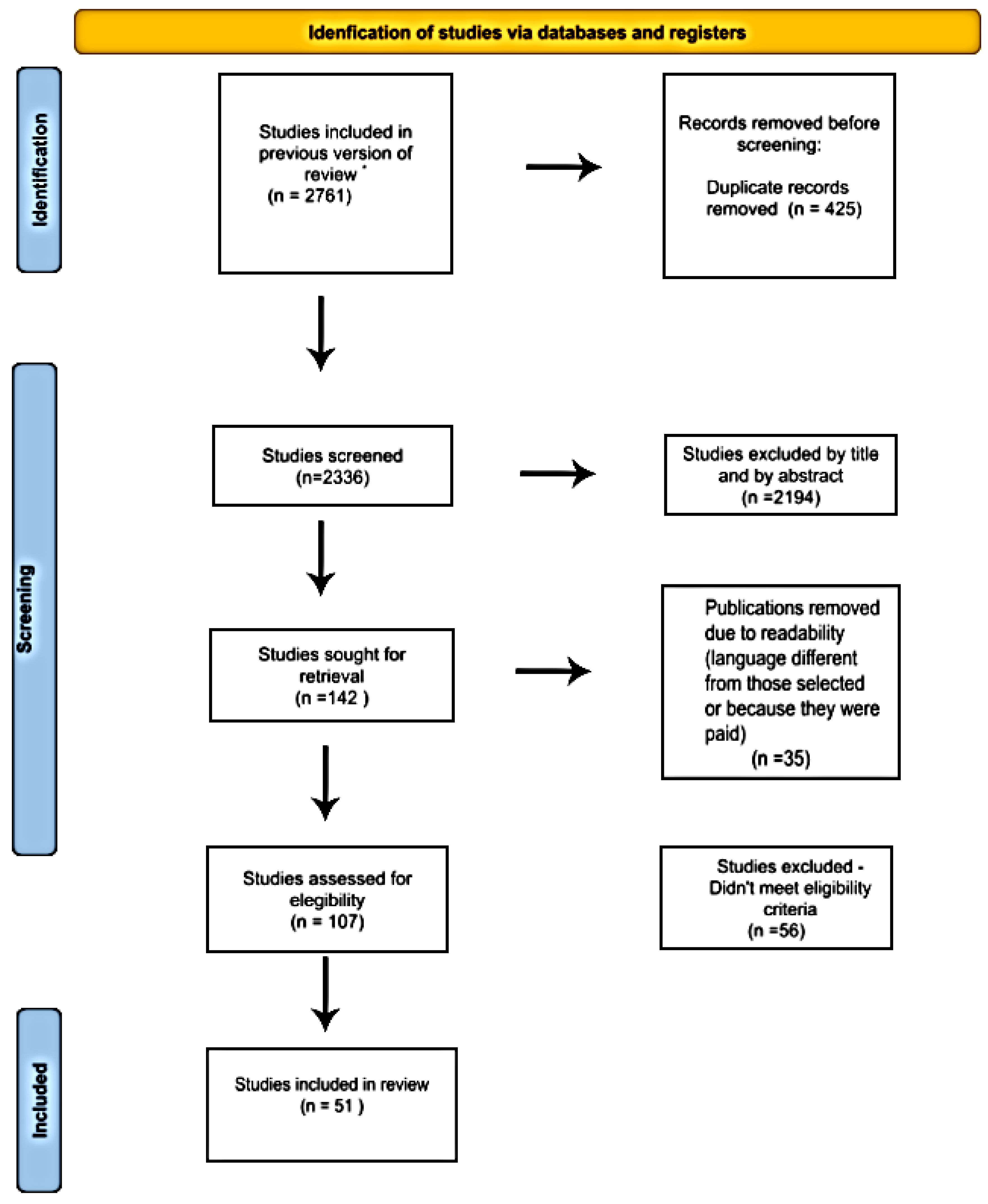



| Database | Search Strategies |
|---|---|
| PUBMED | ((“chronic wound” [MeSH Terms] OR (“chronic wound healing” OR ((“diabetic” [MeSH Terms] OR (“diabetic wound healing”) ((“extract plant” [MeSH Terms] OR (“natural product OR (“plant extract”) OR (“human” [mesh terms] AND (“in vivo” OR in vitro model)) |
| EMBASE | (chronic wound OR “chronic wound healing” OR diabetic wound healing) AND (“extract plant” OR natural product of plant extract) |
| SCOPUS | (chronic wound) AND (chronic wound healing AND “diabetic wound healing” OR (extract plant OR “natural product” OR plant extract) |
| Plant Species | Part | Country-Origin | Extract Type | Extraction Method | Formulation/Concentration | Route of Administration | Treatment/Time (Days) | Finds | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Acacia auriculiformis A. Cunn. ex Benth. | Leaf | India | Methanolic | Maceration | Hydrogel (10%) | Topical | 1x/day (15) | ↓ activity of α- glucosidase and α-amylase ↑ rate of lesion contraction—dose-dependent ↓ time of epithelialization ↓ levels of hydroxyproline | [12] |
| Acalypha langinia Müll.Arg. | Leaf | México | Aqueous | Soxhlet | Solution (0.05–0.5%) | Oral | 2x/day (7) | ↓ congestion and edema ↓ wound area—dose-dependent ↑ protein and DNA content in granulation tissue ↑ tensile strength of incision wounds | [13] |
| Adhatoda nees | Leaf | India | Alcoholic | n.i. * | Solution (400 mg/kg) | Oral | 1x/day (11) | ↑ wound closure ↑ re-epithelialization ↑ tensile strength ↑ granulation tissue weight ↑ hydroxyproline | [14] |
| Allium cepa (L.) | Bulb | India | Alcoholic | n.i. * | Solution (300 mg/kg) | Oral | 1x/day (11) | ↑ wound closure (day 11) ↑ re-epithelialization ↑ tensile strength ↑ granulation tissue weight ↑ hydroxyproline | |
| Teucrium (L.) | Aerial parts | Iran | Hydro- ethanolic | Soxhlet | Ointment (5%–10%) | Topical | 1x/day (14) | ↓ IL-1β, TNF-α ↓ MDA ↑ fibroblasts/collagen ↑ VEGF, IGF-1, FGF-2, GLUT-1 ↑ tensile strength | [15] |
| Aloe vera (L.) Burm.f. | Leaf | Iran | Hydro- ethanolic | Soxhlet | Ointment (5%–10%) | Topical | 1x/day (14) | ↓ IL-1β, TNF-α ↑ VEGF, IGF-1, GLUT-1 ↑ fibroblasts/collagen ↑ wound closure ↑ tensile strength | |
| Aloe vera (L.) Burm.f. | Leaf | Spain | Hydro- ethanolic | Maceration | Nanofiber (5–10%) | Topical | 2x/day (8) | ↑ Fibroblast proliferation ↑ Reepithelisation ↑ Wound closure ↑ Resolution of chronic inflammation Mild ↑ collagen deposition | [16] |
| Angelicae dahuricae | Root | China | Ethanolic | Percolation | Solution (20%) | Oral | 1x/day (14) | ↓healing time ↓ CD68+ macrophages; ↓ IL-1β/TNF-α ↑ Granulation and re-epithelialization ↑ Angiogenesis (↑ CD31 vessels; ↑ pericyte recruitment) ↑ HUVEC proliferation/migration/tube formation (ex vivo/in vitro) ↑ Collagen I deposition | [17] |
| Annona squamosa (L.) | Seed | India | Ethanolic | Maceration | Solution (n.i. *) | Oral | 1x/day (14) | ↑ DNA, protein, collagen, hexosamine, uronic acid ↑ Fibroblasts, macrophages, angiogenesis ↑ Wound contraction and epithelialisation ↑ Tensile strength and collagen organization ↓ Lipid peroxidation | [18] |
| Anthocephalus cadamba | Leaf | India | Aqueous | Infusion | Solution (500 mg/kg) | Topical | 1x/day (28) | ↓area of injury ↑ contraction rate ↑ regeneration, neovascularization, collagen deposition and fibroblast proliferation | [19] |
| Aster koraiensis | Aerial parts | Republic of Korea | Ethanolic | Maceration | Solution (100 mg/kg) | Oral | 1x/day (18) | ↑ Wound closure (day 14) ↑ Keratinocyte migration ↑ Skin thickness/organization ↓ MMP-2/9 expression and activity ↑ Re-epithelialization | [20] |
| Astragalus fasciculifolius | Gum | Iran | Aqueous | Infusion | Cream (5–10%) | Topical | 1x/day (20) | ↑ Wound healing ratio (day 14–20) ↑ Granulation tissue formation ↑ Epithelialization ↑ Collagen deposition/organization ↑ Tissue restoration | [21] |
| Avena sativa (L.) | Seed | India | Ethanolic | Maceration | Hydrogel (200 mg/kg) | Topical | 2x/day (14) | ↓ Inflammatory infiltration and oxidative stress ↑ Fibroblast adhesion and proliferation ↑ Wound contraction ↑ Angiogenesis and epithelialization ↑ Collagen deposition and fiber organization | [22] |
| Buddleja polystachya (F.) | Leaf | Ethiopia | Methanolic | Maceration | Ointment (5–10%) | Topical | 1x/day (18) | ↓ Inflammatory exudate and scab duration ↑ Wound contraction ↑ Fibroblast activity and angiogenesis ↑ Epithelialization rate ↑ Tensile strength and collagen organization | [23] |
| Caesalpinia bonducella (L.) Fleming | Root | India | Methanolic | Soxhlet | Solution (50–100 mg/kg) | Topical | 1x/day (15) | ↓ blood glucose ↑ contraction of the injury ↑ healing | [24] |
| Bark | India | Ethylacetate | Soxhlet | Solution (51–100 mg/kg) | Topical | 1x/day (15) | ↓ blood glucose ↑ contraction of the injury | ||
| Leaf | India | Ethylacetate | Maceration | Hydrogel (52–100 mg/kg) | Topical | 1x/day (15) | ↓ blood glucose ↑ contraction of the injury | ||
| Cyclea peltata (L.) | Leaf | India | Methanolic | Soxhlet | Solution (50 mg/kg) | Topical | 1x/day (15) | ↓ inflammatory period ↑ wound contraction ↑ granulation tissue formation ↑ epithelial closure | |
| Cenostigma macrophyllum Tul. | Leaf | Brazil | Hexanic | Solid–liquid extraction | Emulsion (0.5%) | Topical | 1x/day (28) | ↓ inflammatory cells/resolution of infiltrate ↑ nitric oxide ↑ fibroblasts and granulation tissue ↑ angiogenesis and onset of re-epithelializatio ↑ wound size reduction | [25] |
| Centella asiatica (L.) Urb. | Leaf | India | Ethanolic | Soxhlet | Solution (200 mg/kg) | Oral | 2x/day (14) | ↓ fasting blood glucose ↑ wound contraction; ↓ epithelialization time ↑ granulation tissue (wet/dry weight) ↑ breaking strength (granulation) ↑ hydroxyproline and collagen organization | [26] |
| Chrozophora tinctoria (L.) | Leaf | India | Hydro- methanolic | Soxhlet | Solution (5%) | Oral | 1x/day (21) | ↓ inflammatory infiltration; ↓ inflammatory period ↑ wound contraction; ↓ time to epithelialization ↑ granulation tissue (wet/dry weight) and total protein ↑ collagen (hydroxyproline) ↑ tensile strength | [27] |
| Cotinuos coggygria (S.) | Leaf | Turkey | Ethanolic | Soxhlet | Solution (200 mg/kg) | Oral | 2x/day (14) | ↓ inflammatory infiltrate/edema ↑ GSH ↓ MDA ↑ re-epithelialization and angiogenesis ↑ hydroxyproline | [28] |
| Crocus pallasii (S.) | Leaf | Iran | Methanolic | Maceration | Ointment (2%) | Topical | 2x/day (14) | ↓ MRSA CFU/g ↑ Wound contraction ↓ wound area ↑ Fibroblasts and neovascularization ↑ Hydroxyproline ↑ Biomechanics | [29] |
| Cynodon dactylon (L.) Pers. | Whole plant | India | n.i. * | Maceration | Ghrita (40%) | Topical | 1x/day (21) | ↑ contraction of the injury ↓ epithelialization time | [30] |
| Dodoneae viscosa (J.) | Leaf | India | Ethanolic | Maceration | Ointment (10%) | Topical | 2x/day (16) | ↑ rate of contraction of the injury ↑ collagen content ↑ anti-inflammatory activity | [31] |
| Garcinia mangostana (L.) | Peel | Indonesia | Ethanolic | Maceration | Solution (25%) | Oral | 1x/day (14) | ↓ TNF-α; controlled inflammatory rise ↓ fasting blood glucose ↑ wound closure (≈99% at day 14 vs. ≈64% control) ↑ re-epithelialization speed (inferred from coverage) → remodeling onset earlier; no tensile/collagen data | [32] |
| Ginkgo biloba (L.) | Leaf | China | Aqueous | Soxhlet | Cream (1–5%) | Topical | 1x/day (13) | ↓ inflammatory cells/scab duration ↑ wound contraction (100% by day 13) ↑ re-epithelialization (higher histology score) ↑ collagen alignment/organization ↑ overall healing rate vs. control | [33] |
| Hydnocarpus wightiana Blume | Seed | South Korea | Hydro- ethanolic | Soxhlet | Solution (50 mg/kg) | Topical | 1x/day (14) | ↓ WBC/neutrophils (day 14) ↑ macrophage IL-12/TNF-α (in vitro) ↓ wound area score; dose–response → benefit independent of glycemia → earlier remodeling (closure ≤ 2 weeks) | [34] |
| Jasminum grandiforum (L.) | Flower | India | Ethanolic | Maceration | Solution (250 mg/kg) | Topical | 1x/day (11) | ↑ wound contraction (days 7–14) ↑ granulation dry weight ↑ hydroxyproline (collagen) ↑ incision breaking strength ↑ neo-angiogenesis (histology) | [35] |
| Lantana camara (L.) | Leaf | India | Ethanolic | Soxhlet | Ointment (10%, 15% and 20%) | Topical | 1x/day (23) | → early contraction lag (week 1) ↑ wound contraction after day 7 (dose-dependent) ↓ epithelialization time ↑ complete closure by day 15–17 (higher doses) → remodeling inference; no tensile/collagen data | [36] |
| Lepidium (L.) | Root | India | Hydro- ethanolic | Maceration | Solution (200 mg/kg) | Oral | 1x/day (30) | ↓ bacterial load (days 7 & 14) ↓ inflammatory cell infiltration ↑ wound contraction and wound index ↑ granulation tissue (wet/dry weights) ↑ hydroxyproline & hexosamine (collagen/ECM) | [37] |
| Root | Hydro- ethanolic | Soxhlet | Ointment (5–10%) | Topical | 2x/day (30) | ↓ bacterial load (days 7 & 14) ↓ inflammatory cell infiltration ↑ wound contraction (from day 10; dose-dependent) ↑ granulation tissue (wet/dry weights) ↑ hydroxyproline & hexosamine (collagen/ECM) | |||
| Linum usitatissimum (L.) | Seed | Malasya | Aqueous | Infusion | Oil (200 mg/kg) | Topical | 2x/day (14) | ↓ inflammatory cell infiltration (day 14) ↑ re-epithelialization (early; day 4 diabetic) ↑ surface closure rate ↑ neovascularization (day 14) ↑ collagen organization (histology) | [38] |
| Lycium (L.) | Leaf | Iran | Methanolic | Maceration | Ointment (500 mg/kg) | Topical | 2x/day (14) | ↑ antioxidant activity ↓ area of injury ↑ collagen deposition ↑ epithelialization and vascularization ↑ cell proliferation ↑ acute hemorrhage and edema scores | [39] |
| Merremia macrocarpa (L.) Roberty. | Tuber | Indonesia | Aqueous | Maceration | Solution (0,05%) | Topical | 1x/day (21) | ↑ wound healing % (day 10; 50–100 mg ≈ positive control) ↑ angiogenesis ↑ fibroblast density ↑ collagen fiber density (ECM) ↓ inflammatory delay (dose-responsive) | [40] |
| Merremia macrocalyx (Ruiz & Pav.) O’Donell. | Leaf | Indonesia | Ethanolic | Ultrasonic-assisted | Solution (10%) | Topical | 1x/day (5) | ↑ wound healing % (water fraction 93.4% at day 11) ↓ wound size (days 7–11 vs. control) ↑ contraction/epithelialization speed (inferred) ↑ water fraction > n-hexane > ethyl acetate (day 11) ↑ performance ≈ gentamicin at day 11 | [41] |
| Mikania micrantha Kunth. | Leaf | Indonesia | Ethanolic | Maceration | Nanogel (2%) | Topical | 1x/day (1) | ↑ healing rate | [42] |
| Mimosa pudica (L.) | Leaf | India | Ethanolic | Maceration | Solution (200 mg/kg) | Oral | 2x/day (7) | ↓ inflammatory mediators ↑ antibacterial activity ↓ wound area ↓ time to epithelialization ↑ VEGF | [43] |
| Moringa oleifera Lam. | Leaf | Malasya | Ethanolic | Maceration | Ointment (0.5%, 1% and 2%) | Topical | 1x/day (21) | ↑ antibacterial activity ↓ area of injury dose-dependent ↓ epithelization time ↓ levels of inflammatory mediators ↑ VEGF expression | [44] |
| Nigella (L.) | Seed | Iran | Ethanolic | Maceration | Ointment (20–40%) | Topical | 1x/day (14) | ↓ wound area ↓ healing time ↑ epidermal thickness | [45] |
| Ocimum (L.) | Leaf | India | Ethanolic | Soxhlet | Solution (800 mg/kg) | Oral | 1x/day (7) | ↓ wound area ↓ time to epithelialization ↑ granulation tissue weight ↑ hydroxyproline ↑ tensile strength | [46] |
| Olea europaea (L.) | Leaf | Saudi Arabia | Ethanolic | Maceration | Ointment (2–5%) | Topical | 2x/day (21) | ↓ epithelialization time ↑ wound contraction ↑ granulation tissue (dry weight, protein) ↑ hydroxyproline and tTG (collagen deposition/cross-linking) ↑ TAC; closure/scar positively correlated with HPX/tTG/TAC ↑antioxidant Capacity | [47] |
| Onosma microcarpum (D.) | Root | Iran | Hexanic | Soxhlet | Ointment (20%, 30%, 40% and 60%) | Topical | 1x/day (20) | ↑ wound closure (day 20) ↑ fibroblasts (up to ~1500/mm2) ↑ angiogenesis (up to ~200 vessels/mm2) ↓ residual wound area vs. base → remodeling improvement inferred (no tensile/HPX) | [48] |
| Root | Acetone | Soxhlet | Ointment (30%) | Topical | 1x/day (20) | ↓ area of injury ↓ time to epithelialization ↑ protein content (granulation) ↑ hydroxyproline (collagen) ↑ fabric strength | |||
| Root | Ethanolic | Maceration | Ointment (30%) | Topical | 1x/day (20) | ↑ healing rate ↓ time to epithelialization ↑ collagen stability ↑ antioxidant capacity → remodeling quality improved (surrogate) | |||
| Root | Hydro- ethanolic | Maceration | Ointment (30%) | Topical | 1x/day (20) | ↑ antibacterial activity ↓ area of injury (dose-dependent) ↓ time to epithelialization ↓ inflammatory mediators ↑ VEGF expression | |||
| Phragmites Adans. | Leaf | India | Ethanolic | Soxhlet | Solution (400 mg/kg) | Oral | 2x/day (11) | ↑ granulation tissue (wet/dry) ↓ time to epithelialization ↑ wound contraction ↓ wound area | [49] |
| Phyllanthus (L.) | Fruit | Thailand | Ethanolic | Maceration | Cream (10%) | Topical | 1x/day (30) | ↓ MDA ↓ neutrophils ↑ VEGF ↑ capillary vascularity ↑ wound closure/re-epithelialization | [50] |
| Polygonatum kingianum | Rhizo me | China | Aqueous | Soxhlet | Gel (2–8 g/kg) | Topical | 1x/day (28) | ↓ AGEs/RAGE; ↓ TNF-α, IL-6, IL-2, IFN-γ ↑ Nrf2/HO-1, SOD, GSH, T-AOC; ↓ MDA ↑ wound closure (days 3, 7, 14) ↑ CD34/VEGF/bFGF, angiogenesis; ↑ epidermis/dermis thickness ↓ MMP-2/9; ↑ TIMP-2; ↑ collagen density | [51] |
| Rhizo me | Ethanolic | Soxhlet | Gel (2–8 g/kg) | Topical | 1x/day (28) | ↓ AGEs/RAGE; ↓ TNF-α, IL-6, IL-2, IFN-γ ↑ antioxidant status (Nrf2/HO-1; SOD/GSH/T-AOC); ↓ MDA ↑ wound closure (days 3, 7, 14) ↑ CD34/VEGF/bFGF; ↑ epidermis/dermis thickness ↓ MMP-2/9; ↑ TIMP-2; ↑ collagen density | |||
| Psoralea (L.) | Whole plant | India | Ethanolic | Maceration | Ointment (1%) | Topical | 1x/day (9) | ↑ wound contraction ↑ granulation and epithelial regrowth ↑ tensile strength ↑ collagen organization ↓ overall healing time vs. control | [52] |
| Punica granatum (L.) | Peel | Saudi Arabia | Methanolic | Maceration | Gel (5%) | Topical | 2x/day (21) | ↓ NO/NOS; ↑ antioxidant status ↑ VEGF/EGF (protein & mRNA) ↑ hydroxyproline (early) ↑ wound contraction/closure (>90% by day 21) ↑ re-epithelialization and vascular maturation | [53] |
| Quercus (L.) | Galls | Iran | Hydro- ethanolic | Maceration | Ointment (5–10%) | Topical | 1x/day (14) | ↓ IL-6/TNF-α; ↓ MDA; ↑ TAC ↑ VEGF; ↑ fibroblasts; ↑ angiogenesis ↑ collagen deposition ↑ re-epithelialization → improved early matrix organization | [54] |
| Rehmannia glutinosa (L.) | Root | China | Aqueous | Decoction | Solution (n.i. *) | Oral | 2x/day (30) | ↓ inflammation (carrageenan model) ↓ ulcer area (day 8) ↑ VEGF and apillaries ↑ epithelialization/scar quality ↑ dermal organization (early remodeling) | [55] |
| Rosmarinus officinalis (L.) | Aerial parts | Jordan | Aqueous | Hydrodistillation | Essential oil (5–10%) | Topical | 2x/day (3) | ↓ inflammation/faster re-epithelialization ↑ granulation tissue ↑ angiogenesis ↑ wound contraction ↑ collagen organization | [56] |
| Aerial parts | Aqueous | Solid–liquid extraction | Solution (10%) | Topical | 1x/day (3) | ↓ inflammation/faster re-epithelialization ↑ granulation tissue ↑ wound contraction ↑ collagen deposition ↓ blood glucose (systemic) | |||
| Sida cordifolia (L.) | Aerial parts | India | Methanolic | Maceration | Hydrogel (10%) | Topical | 1x/day (20) | ↓ time to epithelialization ↑ wound contraction ↑ hydroxyproline (collagen) ↑ tensile strength ↑ epithelial/collagen histology | [57] |
| Stachytarpheta jamaicensis (L.) Vahl | Leaf | India | Hydro-ethanolic | Soxhlet | Solution (2–5%) | Topical | 2x/day (20) | ↓ time to epithelialization ↑ wound contraction ↑ granulation tissue mass ↑ collagen/hexosamine/protein/DNA ↑ tensile strength | [58] |
| Stryphnodendron adstringens (Mart.) Coville | Peel | Brazil | Hydro- ethanolic | Maceration | Gel (5%) | Topical | 2x/day (16) | ↑ angiogenesis ↑ re-epithelialization ↑ fibroblast proliferation ↑ overall healing progression | [59] |
| Stryphnodendron adstringens (Mart.) Coville | Leaf | Brazil | Ethanolic | Maceration | Solution (1%) | Oral | 2x/day (14) | ↑ COX-2 (d4–10) and VEGF (d7) ↑ keratinocyte migration/proliferation; complete re-epithelialization by d10–14 ↑ type I collagen and fiber organization dermal permeation confirmed (topical gel) overall earlier inflammatory–proliferative transition | [60] |
| Syzygium aqueum (Burm.f.) Alston | Peel | India | Methanolic | Soxhlet | Ointment (1–2%) | Topical | 1x/day (21) | ↓ inflammatory persistence; ↓ epithelialization time (~15.5 d at 2%) ↑ wound contraction (from d10) ↑ fibroblasts and neovascularization (histology) ↑ complete closure by d21 ↑ collagen bundle organization | [61] |
| Tridax procumbens (L.) | Leaf | India | Ethanolic | Soxhlet | Solution (2.5–5%) | Topical | 2x/day (14) | ↓ wound index; ↓ epithelialization time ↑ wound contraction ↑ hydroxyproline/protein/DNA (granulation) ↑ tensile strength (incision model) ↑ overall healing rate (diabetic) | [62] |
| Typhonium trilobatum (L.) | Whole plant | India | Methanolic | Soxhlet | Solution (100 mg/kg) | Topical | 1x/day (9) | ↓ epithelialization time (diabetic; MeOH/EtOAc > CHCl3) ↑ wound contraction (MeOH/EtOAc) ↑ granulation/epithelial coverage (histology) ↑ tensile strength (incision; MeOH/EtOAc > CHCl3) effective under infected diabetic wounds (excision) | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, R.G.R.; Costa, F.F.; Fuchs, L.A.; Rodrigues, L.S.; Moraes, R.A.N.; Junior, P.S.d.S.A.; Maciel, M.C.G.; Amaral, F.M.M.; Coutinho, D.F.; Reis, A.S. Mechanistic Insights into the Wound Healing Activity of Plant Species in Diabetic Ulcers. Curr. Issues Mol. Biol. 2025, 47, 972. https://doi.org/10.3390/cimb47120972
Chaves RGR, Costa FF, Fuchs LA, Rodrigues LS, Moraes RAN, Junior PSdSA, Maciel MCG, Amaral FMM, Coutinho DF, Reis AS. Mechanistic Insights into the Wound Healing Activity of Plant Species in Diabetic Ulcers. Current Issues in Molecular Biology. 2025; 47(12):972. https://doi.org/10.3390/cimb47120972
Chicago/Turabian StyleChaves, Rodson Glauber Ribeiro, Fernanda Farias Costa, Letícia Andrade Fuchs, Lays Scherrer Rodrigues, Rhuan Antonio Nogueira Moraes, Paulo Sila da Silva Alves Junior, Márcia Cristina Goncalves Maciel, Flavia Maria Mendonça Amaral, Denise Fernandes Coutinho, and Aramys Silva Reis. 2025. "Mechanistic Insights into the Wound Healing Activity of Plant Species in Diabetic Ulcers" Current Issues in Molecular Biology 47, no. 12: 972. https://doi.org/10.3390/cimb47120972
APA StyleChaves, R. G. R., Costa, F. F., Fuchs, L. A., Rodrigues, L. S., Moraes, R. A. N., Junior, P. S. d. S. A., Maciel, M. C. G., Amaral, F. M. M., Coutinho, D. F., & Reis, A. S. (2025). Mechanistic Insights into the Wound Healing Activity of Plant Species in Diabetic Ulcers. Current Issues in Molecular Biology, 47(12), 972. https://doi.org/10.3390/cimb47120972






