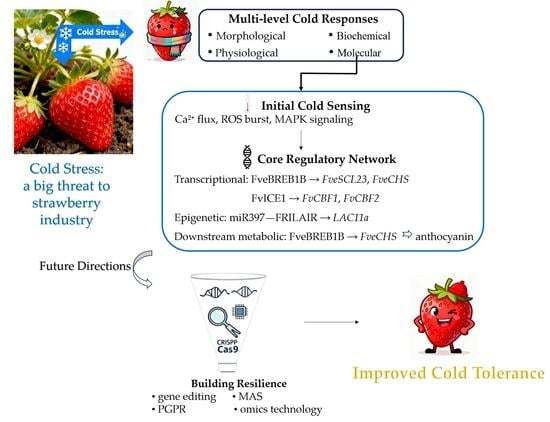
Molecular Mechanisms of Cold Stress Response in Strawberry and Breeding Strategies
Abstract
1. Introduction
Literature Search Methodology
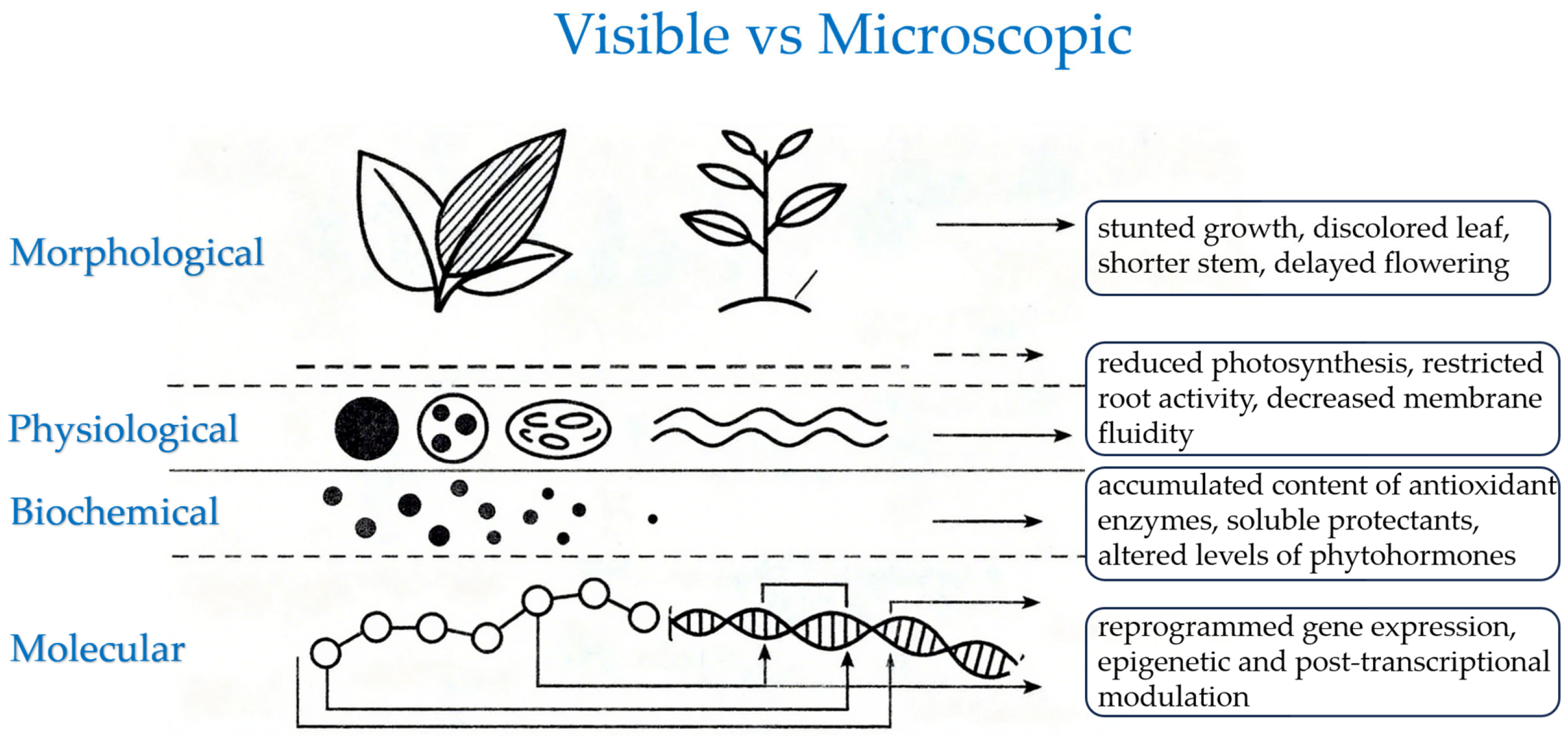
2. Effects of Cold Stress on the Morphological Traits of Strawberry Plants
3. Effects of Cold Stress on the Physiological Traits of Strawberry Plants
4. Effects of Cold Stress on the Metabolic Traits of Strawberry Plants
5. Molecular Pathways in Strawberry Plants upon Cold Stress
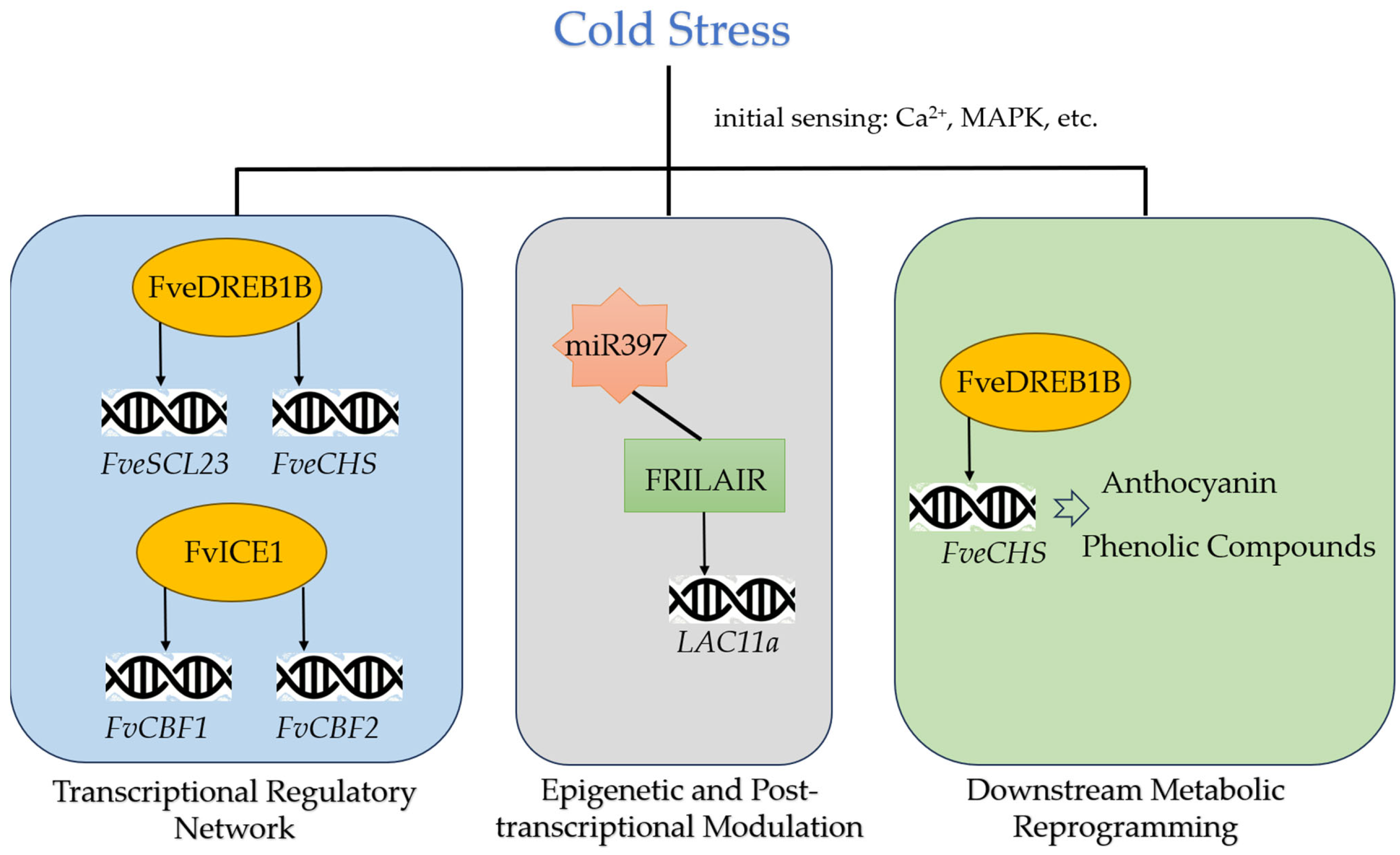
5.1. Initial Cold Sensing and Signal Transduction
5.2. Core Transcriptional Regulatory Network
5.3. Epigenetic and Post-Transcriptional Modulation
5.4. Downstream Metabolic Reprogramming and Integrated Network
6. Discussion
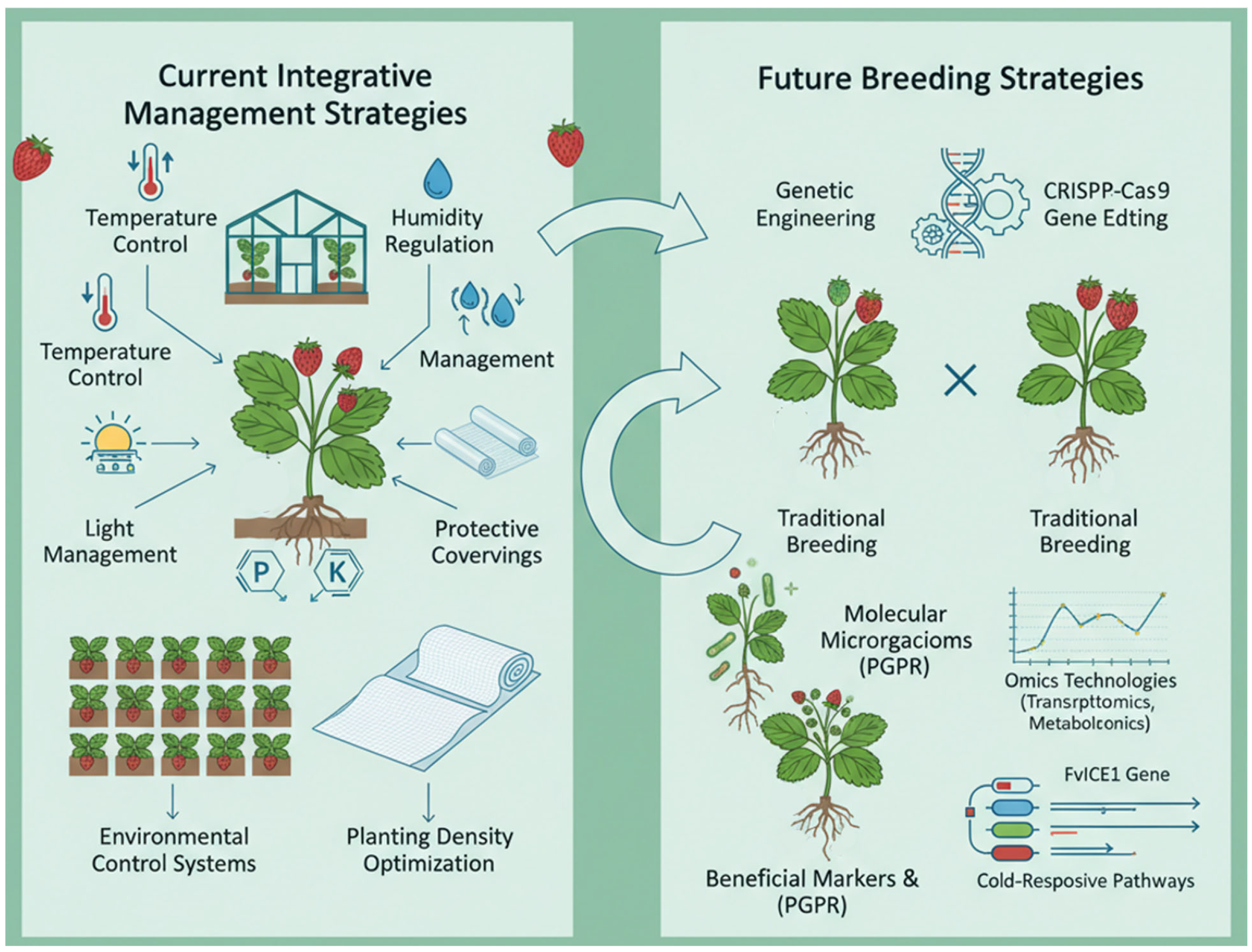
6.1. Integrative Management Strategies for Mitigating Cold Stress Damage in Strawberry Plants
6.2. Future Directions for Breeding Cold-Tolerant Strawberry Plants
6.3. Translational Potential Across Rosaceae and Integrated Breeding Frameworks
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- NetEase. 1 March 2021. Available online: https://www.163.com/dy/article/G3VRT4270519QIKK.html (accessed on 12 August 2025).
- Shanghai Observer. 2 January 2024. Available online: https://export.shobserver.com/baijiahao/html/700614.html (accessed on 15 August 2025).
- China National Radio. 11 January 2013. Available online: https://sc.cnr.cn/2012scfw/touban/2012jiaodiantu/201301/t20130111_511756739.shtml (accessed on 16 August 2025).
- Khajeh Sorkhoeih, M.; Hamidi Moghaddam, A.; Seyedi, A. Different biochemical and morphological responses of the strawberry cultivars to salicylic acid and heat shock. BMC Plant Biol. 2025, 25, 1208. [Google Scholar] [CrossRef]
- Xue, F.; Zhou, Y.; Gong, X.; Gu, X.; Dai, X.; Gu, T. Characterization of the MSI gene family in woodland strawberry Fragaria vesca and its response to cold stresses. Sci. Hortic. 2025, 351, 114384. [Google Scholar] [CrossRef]
- Shah, S.H.; Carlson, J.E.; Niklas, K.J.; Benavides-Mendoza, A.; Ricachenevsky, F.K. Editorial: Deciphering mechanisms of plant adaptation and resistance under cold temperature stress. Front. Plant Sci. 2024, 15, 1460573. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Laczkó-Dobos, H.; Szalontai, B. Lipids, Proteins, and Their Interplay in the Dynamics of Temperature-Stressed Membranes of a Cyanobacterium, Synechocystis PCC 6803. Biochemistry 2009, 48, 10120–10128. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Wei, J.; Zheng, G.; Wu, Z.; Cui, J.; Yang, X.; Li, B.; Zhu, S.; Sa, E.; et al. Effect of membrane rigidification on the BrAFP1 expression and cold-tolerance in Brassica rapa. Front. Plant Sci. 2025, 16, 1527754. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, K.P.; Liu, Y.; Kim, C. Temperature-driven changes in membrane fluidity differentially impact FILAMENTATION TEMPERATURE-SENSITIVE H2-mediated photosystem II repair. Plant Cell 2024, 37, koae323. [Google Scholar] [CrossRef]
- Kang, H.; Thomas, H.R.; Xia, X.; Shi, H.; Zhang, L.; Hong, J.; Shi, K.; Zhou, J.; Yu, J.; Zhou, Y. An integrative overview of cold response and regulatory pathways in horticultural crops. J. Integr. Plant Biol. 2025, 67, 1028–1059. [Google Scholar] [CrossRef]
- Manasa S, L.; Panigrahy, M.; Panigrahi, K.C.S.; Rout, G.R. Overview of Cold Stress Regulation in Plants. Bot. Rev. 2021, 88, 359–387. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Li, M.; Liu, L.; Xu, J.; Cheng, J.; Gang, C.; Yu, Q.; Chen, J.; Peng, C.; et al. Distribution margins as natural laboratories to infer species’ flowering responses to climate warming and implications for frost risk. Agric. For. Meteorol. 2019, 268, 299–307. [Google Scholar] [CrossRef]
- Akhatou, I.; Fernández-Recamales, A. Nutritional and nutraceutical quality of strawberries in relation to harvest time and crop conditions. J. Agric. Food Chem. 2014, 62, 5749–5760. [Google Scholar] [CrossRef] [PubMed]
- Bykova, A.V.; Meleshin, A.A.; Shchennikova, A.V.; Kochieva, E.Z. Effect of Cold Stress on Anthocyanin Content and Anthocyanin Biosynthesis Pathway Gene Expression in Potato Solanum tuberosum L. Leaves. Russ. J. Genet. 2025, 61, 809–819. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, M.; Liu, J.; Kan, J.; Zhang, M.; Xiao, L.; Yang, X.; Qi, X.; Qian, C. Combined Effects of 1-MCP and Modified Atmosphere Packaging on Flavor Quality and Volatile Profile of Cold-Stored Strawberries Revealed by Untargeted GC-MS Analysis. Foods 2025, 14, 2936. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Iwasaki, Y.; Fuke, M.; Ogiwara, I. Analysis of a High-yielding Strawberry (Fragaria × ananassa Duch.) Cultivar ‘Benihoppe’ with Focus on Root Dry Matter and Activity. J. Jpn. Soc. Hortic. Sci. 2014, 83, 142–148. [Google Scholar] [CrossRef]
- Ledesma, N.; Sugiyama, N. Pollen Quality and Performance in Strawberry Plants Exposed to High-temperature Stress. J. Am. Soc. Hortic. Sci. 2005, 130, 341–347. [Google Scholar] [CrossRef]
- Pearce, R.S.; Ashworth, E.N. Cell shape and localisation of ice in leaves of overwintering wheat during frost stress in the field. Planta 1992, 188, 324–331. [Google Scholar] [CrossRef]
- Villouta, C.; Workmaster, B.A.; Atucha, A. Freezing stress damage and growth viability in Vaccinium macrocarpon Ait. bud structures. Physiol. Plant 2021, 172, 2238–2250. [Google Scholar] [CrossRef]
- Takahashi, D.; Soga, K.; Kikuchi, T.; Kutsuno, T.; Hao, P.; Sasaki, K.; Nishiyama, Y.; Kidokoro, S.; Sampathkumar, A.; Bacic, A.; et al. Structural changes in cell wall pectic polymers contribute to freezing tolerance induced by cold acclimation in plants. Curr. Biol. 2024, 34, 958–968.e5. [Google Scholar] [CrossRef]
- Pasandi, M.; Janmohammadi, M. Effects of cold-hardening and plant developments on freezing tolerance of winter plants in a cold climate. Curr. Opin. Agric. 2015, 4, 10–18. [Google Scholar]
- Pu, X.; Fu, Y.; Xu, C.; Li, X.; Wang, W.; De, K.; Wei, X.; Yao, X. Transcriptomic analyses provide molecular insight into the cold stress response of cold-tolerant alfalfa. BMC Plant Biol. 2024, 24, 741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Wu, D.; Zou, X.; Ye, C. Studies on Relationship Between Stomata and Cold Resistance of 3 Pineapple Cultivars. J. Anhui Agric. Sci. 2013, 41, 1–3. [Google Scholar]
- Zhao, F.; Huang, W.; Zhao, X.; Zhang, L.; Guo, Y.; Wang, H.; Wang, X.; Gao, Y. Enhancing nitrogen fertilizer productivity in cotton fields in southern Xinjiang by improving the soil microenvironment through water and nitrogen management. Agric. Water Manag. 2025, 312, 109442. [Google Scholar] [CrossRef]
- Lan, Y.; Gong, F.; Liu, X.; Liu, D.; Liang, G.; Li, C.; Xia, F.; Li, Y.; Fang, C.; Cai, P. Genome-wide characterization of the cytochrome P450 gene family in Solanum melongena and functional analysis of SmCYP82C1 under cold stress. BMC Plant Biol. 2025, 25, 1018. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Wang, Y.; Mao, B.; Zhang, X.; Song, Q.; Cui, F.; Ma, Y.; Dong, J.; Wang, K.; et al. RsWRKY40 coordinates the cold stress response by integrating RsSPS1-mediated sucrose accumulation and the CBF-dependent pathway in radish (Raphanus sativus L.). Mol. Hortic. 2025, 5, 14. [Google Scholar] [CrossRef]
- Shang, X.; Zhao, Z.; Xiao, W.; Zeng, Y.; Li, M.; Jiang, X.; Dahro, B.; Chu, L.; Wang, M.; Li, C.; et al. The CtrCBL1/CtrCIPK6 Complex of Citrus Phosphorylates CtrBBX32 to Regulate CtrSTP1-Mediated Sugar Accumulation and Cold Tolerance. Adv. Sci. 2025, e08372. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Vicente-Valls, J.; Rodríguez-Azorín, L.; Gómez-Cadenas, A.; López-Climent, M.F. Physiological and biochemical adaptive strategies in citrus plants facing cold stress. Plant Growth Regul. 2025, 105, 1575–1590. [Google Scholar] [CrossRef]
- Alhasnawi, A.N. Role of proline in plant stress tolerance: A mini review. Res. Crops 2019, 20, 223–229. [Google Scholar] [CrossRef]
- Vanková, R.; Kosová, K.; Dobrev, P.; Vítámvás, P.; Trávníčková, A.; Cvikrová, M.; Pešek, B.; Gaudinová, A.; Prerostová, S.; Musilová, J.; et al. Dynamics of cold acclimation and complex phytohormone responses in Triticum monococcum lines G3116 and DV92 differing in vernalization and frost tolerance level. Environ. Exp. Bot. 2014, 101, 12–25. [Google Scholar] [CrossRef]
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. GROWTH-REGULATING FACTORS Interact with DELLAs and Regulate Growth in Cold Stress. Plant Cell 2020, 32, 1018–1034. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Chen, X.; Wu, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Foyer, C.H.; et al. SlHY5 Integrates Temperature, Light, and Hormone Signaling to Balance Plant Growth and Cold Tolerance. Plant Physiol. 2019, 179, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Shi, Y.; Liu, Z.; Zhang, X.; Gong, Z.; Yang, S. Strigolactones promote plant freezing tolerance by releasing the WRKY41-mediated inhibition of CBF/DREB1 expression. EMBO J. 2023, 42, e112999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; He, Y.; Liu, Y.; Chen, H. Functional characterization of SmCBF genes involved in abiotic stress response in eggplant (Solanum melongena). Sci. Hortic. 2018, 233, 14–21. [Google Scholar] [CrossRef]
- Liu, J.; Magwanga, R.; Xu, Y.; Wei, T.; Zheng, J.; Hou, Y.; Wang, Y.; Agong, S.; Wang, K.; Zhou, Z.; et al. Genome Wide Identification of Cotton C-Repeat Binding Factor (CBF) and Overexpression of Gthu17439 (GthCBF4) Gene Confer Cold Stress Tolerance in Arabidopsis thaliana. Preprints 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Qu, G.; Chen, S. The cold-responsive C-repeat binding factors in Betula platyphylla Suk. positively regulate cold tolerance. Plant Sci. 2024, 341, 112012. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory Networks Underlying Plant Responses and Adaptation to Cold Stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef]
- Luo, H.; Guan, Y.; Zhang, Z.; Zhang, Z.; Zhang, Z.; Li, H. FveDREB1B improves cold tolerance of woodland strawberry by positively regulating FveSCL23 and FveCHS. Plant Cell Environ. 2024, 47, 4630–4650. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Li, H.; Yao, A.; Ma, Z.; Kang, R.; Guo, Y.; Li, X.; Yu, W.; Han, D. Overexpression of a Fragaria × ananassa AP2/ERF Transcription Factor Gene (FaTINY2) Increases Cold and Salt Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2025, 26, 2109. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF–COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Ma, X.; Chen, C.; Yang, M.; Dong, X.; Lv, W.; Meng, Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Jalalian, S.; Ebrahimzadeh, A.; Zahedi, S.M.; Becker, S.J.; Hayati, F.; Hassanpouraghdam, M.B.; Rasouli, F. Chlamydomonas sp. extract meliorates the growth and physiological responses of ‘Camarosa’ strawberry (Fragaria × ananassa Duch) under salinity stress. Sci. Rep. 2024, 14, 22436. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yang, Z.; Zhang, H.; Zhang, C.; Wang, C.; Wang, N.; Xu, C. Monitoring and Risk Prediction of Low-Temperature Stress in Strawberries through Fusion of Multisource Phenotypic Spatial Variability Features. Plant Phenomics 2025, 7, 100041. [Google Scholar] [CrossRef]
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Davik, J.; Koehler, G.; From, B.; Torp, T.; Rohloff, J.; Eidem, P.; Wilson, R.C.; Sonsteby, A.; Randall, S.K.; Alsheikh, M. Dehydrin, alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.). Planta 2013, 237, 265–277. [Google Scholar] [CrossRef]
- Zimmermann, M.J.; Jathar, V.D.; Baskin, T.I. Thermomorphogenesis of the Arabidopsis thaliana Root: Flexible Cell Division, Constrained Elongation and the Role of Cryptochrome. Plant Cell Physiol. 2024, 65, 1434–1449. [Google Scholar] [CrossRef]
- He, H.; Qiao, Y.; Liu, H.; Liu, C.; Wang, C.; Zhong, Y. Research progress on the Mechansim of Chilling Injury and Alleviating Measures in Peach Fruit. Sci. Technol. Food Ind. 2023, 44, 496–505. [Google Scholar] [CrossRef]
- Lee, S.H.; Singh, A.P.; Chung, G.C.; Kim, Y.S.; Kong, I.B. Chilling root temperature causes rapid ultrastructural changes in cortical cells of cucumber (Cucumis sativus L.) root tips. J. Exp. Bot. 2002, 53, 2225–2237. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, L.; Du, J.; Zhao, X.; Song, Y.; Zhou, H.; Qiao, Y. BAG6-A from Fragaria viridis pollen modulates gametophyte development in diploid strawberry. Plant Sci. 2023, 330, 111667. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Din, N.U.; Ye, W.; Chen, J.; Deng, S.; Zhong, X.; Chen, Y. Effects of low temperature stress on photosynthetic characteristics and antioxidant defense system of flue-cured tobacco. Front. Plant Sci. 2025, 16, 1638344. [Google Scholar] [CrossRef]
- Rai, K.K. Revisiting the Critical Role of ROS and RNS in Plant Defense. J. Plant Growth Regul. 2022, 42, 6202–6227. [Google Scholar] [CrossRef]
- Turhan, E.; Aydogan, C.; Akoglu, A.; Baykul, A.; Evrenosoglu, Y. Relationship of seasonal changes in antioxidative enzymes and cold-hardiness in strawberry plant. J. Food Agric. Environ. 2012, 10, 445–450. [Google Scholar]
- Yang, F.; Tang, L.; He, H.; Kong, F.; Wang, W. Comparison of Resistant Physiological Index among Seven New Strawberry Cultivars after Low Temperature Treatment. Gansu Agric. Sci. Technol. 2021, 52, 14–17. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Gholami, M.; Khandani, Y.; Martinelli, F.; Sarikhani, H. Improving Cold Stress Tolerance of Strawberry (Fragaria × Ananassa Cv. Paros) by Exogenous Melatonin. Appl. Fruit Sci. 2025, 67, 234. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Sheng, J.; Shen, L. Tomato SlMAPK3 Modulates Cold Resistance by Regulating the Synthesis of Raffinose and the Expression of SlWRKY46. J. Agric. Food Chem. 2024, 72, 5185–5196. [Google Scholar] [CrossRef]
- Nagel, M.; Pence, V.; Ballesteros, D.; Lambardi, M.; Popova, E.; Panis, B. Plant Cryopreservation: Principles, Applications, and Challenges of Banking Plant Diversity at Ultralow Temperatures. Annu. Rev. Plant Biol. 2024, 75, 797–824. [Google Scholar] [CrossRef]
- Li, B.; Qu, S.; Kang, J.; Peng, Y.; Yang, N.; Ma, B.; Ruan, Y.L.; Ma, F.; Li, M.; Zhu, L. The MdCBF1/2-MdTST1/2 module regulates sugar accumulation in response to low temperature in apple. Plant J. 2024, 118, 787–801. [Google Scholar] [CrossRef]
- Yang, M.; Song, C.; Lin, Y.; Zhang, Y.; Li, M.; Chen, Q.; Zhang, Y.; Tang, H.; Luo, Y. FaTRAB1, a bZIP transcription factor, enhances anthocyanin biosynthesis in strawberry leaves via tissue-specific regulation. PLoS Genet. 2025, 21, e1011888. [Google Scholar] [CrossRef]
- Liu, S.; Lyu, S.; Zhang, Y.; Liu, S.; Deng, S. Tomato CONSTANS-Like1 promotes anthocyanin biosynthesis under short day and suboptimal low temperature. Plant Physiol. 2025, 198, kiaf190. [Google Scholar] [CrossRef]
- Mao, W.; Han, Y.; Chen, Y.; Sun, M.; Feng, Q.; Li, L.; Liu, L.; Zhang, K.; Wei, L.; Han, Z.; et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell 2022, 34, 1226–1249. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Balcı, G.; Aras, S.; Keles, H. Exogenous EBL (24-Epibrassinolide) Alleviate Cold Damage in Strawberry. Erwerbs-Obstbau 2021, 63, 273–278. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; He, Y.; Hu, W.; Zhang, Y.; Wang, X.; Tang, H. Identification of NADPH oxidase family members associated with cold stress in strawberry. FEBS Open Bio 2018, 8, 593–605. [Google Scholar] [CrossRef]
- Jin, S.; Zhong, X.; Hu, Z.; Jiang, Z. Ca2+ flux in plant responses to abiotic stress. J. Plant Physiol. 2025, 315, 154648. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, H.; Su, C.; Qi, Y.; Liu, X.; Pu, J. Genome-wide identification and expression profile analysis of the NAC transcription factor family during abiotic and biotic stress in woodland strawberry. PLoS ONE 2018, 13, e0197892. [Google Scholar] [CrossRef]
- Prohaska, A.; Petit, A.; Lesemann, S.; Rey-Serra, P.; Mazzoni, L.; Masny, A.; Sánchez-Sevilla, J.F.; Potier, A.; Gaston, A.; Klamkowski, K.; et al. Strawberry phenotypic plasticity in flowering time is driven by the interaction between genetic loci and temperature. J. Exp. Bot. 2024, 75, 5923–5939. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Li, W.; Yang, Q.; Li, Z.; Cheng, Z.; Lv, L.; Zhang, L.; Han, D. Isolation and preliminary functional analysis of FvICE1, involved in cold and drought tolerance in Fragaria vesca through overexpression and CRISPR/Cas9 technologies. Plant Physiol. Biochem. 2023, 196, 270–280. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Ou, Y.; Yin, M.; Chen, T.; Zeng, X.; Li, R.; He, Y. A pair of readers of bivalent chromatin mediate formation of Polycomb-based “memory of cold” in plants. Mol. Cell 2023, 83, 1109–1124.e4. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, C.J.; Shen, M.; Park, H.J.; Cha, J.Y.; Iniesto, E.; Rubio, V.; Mengiste, T.; Zhu, J.K.; Bressan, R.A.; et al. Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc. Natl. Acad. Sci. USA 2018, 115, E5400–E5409. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, J.; Li, D.; Wang, Z.; Peng, C.; Zhu, G. The haplotype-resolved T2T genome assembly of the wild potato species Solanum commersonii provides molecular insights into its freezing tolerance. Plant Commun. 2024, 5, 100980. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qu, Z.; Lei, J.; He, R.; Adelson, D.L.; Zhu, Y.; Yang, Z.; Wang, D. The long noncoding RNA FRILAIR regulates strawberry fruit ripening by functioning as a noncanonical target mimic. PLoS Genet. 2021, 17, e1009461. [Google Scholar] [CrossRef]
- Li, J.; Lai, T.; Song, H.; Xu, X. MiR164 is involved in delaying senescence of strawberry (Fragaria ananassa) fruit by negatively regulating NAC transcription factor genes under low temperature. Russ. J. Plant Physiol. 2017, 64, 251–259. [Google Scholar] [CrossRef]
- Kanbar, A.; Weinert, C.H.; Kottutz, D.; Thinh, L.; Abuslima, E.; Kabil, F.; Hazman, M.; Egert, B.; Trierweiler, B.; Kulling, S.E.; et al. Cold tolerance of woodland strawberry (Fragaria vesca) is linked to Cold Box Factor 4 and the dehydrin Xero2. J. Exp. Bot. 2024, 75, 5857–5879. [Google Scholar] [CrossRef]
- Malekzadeh, M.R.; Roosta, H.R.; Kalaji, H.M. Enhancing strawberry resilience to saline, alkaline, and combined stresses with light spectra: Impacts on growth, enzymatic activity, nutrient uptake, and osmotic regulation. BMC Plant Biol. 2024, 24, 1038. [Google Scholar] [CrossRef]
- Wei, B.; Bi, Y.; Tang, Z.; Sun, H.; Qian, M.; Wang, J.; Meng, Y.; Zhao, J. Effects of substrate warming system on growth and development of strawberry frame cultivation in greenhouse. Acta Agric. Shanghai 2020, 36, 139–145. [Google Scholar]
- Koehler, G.; Wilson, R.C.; Goodpaster, J.V.; Sønsteby, A.; Lai, X.; Witzmann, F.A.; You, J.S.; Rohloff, J.; Randall, S.K.; Alsheikh, M. Proteomic study of low-temperature responses in strawberry cultivars (Fragaria x ananassa) that differ in cold tolerance. Plant Physiol. 2012, 159, 1787–1805. [Google Scholar] [CrossRef]
- Xing, S.; Jia, M.; Wei, L.; Mao, W.; Abbasi, U.A.; Zhao, Y.; Chen, Y.; Cao, M.; Zhang, K.; Dai, Z.; et al. CRISPR/Cas9-introduced single and multiple mutagenesis in strawberry. J. Genet. Genom. 2018, 45, 685–687. [Google Scholar] [CrossRef]
- Qiang, S.; Xie, H. A Method for Epigenetic Manipulation of Plant Phenotypic Plasticity Traits. CN Patent CN110637087A, 3 July 2018. [Google Scholar]
- Marian, M.; Antonielli, L.; Pertot, I.; Perazzolli, M. Amplicon sequencing and culture-dependent approaches reveal core bacterial endophytes aiding freezing stress tolerance in alpine Rosaceae plants. mBio 2025, 16, e0141824. [Google Scholar] [CrossRef]
- Nanjing University of Information Science and Technology. A Manufacturing Method and Drawings for a Phenotype-Based Fusion Approach and Device for Early Warning of Cold Damage Risk and Stress Detection in Strawberries. CN Patent 43881125, 31 December 2024.
- Nanjing University of Information Science and Technology. A Method and Device for Early Warning and Stress Detection of Strawberry Chilling Injury Risk Based on Phenotypic Fusion. CN Patent CN202411710169.6, 2 May 2025.
- Qiao, J.; Guo, M.; Wu, Y.; Gao, J.; Yue, Z. Research on Strawberry Cold Chain Transportation Quality Perception Method Based on BP Neural Network. Appl. Sci. 2022, 12, 8872. [Google Scholar] [CrossRef]
- Elashmawy, R.; Doron, M.; Kanjilal, R.; Brecht, J.K.; Uysal, I. The digital cold chain: Sensor-driven product quality with AI. Postharvest Biol. Technol. 2025, 230, 113714. [Google Scholar] [CrossRef]
- Han, X.; Liang, X.; Li, D.; Song, M.; Ma, Z.; Li, R.; Meng, H.; Cai, Y.; Song, B.; Liu, Z.; et al. A native visual screening reporter-assisted CRISPR/Cas9 system for high-efficient genome editing in strawberry. Mol. Hortic. 2025, 5, 29. [Google Scholar] [CrossRef]
| Gene/Protein | Species | Type | Main Function in Cold Response | Evidence (Overexpression/Knockout) | Interacting Partners/Pathway |
|---|---|---|---|---|---|
| FvICE1 | F. vesca | TF (bHLH) | Master regulator; enhances tolerance, positively regulates FvCBF1/2 | OE: Tolerance ↑, KO: Tolerance ↓ [74] | Upstream of CBFs |
| FveDREB1B | F. vesca | TF (AP2/ERF) | Binds promoters of FveSCL23, FveCHS | OE: Tolerance ↑ [42] | CBF/DREB core |
| FaTINY2 | F. x ananassa | TF (AP2/ERF) | Enhances antioxidant capacity (SOD, CAT, POD), increases proline | OE in Arabidopsis: Tolerance ↑ [43] | CBF/DREB-related |
| FvMAPK3 | F. vesca | Kinase | Phosphorylated by FvMKK4/FvSnRK2.6; phosphorylates FvMYB10 | Functional analysis [66] | MAPK signaling |
| FvMSI4/FVE | F. vesca | Scaffold | Recruits FvHDA6/FvHOS1 complex; represses flowering | Functional analysis [6] | Epigenetic repression |
| miR164 | F. x ananassa | miRNA | Negatively regulates NAC TFs; delays fruit senescence | Expression analysis [79] | Post-transcriptional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yu, J.; Wang, S.; Qiao, R.; Shen, J.; Li, W.; Zhou, F.; Li, X. Molecular Mechanisms of Cold Stress Response in Strawberry and Breeding Strategies. Curr. Issues Mol. Biol. 2025, 47, 966. https://doi.org/10.3390/cimb47110966
Zhang X, Yu J, Wang S, Qiao R, Shen J, Li W, Zhou F, Li X. Molecular Mechanisms of Cold Stress Response in Strawberry and Breeding Strategies. Current Issues in Molecular Biology. 2025; 47(11):966. https://doi.org/10.3390/cimb47110966
Chicago/Turabian StyleZhang, Xiang, Jiajie Yu, Shuang Wang, Rongjia Qiao, Jianjun Shen, Weixiao Li, Fei Zhou, and Xiaohong Li. 2025. "Molecular Mechanisms of Cold Stress Response in Strawberry and Breeding Strategies" Current Issues in Molecular Biology 47, no. 11: 966. https://doi.org/10.3390/cimb47110966
APA StyleZhang, X., Yu, J., Wang, S., Qiao, R., Shen, J., Li, W., Zhou, F., & Li, X. (2025). Molecular Mechanisms of Cold Stress Response in Strawberry and Breeding Strategies. Current Issues in Molecular Biology, 47(11), 966. https://doi.org/10.3390/cimb47110966