Mechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress in the Pathogenesis and Complications of Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Pathophysiology of Obesity-Induced IR and T2DM
2.1. Insulin Signaling Pathways, IR, and the Pathogenesis of T2DM
2.2. Obesity, Chronic Low-Grade Inflammation, OS and the Pathogenesis of T2DM
2.3. The NF-κB Signaling Pathway in the Pathogenesis of T2DM and Its Complications
2.4. The JAK/STAT Signaling Pathway in the Pathogenesis of T2DM and Its Complications
3. Pathophysiology of T2DM Complications
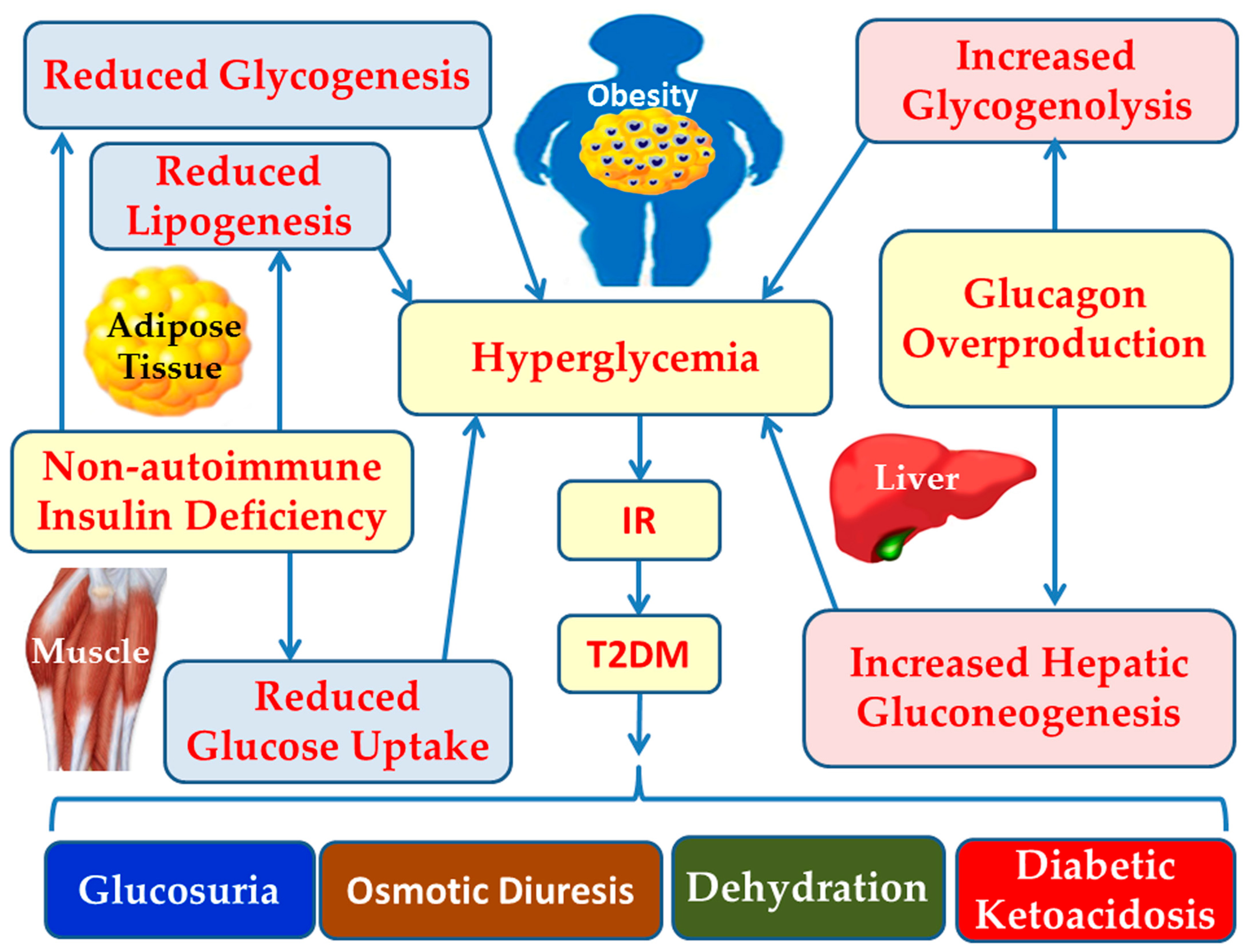
3.1. Pathophysiological Mechanisms Underlying Diabetic Ketoacidosis
3.2. Pathophysiological Mechanisms of Osmotic Diuresis and Muscle Mass Reduction in T2DM
4. Antioxidants in T2DM and the Amelioration of Obesity-Associated T2DM
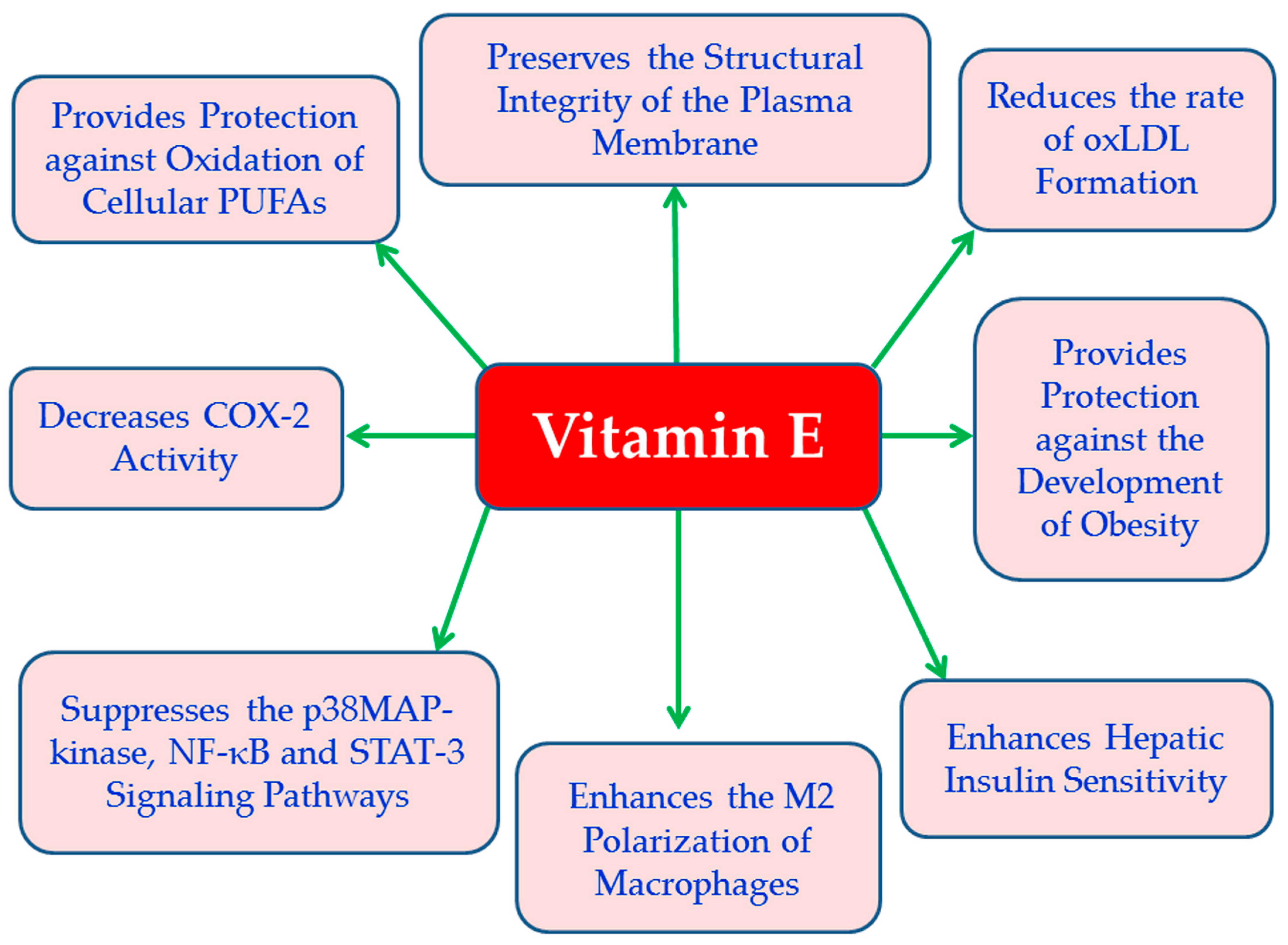
4.1. Vitamin E
4.2. Vitamin C
4.3. N-Acetylcysteine (NAC)
4.4. Zinc
4.5. Alpha-Lipoic Acid
4.6. L-Carnitine
4.7. Coenzyme Q10 (CoQ10)
4.8. Superoxide Dismutases (SODs)
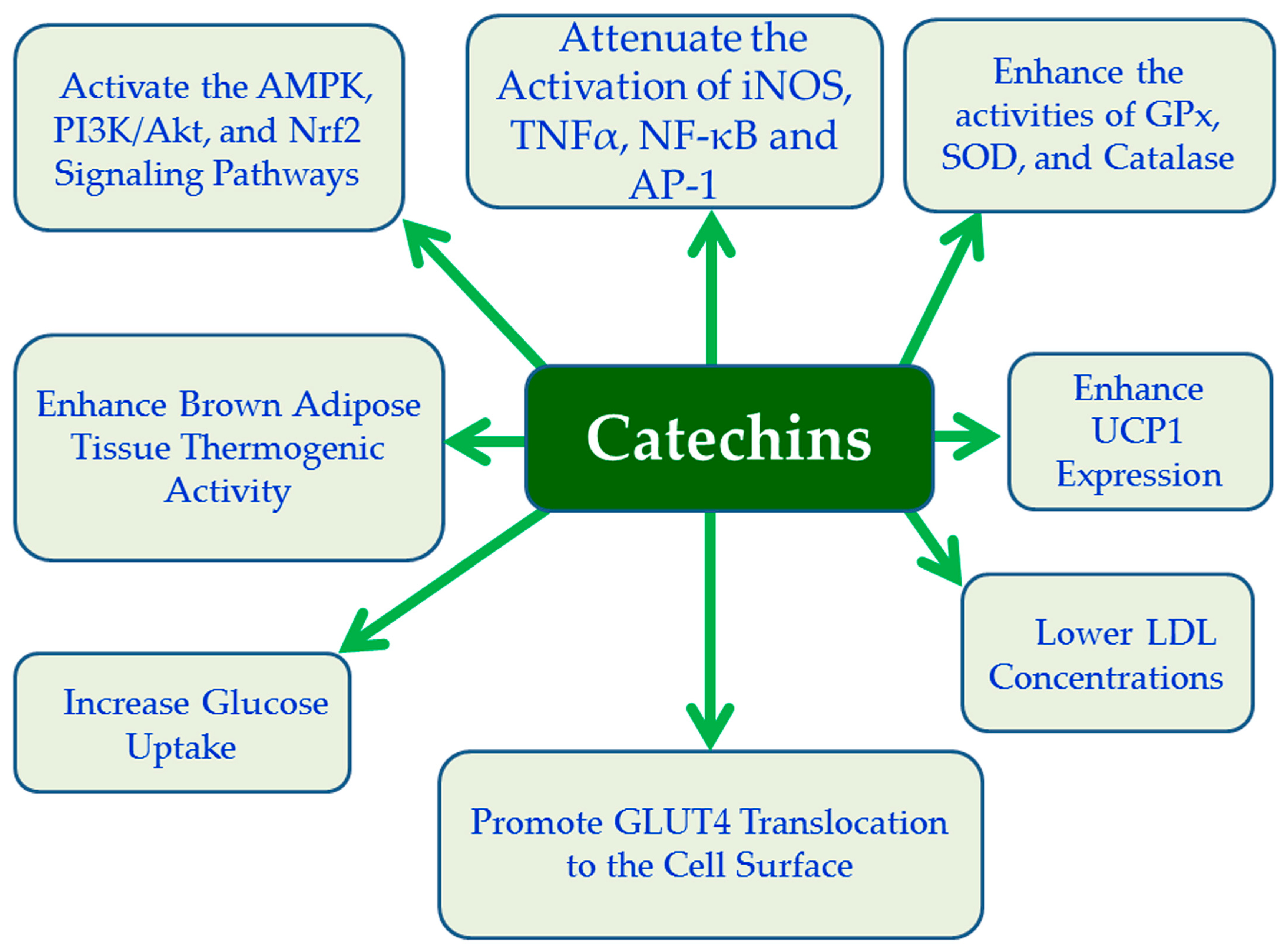
4.9. Catechins
4.10. Quercetin
4.11. Curcumin
4.12. Resveratrol (RSV)
4.13. Lycopene
5. Conclusions, Potential Applications and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Karamanou, M.; Protogerou, A.; Tsoukalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Priya, G.; Kalra, S.; Dasgupta, A.; Grewal, E. Diabetes Insipidus: A Pragmatic Approach to Management. Cureus 2021, 13, e12498. [Google Scholar] [CrossRef]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. Available online: https://www.diabetes.ca/health-care-providers/clinical-practice-guidelines/chapter-3#panel-tab_FullText (accessed on 10 September 2025). [CrossRef]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of Diabetes Mellitus. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279119/ (accessed on 15 September 2025).
- Yudkin, J.S. “Prediabetes”: Are There Problems with This Label? Yes, the Label Creates Further Problems! Diabetes Care 2016, 39, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Targeted. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Arroyave, F.; Montaño, D.; Lizcano, F. Diabetes Mellitus Is a Chronic Disease that Can Benefit from Therapy with Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 8685. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Rekka, E.A. The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients 2024, 16, 2740. [Google Scholar] [CrossRef]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Hamilton, A.; Ramracheya, R.; Tarasov, A.I.; Brereton, M.; Haythorne, E.; Chibalina, M.V.; Spégel, P.; Mulder, H.; Zhang, Q.; et al. Dysregulation of Glucagon Secretion by Hyperglycemia-Induced Sodium-Dependent Reduction of ATP Production. Cell Metab. 2019, 29, 430–442.e4. [Google Scholar] [CrossRef]
- Hædersdal, S.; Lund, A.; Knop, F.K.; Vilsbøll, T. The Role of Glucagon in the Pathophysiology and Treatment of Type 2 Diabetes. Mayo Clin. Proc. 2018, 93, 217–239. [Google Scholar] [CrossRef]
- Mouri, M.; Badireddy, M. Hyperglycemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430900/ (accessed on 5 September 2025).
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279052/ (accessed on 20 September 2025).
- Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (Review). Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef]
- He, L. Alterations of gut microbiota by overnutrition impact gluconeogenic gene expression and insulin signaling. Int. J. Mol. Sci. 2021, 22, 2121. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel excess and β-cell dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53, S16–S21. [Google Scholar] [CrossRef]
- Liu, X.; Gong, M.; Wu, N. Research progress on the relationship between free fatty acid profile and type 2 diabetes complicated by coronary heart disease. Front. Endocrinol. 2024, 15, 1503704. [Google Scholar] [CrossRef]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 3170. [Google Scholar] [CrossRef] [PubMed]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose tissue macrophages and their role in obesity-associated insulin resistance: An overview of the complex dynamics at play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef]
- Gupta, D.; Kono, T.; Evans-Molina, C. The role of peroxisome proliferatoractivated receptor γ in pancreatic β cell function and survival: Therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes. Metab. 2010, 12, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Aziz, S.G.-G.; Nade, R. The effect of tropisetron on oxidative stress, SIRT1, FOXO3a, and claudin-1 in the renal tissue of STZ-induced diabetic rats. Cell Stress Chaperones 2021, 26, 217–227. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Fu, Y.; Xie, L.; Kong, Y.; Yang, X. Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications. Curr. Issues Mol. Biol. 2025, 47, 885. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, M.S.; Carvalho, M.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCε in rat dorsal root ganglion neurons. J. Neuroinflammation 2021, 18, 106. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Y.-H.; Wang, C.-Y.; Yu, Y.; Li, Y.-Z.; Cui, W.; Li, Q.; Yu, Y.H. MicroRNA-7a-5p ameliorates diabetic peripheral neuropathy by regulating VDAC1/JNK/c-JUN pathway. Diabet. Med. 2023, 40, e14890. [Google Scholar] [CrossRef]
- Eid, S.A.; Massry, M.E.I.; Hichor, M.; Haddad, M.; Grenier, J.; Dia, B.; Barakat, R.; Boutary, S.; Chanal, J.; Aractingi, S.; et al. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves Schwann cell integrity in diabetic mice. Diabetes 2020, 69, 448–464. [Google Scholar] [CrossRef]
- Villalobos-Labra, R.; Subiabre, M.; Toledo, F.; Pardo, F.; Sobrevia, L. Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity. Mol. Asp. Med. 2019, 66, 49–61. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; Han, G.-H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, N.; Zhao, D. Function of NLRP3 in the Pathogenesis and Development of Diabetic Nephropathy. Med. Sci. Monit. 2017, 23, 3878–3884. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Sheu, M.L.; Ho, F.M.; Yang, R.S.; Chao, K.F.; Lin, W.W.; Lin-Shiau, S.Y.; Liu, S.-H. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 539–545. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Lai, T.-T.; Li, C.-J. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Lin, R.R.; Parikh, B.H.; Wey, Y.S.; Tun, B.B.; Wong, T.Y.; Luu, C.D.; Agrawal, R.; Ghosh, A.; Mortellaro, A.; et al. The NLRP3 inflammasome may contribute to pathologic neovascularization in the advanced stages of diabetic retinopathy. Sci. Rep. 2018, 8, 2847. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhao, H.; Song, H. Shared signaling pathways and targeted therapy by natural bioactive compounds for obesity and type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2022, 64, 5039–5056. [Google Scholar] [CrossRef] [PubMed]
- Bako, H.Y.; Ibrahim, M.A.; Isah, M.S.; Ibrahim, S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019, 239, 117045. [Google Scholar] [CrossRef]
- Zhang, H.; Nair, V.; Saha, J.; Atkins, K.B.; Hodgin, J.B.; Saunders, T.L.; Myers, M.G., Jr.; Werner, T.; Kretzler, M.; Brosius, F.C. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 2017, 92, 909–921. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Zhao, F.; Zhao, Z.; Tian, J.; Jia, K. Nobiletin suppresses high-glucose-induced inflammation and ECM accumulation in human mesangial cells through STAT3/NF-κB pathway. J. Cell Biochem. 2019, 120, 3467–3473. [Google Scholar] [CrossRef]
- Lee, E.G.; Luckett-Chastain, L.R.; Calhoun, K.N.; Frempah, B.; Bastian, A.; Gallucci, R.M. Interleukin 6 function in the skin and isolated keratinocytes is modulated by hyperglycemia. J. Immunol. Res. 2019, 2019, 5087847. [Google Scholar] [CrossRef]
- Sawaya, A.P.; Stone, R.C.; Brooks, S.R.; Pastar, I.; Jozic, I.; Hasneen, K.; O’Neill, K.; Mehdizadeh, S.; Head, C.R.; Strbo, N.; et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat. Commun. 2020, 11, 4678. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 10 September 2025).
- Liakopoulos, V.; Franzén, S.; Svensson, A.M.; Miftaraj, M.; Ottosson, J.; Näslund, I.; Gudbjörnsdottir, S.; Eliasson, B. Pros and cons of gastric bypass surgery in individuals with obesity and type 2 diabetes: Nationwide, matched, observational cohort study. BMJ Open 2019, 9, e023882. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Stavropoulos, K.; Zografou, I.; Doumas, M.; Karagiannis, A. Prognostic value of arterial stiffness measurements in cardiovascular disease, diabetes, and its complications: The potential role of sodium-glucose co-transporter-2 inhibitors. J. Clin. Hypertens. 2020, 22, 562–571. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Kushner, A.; West, W.P.; Suheb, M.Z.K.; Pillarisetty, L.S. Virchow Triad. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539697/ (accessed on 10 September 2025).
- Chiu, J.-J.; Chien, S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Yachmaneni, A., Jr.; Jajoo, S.; Mahakalkar, C.; Kshirsagar, S.; Dhole, S. A Comprehensive Review of the Vascular Consequences of Diabetes in the Lower Extremities: Current Approaches to Management and Evaluation of Clinical Outcomes. Cureus 2023, 15, e47525. [Google Scholar] [CrossRef]
- Bodman, M.A.; Dreyer, M.A.; Varacallo, M.A. Diabetic Peripheral Neuropathy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442009/ (accessed on 10 September 2025).
- Akkus, G.; Sert, M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J. Diabetes 2022, 13, 1106–1121. [Google Scholar] [CrossRef]
- Koyama, K.; Anno, T.; Kimura, Y.; Kawasaki, F.; Kaku, K.; Tomoda, K.; Kaneto, H. Pathology of Ketoacidosis in Emergency of Diabetic Ketoacidosis and Alcoholic Ketoacidosis: A Retrospective Study. J. Diabetes Res. 2024, 2024, 8889415. [Google Scholar] [CrossRef] [PubMed]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556002/ (accessed on 10 September 2025).
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Lizzo, J.M.; Goyal, A.; Gupta, V. Adult Diabetic Ketoacidosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560723/ (accessed on 12 September 2025).
- Ghimire, P.; Dhamoon, A.S. Ketoacidosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534848/ (accessed on 13 September 2025).
- Self, W.H.; Evans, C.S.; Jenkins, C.A.; Brown, R.M.; Casey, J.D.; Collins, S.P. Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials. JAMA Netw. Open 2020, 3, e2024596. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Electrolyte and Acid–Base Disturbances in Patients with Diabetes Mellitus. N. Engl. J. Med. 2015, 373, 548–559, Erratum in N. Engl. J. Med. 2019, 381, 1598. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.H.I.; Mohebbi, N. Kidney metabolism and acid–base control: Back to the basics. Pflug. Arch. 2022, 474, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hashmi, M.F.; Aggarwal, S. Hyperchloremic Acidosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482340/ (accessed on 10 September 2025).
- Elendu, C.; David, J.A.; Udoyen, A.-O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.; Ibhiedu, J.O.; Ibhiedu, A.O.; Nwosu, P.U.; et al. Comprehensive review of diabetic ketoacidosis: An update. Ann. Med. Surg. 2023, 85, 2802–2807. [Google Scholar] [CrossRef]
- Liman, M.N.P.; Jialal, I. Physiology, Glycosuria. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557441/ (accessed on 15 September 2025).
- Hieshima, K.; Sugiyama, S.; Yoshida, A.; Kurinami, N.; Suzuki, T.; Ijima, H.; Miyamoto, F.; Kajiwara, K.; Jinnouchi, K.; Jinnouchi, T.; et al. Elevation of the renal threshold for glucose is associated with insulin resistance and higher glycated hemoglobin levels. J. Diabetes Investig. 2020, 11, 617–625. [Google Scholar] [CrossRef]
- Hans, F. Polyuria and Diabetes: Understanding the Connection and Implications. Afr. J. Diabetes Med. 2024, 32, 4. [Google Scholar]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. 2020, 472, 1345–1370. [Google Scholar] [CrossRef]
- Gubbi, S.; Hannah-Shmouni, F.; Koch, C.A.; Joseph, G.; Verbalis, J.G. Diagnostic Testing for Diabetes Insipidus. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537591/ (accessed on 15 September 2025).
- Wendt, A.; Eliasson, L. Pancreatic alpha cells and glucagon secretion: Novel functions and targets in glucose homeostasis. Curr. Opin. Pharmacol. 2022, 63, 102199. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef] [PubMed]
- Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and pharmacokinetics of vitamin E: Nanoformulations to enhance bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols–bioactive dietary compounds. What is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and metabolic health: Relevance of interactions with other micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Bucciarelli, T.; Mancini, B.; Corradi, F.; Di Ilio, C.; Mattei, P.A.; D’Orazio, N. Antioxidant vitamin suppementation in cardiovascular diseases. Ann. Clin. Lab. Sci. 2007, 37, 89–95. [Google Scholar]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. A Review on Vitamin E Natural Analogues and on the Design of Synthetic Vitamin E Derivatives as Cytoprotective Agents. Mini Rev. Med. Chem. 2021, 21, 10–22. [Google Scholar] [CrossRef]
- Galmes, S.; Serra, F.; Paou, A. Vitamin E metabolic effects and genetic variants: A challenge for precision nutrition in obesity and associated disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant and anti-inflammatory activities and the role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Kanchi, M.M.; Shanmugam, M.K.; Rane, G.; Sethi, G.; Kumar, A.P. Tocotrienols: The unsaturated sidekick shifting new paradigms in vitamin E therapeutics. Drug Discov. Today 2017, 22, 1765–1781, Erratum in Drug Discov. Today 2019, 24, 1421. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef]
- Alcalá, M.; Sanchez-Vera, I.; Sevillano, J.; Herrero, L.; Serra, D.; Ramos, M.P.; Viana, M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity 2015, 23, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Budin, S.B.; Othman, F.; Louis, S.R.; Bakar, M.A.; Das, S.; Mohamed, J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics 2009, 64, 235–244. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. 2015, 39, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic Efficacy of Antioxidants in Ameliorating Obesity Phenotype and Associated Comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Abdullah, M.; Jamil, R.J.; Attia, J.F. Vitamin C (ascorbic acid). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499877/ (accessed on 15 September 2025).
- Rafighi, Z.; Shiva, A.; Arab, S.; Mohd, Y.R. Association of dietary vitamin C and E intake and antioxidant enzymes in type 2 diabetes mellitus patients. Glob. J. Health Sci. 2013, 5, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Naziroğlu, M.; Simşek, M.; Simşek, H.; Aydilek, N.; Ozcan, Z.; Atilgan, R. The effects of hormone replace therapy combined with vitamins C and E on antioxidants levels and lipid profiles in postmenopausal women with type 2 diabetes. Clin. Chim. Acta 2004, 344, 63–71. [Google Scholar] [CrossRef]
- Harding, A.H.; Wareham, N.J.; Binham, S.A.; Khaw, K.T.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetabel consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer–Norfolk prospective study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef]
- Guerini, M.; Gondrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcystein (NAC) and its role in clinical practice management of cystic fibrosis (CF): A review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef]
- Zhang, Q.; Ju, Y.; Ma, Y.; Wang, T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicina 2018, 97, e13087. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R. Biological activities and potential oral applications of N-acetylcystein: Progress and prospects. Oxid. Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Mondal, S.; Hazra, A.; Paul, P.; Saha, B.; Roy, S.; Bhowmick, P.; Bhowmick, M. Formulation and evaluation of n-acetyl cysteine loaded bi-polymeric physically crosslinked hydrogel with antibacterial and antioxidant activity for diabetic wound dressing. Int. J. Biol. Macromol. 2024, 279, 135418. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The beneficial effects of N-acetyl cystein (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef]
- Ismael, M.A.; Talbot, S.; Carbonneau, C.L.; Beauséjour, C.M.; Couture, R. Blockade of sensory abnormalities and kinin B1 receptor expression by N-Acetyl-L-Cystein and ramipril in a rat model of insulin resistance. Eur. J. Pharmacol. 2008, 589, 66–72. [Google Scholar] [CrossRef]
- Wang, T.; Mao, X.; Li, H.; Qiao, S.; Xu, A.; Wang, J.; Lei, S.; Liu, Z.; Ng, K.F.; Wong, G.T.; et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic. Biol. Med. 2013, 63, 291–303. [Google Scholar] [CrossRef]
- Kadota, Y.; Toriuchi, Y.; Aki, Y.; Mizuno, Y.; Kawakami, T.; Nakaya, T.; Sato, M.; Suzuki, S. Metallothioneins regulate the adipoogenic defferentation of 3T3-L1 cells via the insulin signaling pathway. PLoS ONE 2017, 12, e0176079. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, P.; Wang, Y.; Chen, Y. Clinical efficacy of acetylcysteine combined with tetrandrine tablets in the treatment of silicosis and the effect on serum IL-6 and TNF-alpha. Exp. Ther. Med. 2019, 18, 3383–3388. [Google Scholar] [CrossRef]
- Hang, W.; Shu, H.; Wen, Z.; Liu, J.; Jin, Z.; Shi, Z.; Chen, C.; Wang, D.W. N-acetyl cysteine ameliorates high-fat diet-induced nonalcoholic fatty liver disease and intracellular triglyceride accumulation by preserving mitochondral function. Front. Pharmacol. 2021, 12, 636204. [Google Scholar] [CrossRef]
- Korou, L.M.; Agrogiannis, G.; Koros, C.; Kitraki, E.; Vlachos, I.S.; Tzanetakou, I.; Karatzas, T.; Pergialiotis, V.; Dimitroulis, D.; Perrea, D.N. Impact of N-acetylcysteine and sesame oil on lipid metabolism and hypothalamic-pituitary-adrenal axis homeostasis in middle-aged hypercholesterolemic mice. Sci. Rep. 2014, 4, 6806. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in platelet biology: Functional aspects and methodological insights. Int. J. Mol. Sci. 2020, 2, 4866. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.J.; Sontakke, T.; Biradar, A.; Nalage, D. Zinc: An essential trace element for human health and beyond. Food Health 2023, 5, 13. [Google Scholar] [CrossRef]
- Fung, E.B.; Gildergorin, G. Zinc status affects glucose homeostasis and insulin secretion in patients with thalassemia. Nutrients 2015, 7, 4296–4307. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Muñoz, I.G.; Moran, J.F.; Becana, M.; Montoya, G. The crystal structure of an eukaryotic iron superoxide dismutase suggests intersubunit cooperation during catalysis. Protein Sci. 2005, 14, 387–394. [Google Scholar] [CrossRef]
- do Nascimento Marreiro, D.; Clímaco Cruz, K.J.; Silva Morais, J.B.; Batista Beserra, J.; Soares Severo, J.; de Oliveira, A.R.S. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in cellular regulation: The nature and significance of “zinc signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Anton, I.C.; Mititelu-Tartau, L.; Popa, E.G.; Poroch, M.; Poroch, V.; Pelin, A.M.; Pavel, L.L.; Drochioi, I.C.; Botnariu, G.E. Zinc chloride endances the antioxidant status, improving the functional and structural organic disturbances in streptozotocin-induced diabetes in rats. Medicina 2022, 58, 1620. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systemic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Dajnowicz-Brzezik, P.; Żebrowska, E.; Maciejczyk, M.; Zalewska, A.; Chabowski, A. α-lipoic acid supplementation reduces oxidative stress and inflammation in red skeletal muscle of insulin-resistant rats. Biochem. Biophys. Res. Commun. 2025, 742, 151107. [Google Scholar] [CrossRef] [PubMed]
- Abdali, D.; Samson, S.; Grover, A.K. How effective are antioxidant supplements in obesity and diabetes? Med. Princ. Pract. 2015, 24, 201–215. [Google Scholar] [CrossRef]
- Salehi, B.; Yilmaz, Y.B.; Antika, G.; Tumer, T.B.; Mahomoodally, M.F.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Superti, F.; Russo, R. Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits. Antioxidants 2024, 13, 1228. [Google Scholar] [CrossRef]
- Shahid, A.; Nasir, K.; Bhatia, M. Therapeutic Potential of Alpha-Lipoic Acid: Unraveling Its Role in Oxidative Stress and Inflammatory Conditions. Curr. Issues Mol. Biol. 2025, 47, 322. [Google Scholar] [CrossRef]
- Amrousy, D.E.; El-Afify, D. Effects of alpha lipoic acid as a supplement in obese children and adolescents. Cytokine 2020, 130, 155084. [Google Scholar] [CrossRef] [PubMed]
- Ansar, H.; Mazloom, Z.; Kazemi, F.; Hejazi, N. Effect of α-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011, 32, 584–588. [Google Scholar]
- Zhang, Y.; Han, P.; Wu, N.; He, B.; Lu, Y.; Li, S.; Liu, Y.; Zhao, S.; Liu, L.; Li, Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity 2011, 19, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.J.; Lee, S.A.; Kim, E.H.; Cho, E.H.; Jeong, E.J.; Kim, D.W.; Kim, M.S.; Park, J.Y.; Park, K.G.; et al. Effects of α-lipoic acid on body weight in obese subjects. Am. J. Med. 2011, 124, 85–88. [Google Scholar] [CrossRef]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-lipoic acid and glucose metabolism: A comprehensive update on biochemical and therapeutic features. Nutrients 2023, 15, 18. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Gholami, A.; Hariri, M. Alpha-lipoic acid effect on leptin and adiponectin concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2020, 76, 649–657. [Google Scholar] [CrossRef]
- Pershadsingh, H.A. Alpha-lipoic acid: Physiologic mechanisms and indications for the treatment of metabolic syndrome. Expert. Opin. Investig. Drugs 2007, 16, 291–302. [Google Scholar] [CrossRef]
- Cha, Y.S. Effects of L-carnitine on obesity, diabetes, and as an ergogenic aid. Asia Pac. J. Clin. Nutr. 2008, 17, 306–308. [Google Scholar]
- Li, N.; Zhao, H. Role of carnitine in non-alcoholic fatty liver disease and other related diseases: An update. Front Med. 2021, 8, 689042. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Nazhand, A.; Bouto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. The nutraceutical value of carnitine and its use in dietary supplements. Molecules 2020, 25, 2127. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Asemi, Z.; Mansournia, M.A.; Hallajzadeh, J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Hadi, A.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: An updated systemic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104554. [Google Scholar] [CrossRef]
- Karalis, D.T.; Karalis, T.; Karalis, S.; Kleisiari, A.S. L-Carnitine as a Diet Supplement in Patients With Type II Diabetes. Cureus 2020, 12, e7982. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state and the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Mantle, D.; Dybring, A. Bioavailability of coenzyme Q10: An overview of the absorption process and subsequent metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and immune function: An overview. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and thera- peutic strategies. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Alam, A.; Rahman, M. Mitochondrial dysfunction in obesity: Potential benefit and mechanism of co-enzyme Q10 supplementation in metabolic syndrome. J. Diabetes Metab. Disord. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, D.; Waid, P.H.; Jordão, A.A., Jr. Mechanisms of action and effects of the administration of Coenzyme Q10 on metabolic syndrome. J. Nutr. Intermed. Metab. 2018, 13, 26–32. [Google Scholar] [CrossRef]
- Failla, M.L.; Chitchumroonchokchai, C.; Aoki, F. Increased bioavailability of ubiquinol compared to that of ubiquinone is due to more efficient micellarization during digestion and greater GSH-dependent uptake and basolateral secretion by Caco-2 cells. J. Agric. Food Chem. 2014, 62, 7174–7182. [Google Scholar] [CrossRef]
- Williams, L.; Bradford, J. Should I take coenzyme Q10 with a statin? JAAPA 2007, 19, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrilo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- McRae, M.P. Coenzyme Q10 Supplementation in Reducing Inflammation: An Umbrella Review. J. Chiropr. Med. 2022, 22, 131–137. [Google Scholar] [CrossRef]
- Mantle, D.; Kozhevnikova, S.; Larsen, S. Coenzyme Q10 and Obesity: An Overview. Antioxidants 2025, 14, 871. [Google Scholar] [CrossRef]
- Li, Y.; El-Sehrawy, A.A.M.A.; Shankar, A.; Mohammed, J.S.; Hjazi, A.; Singh, M.; Sapaev, I.B.; Mustafa, Y.F.; Abosaoda, M.K. Effects of Coenzyme Q10 Supplementation on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Clin. Ther. 2025, 47, 235–243. [Google Scholar] [CrossRef]
- Gholami, M.; Khosrowbeygi, A. Review on the mechanisms of the effects of coenzyme Q10 supplementation on serum levels of leptin and adiponectin. Rom. J. Diabetes Nutr. Metab. Dis. 2023, 30, 124–131. [Google Scholar]
- Nie, X.; Dong, X.; Hu, Y.; Xu, F.; Hu, C.; Shu, C. Coenzyme Q10 Stimulate Reproductive Vatality. Drug Des. Devel. Ther. 2023, 17, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.; Tabrizi, R.; Maleki, E.; Lankarani, K.B.; Heydari, S.T.; Moradinazar, M.; Akbari, M. Effects of coenzyme Q10 supplementation on lipid profiles and liver enzymes of nonalcoholic fatty liver disease (NAFLD) patients: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2023, 11, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Chen, L.H.; Chiou, S.H.; Chiou, G.Y.; Chen, Y.C.; Chou, H.Y.; Chen, L.K.; Chen, H.Y.; Chiu, T.H.; Tsai, C.S.; et al. Conzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011, 55, S227–S240. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Kukai, M. Superoxide dismutase: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Kiningham, K.K.; XU, Y.; Daosukho, C.; Popova, B.; Clair, D.K.S. Nuclear factor κB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem. J. 2001, 353, 147–156. [Google Scholar] [CrossRef]
- Coubriet, C.M.; Delmastro-Greenwood, M.M.; Previte, D.M.; Marré, M.; O’Connor, E.C.; Novak, E.A.; Vincent, G.; Mollen, K.P.; Lee, S.; Dong, H.H.; et al. Treatment with a Catalytic Superoxide Dismutase (SOD) Mimetic Improves Liver Steatosis, Insulin Sensitivity, and Inflammation in Obesity-Induced Type 2 Diabetes. Antioxidants 2017, 6, 85. [Google Scholar] [CrossRef]
- Samuni, Y.; Cook, J.A.; Choudhuri, R.; Degraff, W.; Sowers, A.L.; Krishna, M.C.; Mitchell, J.B. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic. Biol. Med. 2010, 49, 667–673. [Google Scholar] [CrossRef]
- Cui, R.; Gao, M.; Qu, S.; Liu, D. Overexpression of superoxide dismutase 3 gene blocks high-fat diet-induced obesity, fatty liver and insulin resistance. Gene Ther. 2014, 21, 840–848. [Google Scholar] [CrossRef]
- Perriotte-Olson, C.; Adi, N.; Manickam, D.S.; Westwood, R.A.; Desouza, C.V.; Natarajan, G.; Crook, A.; Kabanov, A.V.; Saraswathi, V. Nanoformulated copper/zinc superoxide dismutase reduces adipose inflammation in obesity. Obesity 2016, 24, 148–156. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Gastrejón-Téllez, V.; Soto, M.E.; Rudio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, S.; Wu, J.; Liu, L.; Song, Y.; Li, T.; Ha, L.; Liu, X.; Wang, F.; Tian, J.; et al. NRF2 plays a critical role in both self and EGCG protection against diabetic testicular damage. Oxid. Med. Cell Longev. 2017, 2017, 3172692. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The role of catechins in regulating diabetes: An update review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 1378–1387. [Google Scholar] [CrossRef]
- Onakpoya, I.; Spencer, E.; Heneghan, C.; Tompson, M. The effect of green tea on blood pressure and lipid profile: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 823–836. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Papakyriakopoulou, P.; Veldakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P.E. Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Tomou, Ε.-Μ.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banch, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Weng, X.; Bao, X.; Bai, X.; Lv, Y.; Zhang, S.; Chen, Y.; Zhao, C.; Zeng, M.; Huang, J.; et al. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022, 57, 102511, Erratum in Redox Biol. 2022, 58, 102548. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kruzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant potential of curcumin–a meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; He, J.; Zhao, Y. Quercetin Regulates Lipid Metabolism and Fat Accumulation by Regulating Inflammatory Responses and Glycometabolism Pathways: A Review. Nutrients 2024, 16, 1102. [Google Scholar] [CrossRef] [PubMed]
- Damiano, F.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Gnoni, A. Quercetin inhibition of SREBPs and ChREBP expression results in reduced cholesterol and fatty acid synthesis in C6 glioma cells. Int. J. Biochem. Cell Biol. 2019, 117, 105618. [Google Scholar] [CrossRef]
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin improves glucose and lipid metabolism of diabetic rats: Involvement of Akt signaling and SIRT1. J. Diabetes Res. 2017, 2017, 3417306. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef]
- Dhanya, R.; Kartha, C.C. Quercetin improves oxidative stress-induced pancreatic beta cell alterations via MTOR-signaling. Mol. Cell Biochem. 2021, 476, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Abdollahzadeh, S.M.; Dabbaghmanesh, M.-H.; Rezaianzadeh, A. The effect of quercetin supplementation on oxidative stress, glycemic control, lipid profile and insulin resistance in type 2 diabetes: A randomized clinical trial. J. Health Sci. Surveill. Syst. 2014, 2, 8–14. [Google Scholar]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef]
- Shi, G.J.; Li, Y.; Cao, Q.-H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-R.; Liu, S.; Yang, H.-W.; Chen, X.-L. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen. Res. 2020, 16, 1344–1350. [Google Scholar] [CrossRef]
- Chen, P.; Chen, J.; Zheng, Q.; Chen, W.; Wang, Y.; Xu, X. Pioglitazone, Extract of Compound Danshen Dripping Pill, and Quercetin Ameliorate Diabetic Nephropathy in Diabetic Rats. J. Endocrinol. Investig. 2013, 36, 422–427. [Google Scholar]
- Mustafa, N.H.; Siti, H.N.; Kamisah, Y. Role of Quercetin in Diabetic Cardiomyopathy. Plants 2025, 14, 25. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk Factors and Inflammatory Biomarkers inWomen with Type 2 Diabetes: A Double-Blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar] [PubMed]
- Fatima, M.T.; Bhat, A.A.; Nisar, S.; Fakhro, K.A.; Akil, A.S.A.-S. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon 2022, 9, e12698. [Google Scholar] [CrossRef]
- Yang, J.; Miao, X.; Yang, F.-J.; Cao, J.-F.; Liu, X.; Fu, J.-L.; Su, G.-F. Therapeutic potential of curcumin in diabetic retinopathy (Review). Int. J. Mol. Med. 2021, 47, 75. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How curcumin targets inflammatory mediators in diabetes: Therapeutic insights and possible solutions. Molecules 2022, 27, 4058. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef]
- Dahza, M.J.; Ghalandari, H.; Mohammad, H.G.; Amini, M.R.; Askarpour, M. Effects of curcumin/turmeric supplementation on lipid profile: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther. Med. 2023, 75, 102955. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3t3-L1 adipocytes through NF-kappaB and JNK pathway. BioMed Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-E-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Dos Santos Haber, J.F.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V. The effects of curcumin on diabetes mellitus: A systematic review. Front. Endocrinol. 2021, 12, 66948. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.-A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461, Erratum in Cell Rep. 2017, 20, 279. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites. Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Beltran-Velasco, A.I.; Redondo-Florez, L.; Martin-Rodriguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Ren, Z.-Q.; Zheng, S.-Y.; Sun, Z.; Luo, Y.; Wang, Y.; Yi, P.; Li, Y.S.; Huang, C.; Xiao, W.F. Resveratrol: Molecular Mechanisms, Health Benefits, and Potential Adverse Effects. MedComm (2020) 2025, 6, e70252. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.S.; Skolnick, A.H.; Kirtane, A.J.; Murphy, S.A.; Barron, H.V.; Giugliano, R.P.; Cannon, C.P.; Braunwald, E.; Gibson, C.M. TIMI Study Group. U-Shaped Relationship of Blood Glucose with Adverse Outcomes among Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2005, 46, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleem, I.A.; Brakat, A.M.; Adayel, H.M.; Asla, M.M.; Rizk, M.A.; Aboalfetoh, A.Y. The Effects of Resveratrol on Glycemic Control and Cardiometabolic Parameters in Patients with T2DM: A Systematic Review and Meta-Analysis. Med. Clin. 2022, 158, 576–585. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The Effects of Resveratrol Supplementation in Patients with Type 2 Diabetes, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Khare, N.; Rai, A. Carotenoids: Sources, Bioavailability and Their Role in Human Nutrition [Internet]. In Dietary Carotenoids-Sources, Properties, and Role in Human Health; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Joshi, S.; Dobhal, A.; Kaur, R.; Rawat, M.; Bhati, D.; Wilson, I. Enhancing lycopene stability and extraction: Sustainable techniques and role of nanotechnology. J. Food Compos. Anal. 2025, 148, 108192. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Shafras, M.; Sabaragamuwa, R.; Suwair, M. Role of dietary antioxidants in diabetes: An overview. Food Chem. Adv. 2024, 4, 100666. [Google Scholar] [CrossRef]
- Tafail, T.; Ain, H.B.U.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef] [PubMed]
- Abir, M.H.; Mahamud, A.G.M.S.U.; Tonny, S.H.; Anu, M.S.; Hossain, K.H.S.; Protic, I.A.; Khan, M.S.U.; Baroi, A.; Moni, A.; Uddin, M.J. Pharmacological potentials of lycopene against aging and aging-related disorders: A review. Food Sci. Nutr. 2023, 11, 5701–5735. [Google Scholar] [CrossRef]
- Karaköy, Z.; Cadirci, E.; Dincer, B. A New Target in Inflammatory Diseases: Lycopene. Eurasian J. Med. 2022, 54, S23–S28. [Google Scholar] [CrossRef]
- Chen, G.; Ni, Y.; Nagata, N.; Zhuge, F.; Xu, L.; Nagashimada, M.; Yamamoto, S.; Ushida, Y.; Fuke, N.; Suganuma, H.; et al. Lycopene alleviates obesity-induced Inflammation and insulin resistance by regulating M1/M2 status of macrophages. Mol. Nutr. Food Res. 2019, 63, 1900602. [Google Scholar] [CrossRef]
- Singh, D.P.; Khare, P.; Zhu, J.; Kondepudi, K.K.; Singh, J.; Baboota, R.K.; Boparai, R.K.; Khardori, R.; Chopra, K.; Bishnoi, M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int. J. Obes. 2016, 40, 487–496. [Google Scholar] [CrossRef]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef]
- Wang, J.; Suo, Y.; Zhang, J.; Zou, Q.; Tan, X.; Yuan, T.; Liu, Z.; Liu, X. Lycopene supplementation attenuates western diet-induced body weight gain through increasing the expressions of thermogenic/mitochondrial functional genes and improving insulin resistance in the adipose tissue of obese mice. J. Nutr. Biochem. 2019, 69, 63–72. [Google Scholar] [CrossRef]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Bose, K.; Agrawal, B. Effect of long term supplementation of tomatoes (cooked) on levels of antioxidant enzymes, lipid peroxidation rate, lipid profile and glycated haemoglobin in Type 2 diabetes mellitus. W. Indian Med. J. 2006, 55, 274–278. [Google Scholar] [CrossRef]
- Shidfar, F.; Froghifar, N.; Vafa, M.; Rajab, A.; Hosseini, S.; Shidfar, S.; Gohari, M. The effects of tomato consumption on serum glucose, apolipoprotein B, apolipoprotein A-I, homocysteine and blood pressure in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bal, B.S.; Chopra, S.; Singh, S.; Malhotra, N. Ameliorative effect of lycopene on lipid peroxidation and certain antioxidant enzymes in diabetic patients. J. Diabetes Metab. 2012, 3, 61–75. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Wang, Y. Beneficial effect of lycopene on anti-diabetic nephropathy through diminishing inflammatory response and oxidative stress. Food Funct. 2015, 6, 1150–1156. [Google Scholar] [CrossRef]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Zhou, X.; Chen, R.; Xiong, T.; Hong, M.; Li, Q.; Kong, M.; Han, W.; Sun, G. The association between intake of dietary lycopene and other carotenoids and gestational diabetes mellitus risk during mid-trimester: A cross-sectional study. Br. J. Nutr. 2019, 121, 1405–1412. [Google Scholar] [CrossRef]
- Menezes da Costa, R.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Ren, Y.; Li, Y.; Niu, F.; Li, Z.; Li, M.; Li, X.; Li, Q.; Huang, D.; Yu, Y. RNA-binding protein GIGYF2 orchestrates hepatic insulin resistance through STAU1/PTEN-mediated disruption of the PI3K/AKT signaling cascade. Mol. Med. 2024, 30, 124. [Google Scholar] [CrossRef]
- Su, D.; Zhang, C.-l.; Gao, Y.-c.; Liu, X.-y.; Li, C.-p.; Huangfu, J.; Xiao, R. Gene Expression and Correlation of Pten and Fabp4 in Liver, Muscle, and Adipose Tissues of Type 2 Diabetes Rats. Med. Sci. Monit. 2015, 21, 3616–3621. [Google Scholar] [CrossRef]
- Ahmadirad, H.; Teymoori, F.; Farhadnejad, H.; Shimi, G.; Asghari, G.; Yuzbashian, E.; Zarkesh, M.; Mirmiran, P.; Khalaj, A. The association of dietary insulinemic indices with PI3K, PTEN, and Akt gene expressions in visceral and subcutaneous adipose tissues among individuals undergoing abdominal surgery. Front. Nutr. 2024, 11, 1467686. [Google Scholar] [CrossRef]
- Li, Y.Z.; Cristofano, A.D.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.i.; Awazu, M.; Tamiya, M.; Iwasaki, Y.; Harada, Y.; Kugisaki, S.; Tanimura, S.; Kohno, M. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E643–E651. [Google Scholar] [CrossRef]
- Bengal, E.; Aviram, S. p38α MAPK Regulation of Energy Metabolism in Skeletal Muscle Offers a Therapeutic Path for Type 2 Diabetes. Cells 2025, 14, 1277. [Google Scholar] [CrossRef]
- Bengal, E.; Aviram, S.; Hayek, T. p38 MAPK in Glucose Metabolism of Skeletal Muscle: Beneficial or Harmful? Int. J. Mol. Sci. 2020, 21, 6480. [Google Scholar] [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative stress in diabetes mellitus and its complications: From pathophysiology to therapeutic strategies. Chin. Med. J. 2025, 138, 15–27. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Creo, A.L.; Cortes, T.M.C.; Dasari, S.; Nair, S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 2018, 128, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef]
- Khoi, C.-S.; Lin, T.-Y.; Chiang, C.-K. Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity. Antioxidants 2024, 13, 1486. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation Improves Impaired Mitochondrial Fuel Oxidation and Lowers Insulin Resistance in Patients with Type 2 Diabetes: Results of a Pilot Study. Antioxidants 2022, 11, 154. [Google Scholar] [CrossRef]
- Tippairote, T.; Bjørklund, G.; Gasmi, A.; Semenova, Y.; Peana, M.; Chirumbolo, S.; Hangan, T. Combined Supplementation of Coenzyme Q10 and Other Nutrients in Specific Medical Conditions. Nutrients 2022, 14, 4383. [Google Scholar] [CrossRef]
- Asbaghi, O.; Nazarian, B.; Yousefi, M.; Anjom-Shoae, J.; Rasekhi, H.; Sadeghi, O. Effect of vitamin E intake on glycemic control and insulin resistance in diabetic patients: An updated systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2023, 22, 10. [Google Scholar] [CrossRef]
- Lampousi, A.-M.; Lundberg, T.; Löfvenborg, J.E.; Carlsson, S. Vitamins C, E, and β-Carotene and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100211. [Google Scholar] [CrossRef]
- Ho, J.I.; Ng, E.Y.; Chiew, Y.; Koay, Y.Y.; Chuar, P.F.; Phang, S.C.W.; Ahmad, B.; Kadir, K.A. The effects of vitamin E on non-proliferative diabetic retinopathy in type 2 diabetes mellitus: Are they sustainable with 12 months of therapy. SAGE Open Med. 2022, 10, 20503121221095324. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, J.; Zhang, W. Effect of Vitamin E on Diabetic Nephropathy: A Meta-Analysis. Altern. Ther. Health Med. 2024, 30, 344–349. [Google Scholar] [PubMed]
- Bhatti, S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People With Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef]
- Norratabadi, S.; Ashtary-Larky, D.; Hosseini, F.; Namkhah, Z.; Mohammadi, S.; Salamat, S.; Nadery, M.; Yarmand, S.; Zamani, M.; Wong, A.; et al. The effects of vitamin C supplementation on glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102824. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academic Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225480/?utm_source=chatgpt.com (accessed on 15 September 2025).
- Szkudlinska, M.A.; von Frankenberg, A.D.; Utzchneider, K.M. The Antioxidant N-Acetylcysteine Does Not Improve Glucose Tolerance or β-Cell Function in Type 2 Diabetes. J. Diabetes Complicat. 2016, 30, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Emara, S.M.; Fahmy, S.F.; AbdelSalam, M.M.; El Wakeel, L.M. Effect of high-dose N-acetyl cysteine on the clinical outcome of patients with diabetic peripheral neuropathy: A randomized controlled study. Diabetol. Metab. Syndr. 2025, 17, 79. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Xu, Y.-L.; Li, S.-H.; Hui, R.; Wu, Y.-J.; Huang, X.-H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef]
- Yuan, F.; Wu, W.; Ma, L.; Wang, D.; Hu, M.; Gong, J.; Fang, K.; Xu, L.; Dong, H.; Lu, F. Turmeric and curcuminiods ameliorate disorders of glycometabolism among subjects with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2022, 177, 106121. [Google Scholar] [CrossRef]
- Mokgaloboni, K.; Mashaba, R.G.; Phoswa, W.N.; Lebelo, L.L. Curcumin Attenuates Hyperglycemia and Inflammation in Type 2 Diabetes Mellitus: Quantitative Analysis of Randomized Controlled Trial. Nutrients 2024, 16, 4177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, J.; Haedi, A.R.; Zhou, M. The effect of curcumin supplementation on glycemic indices in adults: A meta-analysis of meta-analyses. Prostaglandins Other Lipid. Mediat. 2024, 175, 106908. [Google Scholar] [CrossRef] [PubMed]
- Moradi Baniasadi, M.; Arzhang, P.; Setayesh, A.; Moradi, M.; Nasli-Esfahani, E.; Azadbakht, L. The effect of turmeric/curcumin supplementation on anthropometric indices in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Diabetes 2025, 15, 34. [Google Scholar] [CrossRef]
- Ramírez-Alarcón, K.; Victoriano, M.; Mardones, L.; Villagran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Cruz-Martins, N.; Sharifi-Rad, J.; Martorell, M. Phytochemicals as Potential Epidrugs in Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 656978. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic Effect of Resveratrol: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules 2022, 27, 5232. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Saleh, S.A.K.; Adly, H.M.; Adi, A.R.; Kutbi, E.; Alshammari, N.; Albahrani, S.J.; Al Shaikh, M.A.; Al-Agsam, M.A.M.; Alharran, A.M. The effect of resveratrol supplementation on obesity indices: A critical umbrella review of interventional meta-analyses. Eat. Weight Disord. 2025, 30, 92. [Google Scholar] [CrossRef]
- Thadhani, V.M. Resveratrol in Management of Diabetes and Obesity: Clinical Applications, Bioavailability, and Nanotherapy. In Resveratrol-Adding Life to Years, Not Adding Years to Life; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yu, C.P.; Hsieh, Y.W.; Lin, S.-P.; Hou, Y.-C. Resveratrol stereoselectively affected (±)warfarin pharmacokinetics and enhanced the anticoagulation effect. Sci Rep. 2020, 10, 15910. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bai, Y.; Yang, K.; Chen, G. Effects of green tea consumption on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Wang, W.-Y.; Fan, W.-Y.; Deng, Q.; Wang, X. Tea consumption and risk of type 2 diabetes: A dose–response meta-analysis of cohort studies. Br. J. Nutr. 2014, 111, 1329–1339. [Google Scholar] [CrossRef]
- Ito, A.; Matsui, Y.; Takeshita, M.; Katashima, M.; Goto, C.; Kuriki, K. Gut microbiota-mediated associations of green tea and catechin intakes with glucose metabolism in individuals without type 2 diabetes mellitus: A four-season observational study with mediation analysis. Arch. Microbiol. 2023, 205, 191. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Jiang, J.; Li, L.; Ying, X.; Tian, H.; Nie, M. Effects of green tea or green tea extract on insulin sensitivity and glycaemic control in populations at risk of type 2 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet 2014, 27, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea on glycemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 23–31. [Google Scholar] [CrossRef]
- Yu, J.; Song, P.; Perry, R.; Penfold, C.; Cooper, A.R. The Effectiveness of Green Tea or Green Tea Extract on Insulin Resistance and Glycemic Control in Type 2 Diabetes Mellitus: A Meta-Analysis. Diabetes Metab. J. 2017, 41, 251–262. [Google Scholar] [CrossRef]
- Schuurman, M.; Nguyen, J.; Wilson, R.B.; Barillaro, M.; Wallace, M.; Borradaile, N.; Wang, R. Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice. Antioxidants 2025, 14, 417. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, N.; Bonyadian, A.; Hojati, A.; Abbasalizad-Farhangi, M.; Heidari, M.; Darzi, M.; Seyedhosseini-Ghaheh, H.; Khajeh, M.; Tabrizi, F.P.F.; Vajdi, M.; et al. The effect of quercetin supplementation on the components of metabolic syndrome in adults: A systematic review and dose–response meta-analysis of randomized controlled trials. J. Funct. Foods 2024, 116, 106175. [Google Scholar] [CrossRef]
- Mantadaki, A.E.; Linardakis, M.; Vafeiadi, M.; Anastasiou, F.; Tsatsakis, A.; Symvoulakis, E. The Impact of Three-Month Quercetin Intake on Quality of Life and Anxiety in Patients with Type II Diabetes Mellitus: An Early Data Analysis from a Randomized Controlled Trial. Cureus 2024, 16, e58219. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Dawi, J.; Misakyan, Y.; Affa, S.; Kades, S.; Narasimhan, A.; Hajjar, F.; Besser, M.; Tumanyan, K.; Venketaraman, V. Oxidative Stress, Glutathione Insufficiency, and Inflammatory Pathways in Type 2 Diabetes Mellitus: Implications for Therapeutic Interventions. Biomedicines 2025, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Aghasizadeh, M.; Nazifkar, F.; Choubdari, M.G.; Assaran-Darban, R.; Tavallaie, S.; Hatamzadeh, H.; Ferns, G.; Mirinezhad, M.R.; Baharara, H. The Effects of Lycopene on Modulating Oxidative Stress and Liver Enzymes Levels in Metabolic Syndrome Patients: A Randomised Clinical Trial. Cell J. 2023, 25, 847–853. [Google Scholar] [CrossRef]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and Metabolic Syndrome: A Systematic Review of the Literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Aghasizadeh, M.; Esmaily, H.; Baharara, H.; Mahammadi-bajgiran, M.; Assaran-Darban, R.; Ferns, G.A.; Hadizaheh, F.; Mobarhan, M.G. The Effects of Lycopene Intake on Blood Pressure and Lipid Profile of Patients with Metabolic Syndrome; a Randomized Clinical Trial. J. Food Nutr. Res. 2023, 11, 602–607. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sesso, H.D. Whole Food versus Supplement: Comparing the Clinical Evidence of Tomato Intake and Lycopene Supplementation on Cardiovascular Risk Factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef] [PubMed]

| Non-Enzymatic Antioxidant Compound | Mechanistic Targets | Clinical Outcomes | Evidence-Based Recommendations | Safety Profile |
|---|---|---|---|---|
| Vitamin E | Vitamin E inhibits activation of p38 MAPK pathway [77], as well as NF-κB, and STAT-3 signaling [78], and decreases the production of pro-inflammatory eicosanoids [79]. | Vitamin E exhibits anti-inflammatory [76], antioxidant [74,75], antidiabetic [80], anti-atherogenic properties [74], and has been shown anti-obesity effects in animal models [81]. | RCTs and metanalyses show that vitamin E supplementation produces moderate improvement in OS markers; however, effects on glycemic control and autonomic function remain inconsistent [185,240]. Possible benefits for diabetic retinopathy and nephropathy have been reported, although the evidence in not yet conclusive [241,242]. | Safety at high doses (>400 IU/day) is uncertain; may interact with anticoagulants such as warfarin [185]. |
| Vitamin C | Unclear and potentially pleitropic [243]. | Vitamin C exhibits antioxidant and antidiabetic effects [86,88], shows hypolipidemic effects in postmenopausal women with T2DM [87] and acts synergistically with vitamin E to enhance antioxidative enzyme activity, including SOD and GSH, in patients with T2DM [86]. | Short-term RCTs and meta-analyses indicate that vitamin C supplementation produces moderate reductions in fasting glucose and certain OS markers in individuals with obesity-associated T2DM. However, effects on HbA1c remain inconsistent, likely in part due to the short duration of most of the studies (<6 months) [244,245]. | Safe at doses below 2000 mg/day for adults aged 19 and older [246]; high doses may cause gastrointestinal discomfort and may increase the risk of oxalate stone formation in susceptible individuals [244]. |
| N-acetylcysteine | N-acetylcysteine inhibits the NF-κB signaling pathway [99], while activating the PI3K/Akt and JNK2/STAT3 pathways, mechanisms relevant to metabolic regulation in T2DM [97]. | N-acetylcysteine exhibits anti-inflammatory and antioxidant properties [89,90], and has demonstrated antidiabetic potential [95]. It scavenges ROS [99] and serves as a precursor for GSH synthesis [89]. Additionally, it enhances the activity of key antioxidant enzymes, including SOD [101]. | Preclinical studies and small human trials—including GlyNAC investigations—indicate reductions in OS and suggest potential improvements in insulin sensitivity and mitochondrial function in the context of T2DM. Nevertheless, clinical evidence supporting direct glycemic benefits remains limited and preliminary [238,247]. | Safe at usual doses (1200–2400 mg/day) [238,248], though gastrointestinal discomfort may occur; caution is advised in individuals with a history of asthma or bronchospasm [249]. |
| Curcumin | Curcumin suppresses key pro-inflammatory signaling pathways relevant to T2DM pathophysiology, including NF-κB and MAPK [184], ERK/JNK [186], IKK [187,190], and JAK/STAT [184]. It also activates regulatory pathways involved in cellular protection and metabolic homeostasis, particularly the PI3K–Akt–GSK3β pathway [186] and the Keap1/Nrf2 antioxidant pathway [184]. | Curcumin exhibits anti-inflammatory [184], antioxidant [184], and metabolic regulatory actions [187,189]. It chelates metal ions and scavenges ROS [184], while enhancing the activity of key antioxidant enzymes, including SOD and catalase [184]. Curcumin promotes glucose uptake and improves insulin sensitivity [186], β-cell function [191], and overall glycemic control [187]. Additionally, it reduces triglycerides, total cholesterol, and LDL cholesterol, while increasing HDL levels [188]. Furthermore, curcumin inhibits glucosidase and aldose reductase [186], and has been associated with reductions in body weight [184,187,189], supporting its relevance in obesity-related T2DM. | Meta-analyses of RCTs indicate that curcumin produces moderate reductions in fasting plasma glucose, HbA1c, and inflammatory markers in individuals with T2DM. However, the magnitude and consistency of these effects vary depending on the dose, formulation, and methodological quality of the included studies [250,251,252,253]. | Curcumin is generally well tolerated at typical doses of approximately 1500 mg per day when used over extended periods (several months) [252,253,254]. However, higher doses may cause gastrointestinal discomfort and have the potential to interact with anticoagulant medications [252,253,254]. |
| Resveratrol | Resveratrol exerts multiple mechanistic actions relevant to obesity-associated T2DM. It modulates the composition and function of the gut microbiome [196], and inhibits key metabolic and inflammatory regulators including the NF-κB pathway [198], and the PPARγ [196]. It also suppresses PTP1B, thereby permitting sustained activation of the IRS-1/PI3K/AKT signaling cascade [198]. Additionally, it activates several protective and metabolic pathways, including AMPK/SIRT1, Keap1/Nrf2 and Akt [197,198]. Resveratrol further supports mitochondrial function and biogenesis through the activation of FOXO1 and PGC-1α [198]. | Resveratrol exhibits antioxidant and anti-inflammatory properties in experimental models of diabetes [197,198]. It promotes lipolysis, while suppressing lipogenesis, thereby contributing to reductions in adiposity and overall body weight [197]. It also exerts protective anti-apoptotic effects on pancreatic β-cells [198]. Furthermore, it demonstrates potent anti-diabetic actions by ameliorating IR and enhancing glucose uptake and metabolism regulation [198]. | Several meta-analyses of RCTs indicate that resveratrol produces modest improvements in glucose homeostasis, blood pressure and -in certain conditions- body weight in individuals with obesity-associated T2DM. However, the variability across studies and the generally small effect sizes do not support its use as a standard therapy [203,255,256,257]. Larger, longer-term, well-designed RCTs are still needed to clarify optimal dosing, duration, and patient subgroups, which might benefit the most. | Human resveratrol supplement doses used in clinical studies vary widely, ranging from approximately 8 mg/day to more than 3000 mg/day, with many trials employing doses between 250 and 1000 mg/day [258]. Resveratrol can modulate CYP enzymes and has shown potential interactions with warfarin in preclinical and some human studies [259]. |
| Catechins | Catechins stimulate the PI3K signaling cascade and activate the Nrf2 pathway [158]. Additionally, they activate the PI3K/Akt and AMPK pathways [159]. | Catechins exert multiple beneficial effects on obesity-associated T2DM by possessing potent antioxidant and anti-inflammatory properties [153,157], thereby contributing to mitigation of metabolic dysfunction [153,157]. They enhance glucose homeostasis by promoting the translocation of GLUT4 from intracellular compartments to the cell membrane, facilitating increased glucose uptake [159]. They demonstrate anti-obesity properties through the upregulation of UCP1, which stimulates thermogenesis and energy expenditure [160]. They support cardiovascular health by lowering SBP via increased NO bioavailability and reduced ET-1 production [161] and they improve lipid profiles by decreasing LDL levels [162]. | Meta-analyses demonstrate small yet consistent reductions in fasting glucose and HbA1c with green tea or catechin supplementation [260], as well as a lower risk of developing type 2 diabetes mellitus (T2DM) associated with habitual intake [261]. Mechanistic and intermediary-marker studies—encompassing gut microbiota modulation, improvements in lipid profiles, and reductions in waist circumference—provide biologically plausible pathways through which catechins may influence metabolic risk in obesity [262]. Overall, the magnitude of these effects is modest [260], and considerable heterogeneity exists across studies due to differences in dosage, study population, intervention duration, and baseline metabolic status. Furthermore, some analyses report no significant improvements in specific glycemic parameters, such as fasting insulin, among individuals with T2DM [263,264,265]. | High dose of EGCG ≥ 800 mg/day extracts have been associated with rare cases of serum transaminases, indicating potential hepatotoxicity at excessive intakes [266]. In addition, the caffeine content of many green tea–derived preparations may pose tolerability issues for sensitive individuals [260]. |
| Quercetin | Quercetin exerts its protective actions against obesity associated T2DM by modulating several key molecular pathways. It activates the KEAP1/Nrf2 [169], IRS-1/PI3K/Akt [163,176], AMPK [176] and HO-1 [180] signaling cascades, while concurrently inhibiting the NF-κΒ [171] and SphK1-S1P [181] pathways. | Quercetin demonstrates significant antioxidant [169] and anti-inflammatory activities [171]. It inhibits lipogenesis and mitigates overall adiposity and the development of NAFLD by suppressing the expression of ACACA, FASN, and SREBP-1c [172,173]; Quercetin also exhibits antidiabetic properties by reducing intestinal glucose absorption, enhancing insulin secretion and sensitivity, promoting GLUT4 translocation to the cell membrane, increasing cellular glucose uptake and protecting pancreatic β-cells [163]. Additionally, it may ameliorate diabetic retinopathy [180] and attenuate diabetic renal fibrosis [181]. Quercetin has been reported to improve endothelial function by increasing NO bioavailability [182] and may also reduce SBP in postmenopausal women with T2DM [183]. | The available clinical evidence consists largely of small randomized controlled trials and pilot studies. Recent meta-analyses and systematic reviews indicate modest improvements in oxidative-stress markers, blood pressure, and select metabolic parameters following quercetin supplementation, although findings remain heterogeneous and dose-dependent [267,268]. Individual trials and early open-label studies in individuals with T2DM similarly report benefits in OS biomarkers, quality-of-life indices, and certain cardiometabolic outcomes; however, these studies are limited by small sample sizes, short durations, and considerable variability in quercetin formulations and dosing regimens [269]. Evidence for glycemic outcomes remains limited [268]. | Quercetin is generally well tolerated in clinical studies, including those involving individuals with T2DM, with adverse events typically mild and primarily limited to gastrointestinal complaints such as nausea or dyspepsia [269]. It may interact with certain medications through cytochrome P450–mediated mechanisms [270]. Human intervention trials most commonly employ doses of 500–1000 mg per day for short-to-moderate durations, typically 4–12 weeks [268]. |
| Lycopene | Lycopene exerts its protective effects in obesity-associated T2DM through multiple molecular mechanisms. It suppresses activation of the NF-κB signaling pathway and downregulates genes involved in lipogenesis, thermogenetic and mitochondrial functional, including Fas, ACACA, PPARγ, PGC1α, Prdm16, UCPs, and Sirt1. Lycopene also reduces the expression of autophagy-related genes such as Atg7, Atg14, P62, Lc3, and Beclin [214]. Furthermore, it modulates cell-survival pathways by influencing proteins associated with apoptosis and cellular stress responses, including RAGE, NF-қB, Bax, Bcl-Xl, and Bcl-2 [216]; In addition, lycopene enhances the expression of HO-1 in the kidneys of individuals with T2DM [220]. | Lycopene exhibits notable antioxidant [206,217] and anti-inflammatory activities [206], enhancing the expression of key antioxidant enzymes, including SOD, GPx, GR, while reducing markers of OS such as the levels of MDA, and XOD [219]. In experimental models, lycopene has been shown to stimulate lipolysis, improve circulating lipid profiles, limit weight gain, and reduce hepatic fat accumulation [213]. Additionally, it demonstrates antidiabetic properties by lowering blood glucose and insulin concentrations [216,222]. | Human clinical evidence for lycopene in obesity-associated T2DM is limited and heterogeneous. Small randomized trials and pilot studies have generally reported improvements in OS biomarkers and some lipid parameters after lycopene supplementation, but effects on clinically relevant glycemic outcomes (fasting glucose, HbA1c, IR) are inconsistent and sparsely reported. Recent systematic reviews reach similar conclusions and highlight the need for larger, longer, well-controlled trials to establish efficacy, optimal dose and formulation, and durability of effects [271,272,273]. | Lycopene is considered safe when consumed through the habitual diet, with reported adverse effects uncommon and generally mild; however, long-term safety data remain limited [271]. Human supplementation studies outside the diabetes setting have typically used daily doses of 10–30 mg for several weeks to a few months [274]. Higher short-term doses of approximately 70–75 mg/day have also been evaluated without major adverse effects, though these findings are based on small, short-duration trials and should be interpreted with caution [275,276]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varra, F.-N.; Theodosis-Nobelos, P.; Varra, V.-K.; Varras, M. Mechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress in the Pathogenesis and Complications of Type 2 Diabetes Mellitus. Curr. Issues Mol. Biol. 2025, 47, 1063. https://doi.org/10.3390/cimb47121063
Varra F-N, Theodosis-Nobelos P, Varra V-K, Varras M. Mechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress in the Pathogenesis and Complications of Type 2 Diabetes Mellitus. Current Issues in Molecular Biology. 2025; 47(12):1063. https://doi.org/10.3390/cimb47121063
Chicago/Turabian StyleVarra, Fani-Niki, Panagiotis Theodosis-Nobelos, Viktoria-Konstantina Varra, and Michail Varras. 2025. "Mechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress in the Pathogenesis and Complications of Type 2 Diabetes Mellitus" Current Issues in Molecular Biology 47, no. 12: 1063. https://doi.org/10.3390/cimb47121063
APA StyleVarra, F.-N., Theodosis-Nobelos, P., Varra, V.-K., & Varras, M. (2025). Mechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress in the Pathogenesis and Complications of Type 2 Diabetes Mellitus. Current Issues in Molecular Biology, 47(12), 1063. https://doi.org/10.3390/cimb47121063





