The CXCL12/CXCR4 Axis in Sepsis-Induced Acute Lung Injury: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
2. Structural and Signaling Framework of the CXCL12/CXCR4 Characteristics of CXCL12
2.1. Structural Characteristics
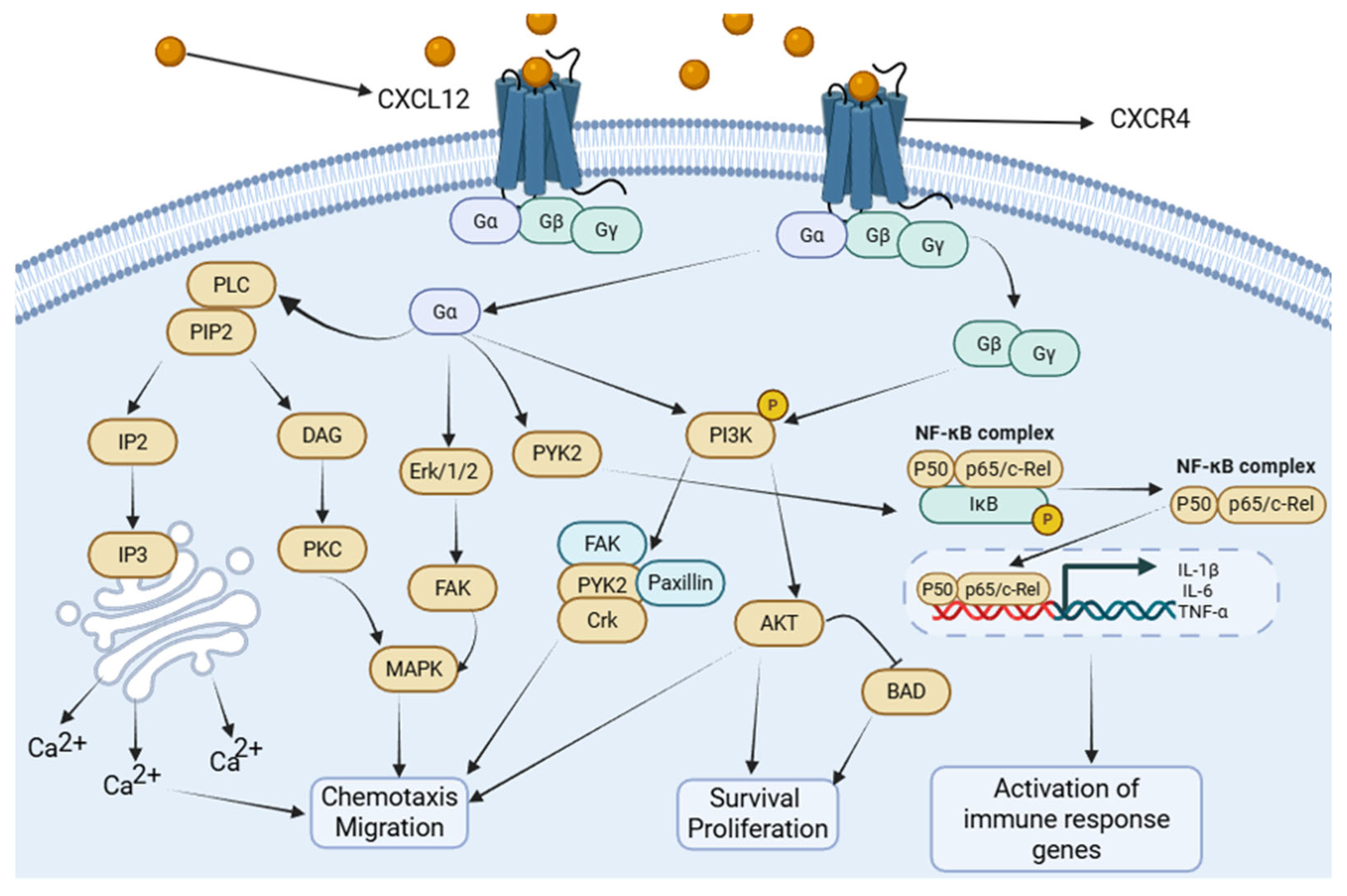
2.2. Downstream Signaling Pathways
2.3. Upstream Regulation of CXCL12 in Sepsis and Acute Lung Injury (ALI)
3. Cell-Type-Specific Roles of the CXCL12/CXCR4 Axis in ALI
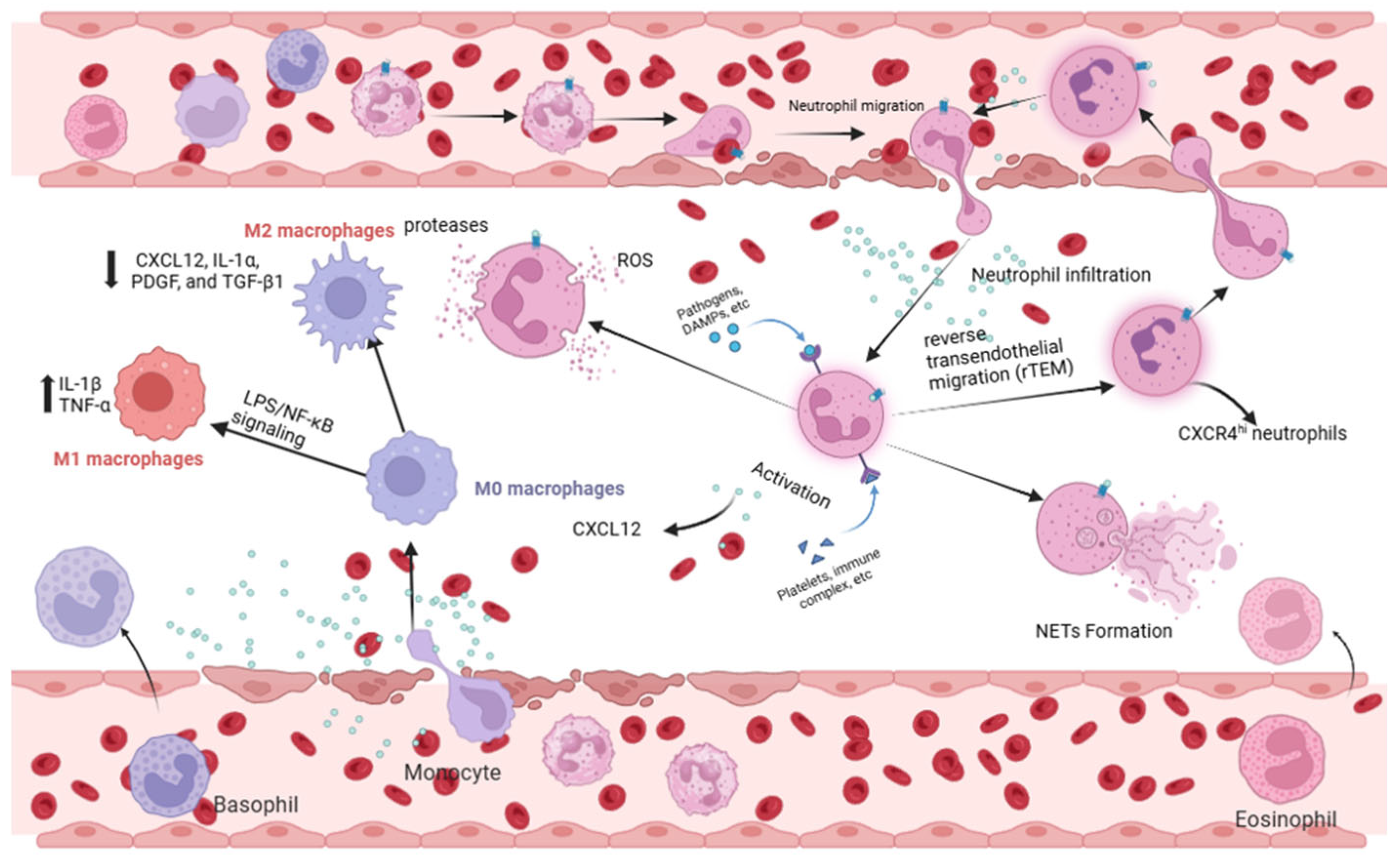
3.1. Acute Lung Injury Neutrophils: Orchestrators of Inflammation and Reverse Migration
3.2. Macrophages: Balancing M1/M2 Polarization
3.3. Alveolar Epithelial Cells: Guardians of Barrier and Repair
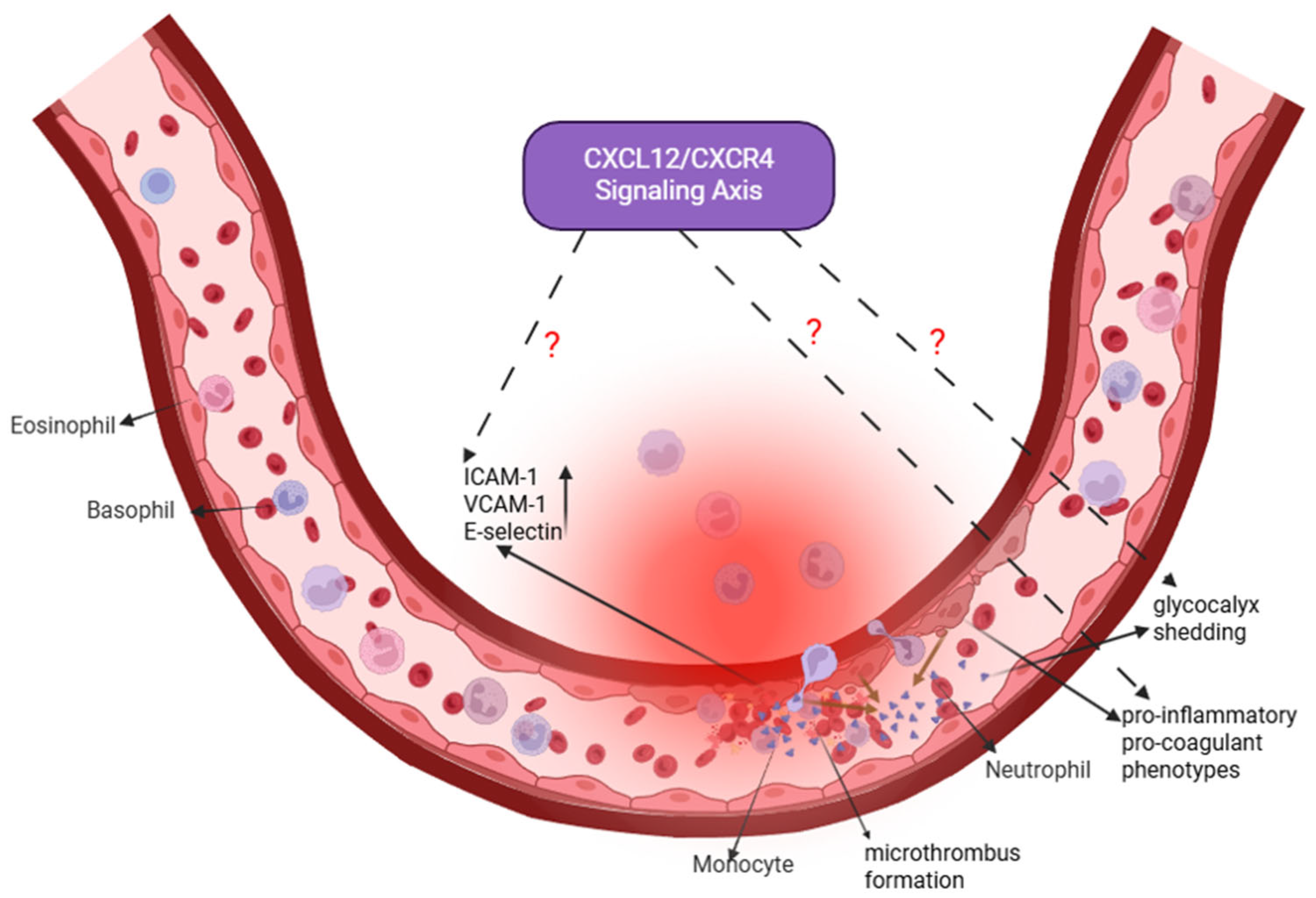
3.4. Pulmonary Vascular Endothelial Cells: A Nexus of Barrier Dysfunction and Potential Regeneration
3.5. CXCR7/ACKR3: Modulator of CXCL12 Bioavailability and Signaling Bias
4. Clinical Translation Perspective: From Mechanistic Insights to Therapeutic Strategies
4.1. Distinguishing Preclinical Evidence from Human Data; Circulating CXCL12/CXCR4 in Human Sepsis/Acute Lung Injury (ALI)
4.2. Therapeutic Modulation of the CXCL12/CXCR4 Axis: Balancing Inflammation Control and Tissue Repair
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AECs | Alveolar Epithelial Cells |
| ALI | Acute Lung Injury |
| ARDS | Acute Respiratory Distress Syndrome |
| AT I | Alveolar Type I cells |
| AT II | Alveolar Type II cells |
| BAD | Bcl-2 Antagonistic Cell Death |
| CLP | Cecal Ligation and Puncture |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CXCR4 | C-X-C Chemokine Receptor Type 4 |
| DAG | Diacylglycerol |
| FAK | Focal Adhesion Kinase |
| GDP | Guanosine Diphosphate |
| GPCR | G Protein-Coupled Receptor |
| GTP | Guanosine Triphosphate |
| HIF-1 | Hypoxia-Inducible Factor-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| ICS II | Icariside II |
| IL-1β | Interleukin-1 beta |
| IL-10 | Interleukin-10 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IP3 | Inositol Trisphosphate |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MSCs | Mesenchymal Stem Cells |
| NETs | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PDGF | Platelet-Derived Growth Factor |
| PI3K | Phosphoinositide 3-Kinase |
| PIP2 | Phosphatidylinositol 4,5-Bisphosphate |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| rTEM | Reverse Transendothelial Migration |
| ROS | Reactive Oxygen Species |
| RvD1 | Resolvin D1 |
| SALI | Sepsis-induced Acute Lung Injury |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primer 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Legrand, M. Epidemiology of sepsis and septic shock. Curr. Opin. Anaesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef]
- Luyt, C.E.; Bouadma, L.; Morris, A.C.; Dhanani, J.A.; Kollef, M.; Lipman, J.; Martin-Loeches, I.; Nseir, S.; Ranzani, O.T.; Roquilly, A.; et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020, 46, 2168–2183. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Lindstedt, S.; Wang, Q.; Niroomand, A.; Stenlo, M.; Hyllen, S.; Pierre, L.; Olm, F.; Bechet, N.B. High resolution fluorescence imaging of the alveolar scaffold as a novel tool to assess lung injury. Sci. Rep. 2024, 14, 6662. [Google Scholar] [CrossRef]
- Chase, M.A.; Wheeler, D.S.; Lierl, K.M.; Hughes, V.S.; Wong, H.R.; Page, K. Hsp72 Induces Inflammation and Regulates Cytokine Production in Airway Epithelium through a TLR4- and NF-κB-Dependent Mechanism. J. Immunol. 2007, 179, 6318–6324. [Google Scholar] [CrossRef]
- Ghosh, M.C.; Makena, P.S.; Gorantla, V.; Sinclair, S.E.; Waters, C.M. CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L846–L856. [Google Scholar] [CrossRef] [PubMed]
- Bleul, C.C.; Fuhlbrigge, R.C.; Casasnovas, J.M.; Aiuti, A.; Springer, T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 1996, 184, 1101–1109. [Google Scholar] [CrossRef]
- Murdoch, C. CXCR4: Chemokine receptor extraordinaire: CXCR4: Chemokine receptor extraordinaire. Immunol. Rev. 2000, 177, 175–184. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Janssens, R.; Struyf, S.; Proost, P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018, 44, 51–68. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Porcelli, D.; Torella, A.R.; Straino, S.; Iachininoto, M.G.; Orlandi, A.; Truffa, S.; Biglioli, P.; Napolitano, M.; Capogrossi, M.C.; et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 2004, 104, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Luster, A.D. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, K.; Rankin, S.M. CXCR 4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur. J. Clin. Investig. 2018, 48, e12949. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, M.; Zhao, X. Role of chemokine systems in cancer and inflammatory diseases. MedComm 2022, 3, e147. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kubo, H.; Kobayashi, S.; Ishizawa, K.; He, M.; Suzuki, T.; Fujino, N.; Kunishima, H.; Hatta, M.; Nishimaki, K.; et al. The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury. Cell. Mol. Immunol. 2011, 8, 305–314. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Q.; Tang, H.; Chen, J.; Wu, Q.; Yuan, X.; Xiong, S.; Ye, Y.; Lv, H. NLRP3 Regulated CXCL12 Expression in Acute Neutrophilic Lung Injury. J. Inflamm. Res. 2020, 13, 377–386. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, C.; Yin, H.; Tan, X.; Yi, J.; Tian, S.; Wang, Y.; Liu, J. Mesenchymal stem cells inhibited the apoptosis of alveolar epithelial cells caused by ARDS through CXCL12/CXCR4 axis. Bioengineered 2022, 13, 9060–9070. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Huang, Z.; Wu, H.; Zhou, Y. Tranilast attenuates lipopolysaccharide-induced lung injury via the CXCR4/JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2022, 26, 220. [Google Scholar] [CrossRef]
- Hostler, A.C.; Hahn, W.W.; Hu, M.S.; Rennert, R.; Fischer, K.S.; Barrera, J.A.; Duscher, D.; Januszyk, M.; Henn, D.; Sivaraj, D.; et al. Endothelial-specific CXCL12 regulates neovascularization during tissue repair and tumor progression. FASEB J. 2024, 38, e70210. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Lagerström, M.C.; Schiöth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357, Erratum in Nat. Rev. Drug Discov. 2008, 7, 542. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Nielsen, B.W.; Matsushima, K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int. Immunol. 1993, 5, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.P. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997, 16, 6996–7007. [Google Scholar] [CrossRef]
- Murphy, J.W.; Yuan, H.; Kong, Y.; Xiong, Y.; Lolis, E.J. Heterologous quaternary structure of CXCL12 and its relationship to the CC chemokine family. Proteins Struct. Funct. Bioinform. 2010, 78, 1331–1337. [Google Scholar] [CrossRef]
- Mellado, M.; Rodríguez-Frade, J.M.; Mañes, S.; Martínez-A, C. Chemokine Signaling and Functional Responses: The Role of Receptor Dimerization and TK Pathway Activation. Annu. Rev. Immunol. 2001, 19, 397–421. [Google Scholar] [CrossRef]
- Lin, C.-H.; Shih, C.-H.; Lin, Y.-C.; Yang, Y.-L.; Chen, B.-C. MEKK1, JNK, and SMAD3 mediate CXCL12-stimulated connective tissue growth factor expression in human lung fibroblasts. J. Biomed. Sci. 2018, 25, 19. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Chen, Y.-B.; Li, P. Quercetin inhibits NTHi-triggered CXCR4 activation through suppressing IKKα/NF-κB and MAPK signaling pathways in otitis media. Int. J. Mol. Med. 2018, 42, 248–258. [Google Scholar] [CrossRef]
- Li, L.; Lv, G.; Wang, B.; Kuang, L. The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem. Biophys. Res. Commun. 2018, 503, 2555–2562. [Google Scholar] [CrossRef]
- Bendall, L.J.; Baraz, R.; Juarez, J.; Shen, W.; Bradstock, K.F. Defective p38 Mitogen-Activated Protein Kinase Signaling Impairs Chemotaxic but not Proliferative Responses to Stromal-Derived Factor-1α in Acute Lymphoblastic Leukemia. Cancer Res. 2005, 65, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.O.; Wang, C. CXCL12/SDF-1α Activates NF-κB and Promotes Oral Cancer Invasion through the Carma3/Bcl10/Malt1 Complex. Int. J. Oral Sci. 2009, 1, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xie, F.; Zhao, J.; Yue, B. Suppressed nuclear factor-kappa B alleviates lipopolysaccharide-induced acute lung injury through downregulation of CXCR4 mediated by microRNA-194. Respir. Res. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Pai, H.K.; Hong, K.O.; Kim, M.A.; Kim, J.H.; Lee, J.I.; Hong, S.P.; Hong, S.D. CXCR-4 knockdown by small interfering RNA inhibits cell proliferation and invasion of oral squamous cell carcinoma cells. J. Oral Pathol. Med. 2008, 38, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Barbero, S.; Bonavia, R.; Bajetto, A.; Porcile, C.; Pirani, P.; Ravetti, J.L.; Zona, G.L.; Spaziante, R.; Florio, T.; Schettini, G. Stromal Cell-derived Factor 1α Stimulates Human Glioblastoma Cell Growth through the Activation of Both Extracellular Signal-regulated Kinases 1/2 and Akt. Cancer Res. 2003, 63, 1969–1974. [Google Scholar] [PubMed]
- Ooppachai, C.; Limtraku, P.; Yodkeeree, S. Dicentrine Potentiates TNF-α-Induced Apoptosis and Suppresses Invasion of A549 Lung Adenocarcinoma Cells via Modulation of NF-κB and AP-1 Activation. Molecules 2019, 24, 4100. [Google Scholar] [CrossRef] [PubMed]
- McClendon, J.; Jansing, N.L.; Redente, E.F.; Gandjeva, A.; Ito, Y.; Colgan, S.P.; Ahmad, A.; Riches, D.W.H.; Chapman, H.A.; Mason, R.J.; et al. Hypoxia-Inducible Factor 1α Signaling Promotes Repair of the Alveolar Epithelium after Acute Lung Injury. Am. J. Pathol. 2017, 187, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Kubarek, Ł.; Jagodzinski, P.P. Epigenetic up-regulation of CXCR4 and CXCL12 expression by 17 beta-estradiol and tamoxifen is associated with formation of DNA methyltransferase 3B4 splice variant in Ishikawa endometrial adenocarcinoma cells. FEBS Lett. 2007, 581, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yuan, Y.; Liao, Q. Alleviation of Lipopolysaccharides-Induced Acute Lung Injury by MiR-454. Cell. Physiol. Biochem. 2016, 38, 65–74. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, J.; Lv, T.; Song, Y. A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 1464. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Krauss-Etschmann, S.; Koller, B.; Hordijk, P.L.; Kuijpers, T.W.; Hoffmann, F.; Hector, A.; Eber, E.; Marcos, V.; Bittmann, I.; et al. Infiltrated Neutrophils Acquire Novel Chemokine Receptor Expression and Chemokine Responsiveness in Chronic Inflammatory Lung Diseases. J. Immunol. 2008, 181, 8053–8067. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiao, M.; Zhou, J.; Wang, P.; Peng, J.; Mao, W.; Hu, Y.; Liu, Y.; Yin, J.; Ke, L.; et al. PFKFB3 promotes sepsis-induced acute lung injury by enhancing NET formation by CXCR4hi neutrophils. Int. Immunopharmacol. 2023, 123, 110737. [Google Scholar] [CrossRef]
- Badr, H.S.; El-Gendy, F.M.; Helwa, M.A. Serum stromal-derived-factor-1 (CXCL12) and its alpha chemokine receptor (CXCR4) as biomarkers in neonatal sepsis. J. Matern. Fetal Neonatal Med. 2018, 31, 2209–2215. [Google Scholar] [CrossRef]
- Hirano, Y.; Ode, Y.; Ochani, M.; Wang, P.; Aziz, M. Targeting junctional adhesion molecule-C ameliorates sepsis-induced acute lung injury by decreasing CXCR4+ aged neutrophils. J. Leukoc. Biol. 2018, 104, 1159–1171. [Google Scholar] [CrossRef]
- Fan, J. Neutrophil in Reverse Migration: Role in Sepsis. Front. Immunol. 2021, 12, 656039. [Google Scholar] [CrossRef]
- Barkaway, A.; Rolas, L.; Joulia, R.; Bodkin, J.; Lenn, T.; Owen-Woods, C.; Reglero-Real, N.; Stein, M.; Vázquez-Martínez, L.; Girbl, T.; et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021, 54, 1494–1510.e7. [Google Scholar] [CrossRef] [PubMed]
- Owen-Woods, C.; Joulia, R.; Barkaway, A.; Rolas, L.; Ma, B.; Nottebaum, A.F.; Arkill, K.P.; Stein, M.; Girbl, T.; Golding, M.; et al. Local microvascular leakage promotes trafficking of activated neutrophils to remote organs. J. Clin. Investig. 2020, 130, 2301–2318. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, Y.; Wang, D.M.; Liu, K.; Yang, X.; Zheng, C.X.; He, Z.B.; Guo, Z.Y.; Yang, Y. MCTR1 ameliorates LPS-induced lung injury by inhibiting neutrophil reverse transendothelial migration. Int. Immunopharmacol. 2025, 157, 114777. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, K.; Liao, L.; Zhang, T.; Yang, M.; Sun, C. Lipoxin A4 receptor agonist BML-111 induces autophagy in alveolar macrophages and protects from acute lung injury by activating MAPK signaling. Respir. Res. 2018, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, X.; Bolli, E.; Fendt, S.-M.; Van Ginderachter, J.A. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front. Immunol. 2017, 8, 289. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, X.; Zhang, P.; Li, Q.; Liang, X.; Liu, J. MiR-212-3p inhibits LPS-induced inflammatory response through targeting HMGB1 in murine macrophages. Exp. Cell Res. 2017, 350, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, P.; Harandi, O.F.; Agarwal, S.; Rus, F.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Caffrey, D.R.; Golenbock, D.T. miR-718 represses proinflammatory cytokine production through targeting phosphatase and tensin homolog (PTEN). J. Biol. Chem. 2017, 292, 5634–5644. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Tang, H.; Shen, Y.; Gong, Z.; Xie, N.; Zhang, X.; Wang, W.; Kong, W.; Zhou, Y.; et al. The N6-methyladenosine (m6 A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am. J. Physiol. Cell Physiol. 2019, 317, C762–C775. [Google Scholar] [CrossRef]
- Shen, Y.; Song, J.; Wang, Y.; Chen, Z.; Zhang, L.; Yu, J.; Zhu, D.; Zhong, M. M2 macrophages promote pulmonary endothelial cells regeneration in sepsis-induced acute lung injury. Ann. Transl. Med. 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xie, G.; Xiao, H.; Ding, F.; Bao, W.; Zhang, M. CXCR4 knockdown prevents inflammatory cytokine expression in macrophages by suppressing activation of MAPK and NF-κB signaling pathways. Cell Biosci. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Matthay, M.A. What drives neutrophils to the alveoli in ARDS? Thorax 2017, 72, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.A.; Sun, H.; Chiu, S.; DeCamp, M.M.; Sporn, P.H.; Sznajder, J.I.; Bharat, A. Decreased CXCL12 is associated with impaired alveolar epithelial cell migration and poor lung healing after lung resection. Surgery 2015, 158, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The pulmonary endothelium in acute respiratory distress syndrome: Insights and therapeutic opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yin, J.; Zheng, Z.; Li, L.; Feng, X. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: Pathogenesis and therapeutic implications. Cell Commun. Signal. 2024, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.F.; Wang, J.H.; Sun, S.; Xu, Y.T.; Wan, D.; Feng, S.; Tian, Z.; Zhu, H.F. Catalpol attenuates ischemic stroke by promoting neurogenesis and angiogenesis via the SDF–1α/CXCR4 pathway. Phytomedicine 2024, 128, 155362. [Google Scholar] [CrossRef]
- Liu, G.Q.; Lu, P.R.; Li, L.B.; Zhang, X.G. Inhibited experimental corneal neovascularization by neutralizing anti-SDF-1α antibody. Int. J. Ophthalmol. 2012, 5, 7–12. [Google Scholar] [PubMed]
- Inagawa, R.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Yano, H.; Ando, Y.; Usui, T.; Hotta, Y.; Miyazaki, N.; et al. Ultrastructural Alteration of Pulmonary Capillary Endothelial Glycocalyx During Endotoxemia. Chest 2018, 154, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.C.; Rockstrom, M.D.; Schmidt, E.P.; Hippensteel, J.A. Endothelial glycocalyx degradation during sepsis: Causes and consequences. Matrix Biol. Plus 2021, 12, 100094. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kakuuchi, M.; Maruyama, I. Endotheliopathy in septic conditions: Mechanistic insight into intravascular coagulation. Crit. Care 2021, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Lima e Silva, R.; Shen, J.; Hackett, S.F.; Kachi, S.; Akiyama, H.; Kiuchi, K.; Yokoi, K.; Hatara, M.C.; Lauer, T.; Aslam, S.; et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007, 21, 3219–3230. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Dong, P. CircMETTL14(11)S upregulated METTL14 and induced CXCR4 to aggravate endothelial inflammation and atherosclerosis. Int. Immunopharmacol. 2024, 126, 110979. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; de Fabritus, L.; Kumar, P.A.; Werner, Y.; Ma, M.; Li, D.; Siret, C.; Simic, M.; Li, B.; Kerdiles, Y.M.; et al. Brain endothelial CXCL12 attracts protective natural killer cells during ischemic stroke. J. Neuroinflamm. 2023, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Kim, J.; Ahn, S.; Craig, S.; Lam, C.M.; Gerard, N.P.; Gerard, C.; Lefkowitz, R.J. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA 2010, 107, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Abbasifard, M.; Mohammadi-Shahrokhi, V.; Khorramdelazad, H. Stromal-derived factor-1: The glycoprotein fueling autoimmune storms via CXCR4 and CXCR7. Int. J. Biol. Macromol. 2025, 321, 146374. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Elad, H.; Brazowski, E.; Tulchinsky, H.; Vigodman, S.; Kopylov, U.; Halpern, Z.; Guzner-Gur, H.; Dotan, I. Reciprocal regulation of CXCR4 and CXCR7 in intestinal mucosal homeostasis and inflammatory bowel disease. J. Leukoc. Biol. 2011, 90, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Orengo, L.; Holman, D.W.; Dorsey, D.; Zhou, L.; Zhang, P.; Wright, M.; McCandless, E.E.; Patel, J.R.; Luker, G.D.; Littman, D.R.; et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med. 2011, 208, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Penfold, M.E.; Matsuda, A.; Ohyanagi, N.; Kaneko, K.; Miyabe, Y.; Matsumoto, K.; Schall, T.J.; Miyasaka, N.; Nanki, T. Pathogenic role of CXCR7 in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 3211–3220. [Google Scholar] [CrossRef]
- Levoye, A.; Balabanian, K.; Baleux, F.; Bachelerie, F.; Lagane, B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 2009, 113, 6085–6093. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, F.; He, D.; Zhang, L.; Shen, J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019, 109, 1233–1239. [Google Scholar] [CrossRef]
- Pouzol, L.; Sassi, A.; Baumlin, N.; Tunis, M.; Strasser, D.S.; Lehembre, F.; Martinic, M.M. CXCR7 Antagonism Reduces Acute Lung Injury Pathogenesis. Front. Pharmacol. 2021, 12, 748740. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Adrover, J.M.; Felice, C.; Christensen, L.N.; He, X.Y.; Merrill, J.R.; Wilkinson, J.E.; Janowitz, T.; Lyons, S.K.; Egeblad, M.; et al. PTP1B inhibitors protect against acute lung injury and regulate CXCR4 signaling in neutrophils. JCI Insight 2022, 7, e158199. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Takao, K.; Miyakawa, T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 1167–1172, Correction in Proc. Natl. Acad. Sci. USA 2015, 112, E1163–E1167. [Google Scholar] [CrossRef] [PubMed]
- Cotoia, A.; Mirabella, L.; Altamura, S.; Villani, R.; Marchese, F.; Ferrara, G.; Mariano, K.; Livio, T.; Cinnella, G. Circulating stem cells, HIF-1, and SDF-1 in septic abdominal surgical patients: Randomized controlled study protocol. Trials 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Chen, Y.; Lu, Z.; Sun, Y.; Zhong, C.; Lv, Z.; Pan, H.; Chen, J.; Yao, D.; et al. Icariside II alleviates lipopolysaccharide-induced acute lung injury by inhibiting lung epithelial inflammatory and immune responses mediated by neutrophil extracellular traps. Life Sci. 2024, 346, 122648. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, S.; Shu, H.; Liu, H.; Yuan, S.; Chen, X.; Li, R.; Wu, X.; Guo, L.; Wang, Y. Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J. Surg. Res. 2014, 188, 213–221, Erratum in J. Surg. Res. 2020, 252, 285. [Google Scholar]
- Lv, Z.; Zhang, B.; Zhang, H.; Mao, Y.; Yu, Q.; Dong, W. Exploration of key mechanisms underlying the therapeutic effects of AMD3100 on attenuating lipopolysaccharide-induced acute lung injury in mice. PeerJ 2024, 12, e18698. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action. of Glucocorticoids—New Mechanisms for Old. Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiu, D.; Li, Y.; Song, J.; Lu, Y.; Bi, J.; Chen, C.; Wang, J.; Jiang, H.; Song, Y.; et al. Tissue factor pathway inhibitor promotes the migration, proliferation and colonization of MSCs by regulating CXCL12/CXCR4 signaling pathway. Biochem. Biophys. Res. Commun. 2025, 777, 152319. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tao, X.; Li, B.; Cao, H.; Chen, H.; Zou, Y.; Tao, H.; Mu, M.; Wang, W.; Xu, K. C-X-C-Chemokine-Receptor-Type-4 Inhibitor AMD3100 Attenuates Pulmonary Inflammation and Fibrosis in Silicotic Mice. J. Inflamm. Res. 2022, 15, 5827–5843. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomized controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Ding, X.F.; Liang, H.Y.; Wang, D.; Zhang, X.J.; Li, L.F.; Kan, Q.C.; Wang, L.X.; Sun, T.W. Efficacy and safety of low-dose corticosteroids for acute respiratory distress syndrome: A systematic review and meta-analysis. World J. Emerg. Med. 2021, 12, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jayasimhan, D.; Mallett, S.; Hall, J. Corticosteroids in adults with acute respiratory distress syndrome: An updated evidence summary. Ther. Adv. Respir. Dis. 2023, 17, 17534666231162431. [Google Scholar]
- Sitaru, S.; Budke, A.; Bertini, R.; Sperandio, M. Therapeutic inhibition of CXCR1/2: Where do we stand? Front. Immunol. 2023, 14, 1163990. [Google Scholar] [CrossRef] [PubMed]
- Alsabani, M.; Abrams, S.T.; Cheng, Z.; Morton, B.; Lane, S.; Alosaimi, S.; Yu, W.; Wang, G.; Toh, C.H. Reduction of NETosis by targeting CXCR1/2 reduces thrombosis, lung injury, and mortality in experimental human and murine sepsis. Br. J. Anaesth. 2022, 128, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Genkel, V.; Dolgushin, I.; Baturina, I.; Savochkina, A.; Nikushkina, K.; Minasova, A.; Pykhova, L.; Sumerkina, V.; Kuznetsova, A.; Shaposhnik, I. Circulating Ageing Neutrophils as a Marker of Asymptomatic Polyvascular Atherosclerosis in Statin-Naïve Patients without Established Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 10195. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.R. Pharmacologic therapies on the horizon for acute lung injury/acute respiratory distress syndrome. J. Investig. Med. 2009, 57, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Tsutsui, M.; Shimokawa, H.; Yamashita, T.; Tanimoto, A.; Tasaki, H.; Ozumi, K.; Sabanai, K.; Morishita, T.; Suda, O.; et al. Statin treatment upregulates vascular neuronal nitric oxide synthase through the Akt/NF-κB pathway. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 92–98. [Google Scholar] [CrossRef] [PubMed]

| Drug Class/Strategy (Examples) | Mechanistic Focus in ALI/ARDS | CXCL12/CXCR4 Modulation | Key Evidence | Major Limitations | References |
|---|---|---|---|---|---|
| Direct CXCR4 Antagonists (AMD3100) | Blocks CXCL12–CXCR4; reduces leukocyte recruitment; promotes neutrophil reverse migration | Direct, complete inhibition | Reduces inflammation and fibrosis in LPS/CLP/viral ALI; clinically approved for mobilization | May hinder repair (stem cell homing); timing-sensitive; potential immune disruption | [54,56,99,100,103] |
| Natural Compounds/SPMs (ICS II, RvD1) | ICS II inhibits NETs; RvD1 enhances resolution pathways | Indirect/partial modulation | Reduce neutrophil activation and lung injury in rodent ALI | Multi-target actions; variable purity and bioavailability | [97,98] |
| Epigenetic/Molecular Modulators (miR-194/454, PTP1B inhibitors) | Reprogram immune inflammation; restrain CXCR4 signaling; neutrophil senescence | Upstream tuning of CXCR4 expression/signaling | Improve permeability, cytokine burden, and survival | Delivery limitations; off-target effects; early translational stage | [39,45,94] |
| Corticosteroids (Dexamethasone) | Broad suppression of NF-κB/MAPK and cytokines | Indirect suppression | Proven benefit in severe ARDS (COVID-19 & non-COVID trials) | Infection risk; hyperglycemia; non-specific; may delay repair | [104,105,106] |
| Other Targeted Agents (CXCR1/2 inhibitors; Statins) | Block CXCL8–driven neutrophil trafficking; stabilize endothelium | Pathway-adjacent effects intersecting CXCL12/CXCR4 | CXCR1/2 inhibitors effective in murine ALI; statins mixed/negative in ARDS | Limited efficacy/safety for CXCR1/2i; statins lack mortality benefit | [107,108,109,110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, R.; Fan, Q.; Chen, Q.; Nie, Z.; Dan, L.; Xie, S. The CXCL12/CXCR4 Axis in Sepsis-Induced Acute Lung Injury: Mechanisms and Therapeutic Potential. Curr. Issues Mol. Biol. 2025, 47, 1052. https://doi.org/10.3390/cimb47121052
Luo R, Fan Q, Chen Q, Nie Z, Dan L, Xie S. The CXCL12/CXCR4 Axis in Sepsis-Induced Acute Lung Injury: Mechanisms and Therapeutic Potential. Current Issues in Molecular Biology. 2025; 47(12):1052. https://doi.org/10.3390/cimb47121052
Chicago/Turabian StyleLuo, Renwei, Qinglu Fan, Qingyun Chen, Zhihao Nie, Lingxuan Dan, and Songping Xie. 2025. "The CXCL12/CXCR4 Axis in Sepsis-Induced Acute Lung Injury: Mechanisms and Therapeutic Potential" Current Issues in Molecular Biology 47, no. 12: 1052. https://doi.org/10.3390/cimb47121052
APA StyleLuo, R., Fan, Q., Chen, Q., Nie, Z., Dan, L., & Xie, S. (2025). The CXCL12/CXCR4 Axis in Sepsis-Induced Acute Lung Injury: Mechanisms and Therapeutic Potential. Current Issues in Molecular Biology, 47(12), 1052. https://doi.org/10.3390/cimb47121052





