The Complex Role of MAPK/ERK Signaling Pathway in Different Types of Thrombocytopenia
Abstract
1. Introduction
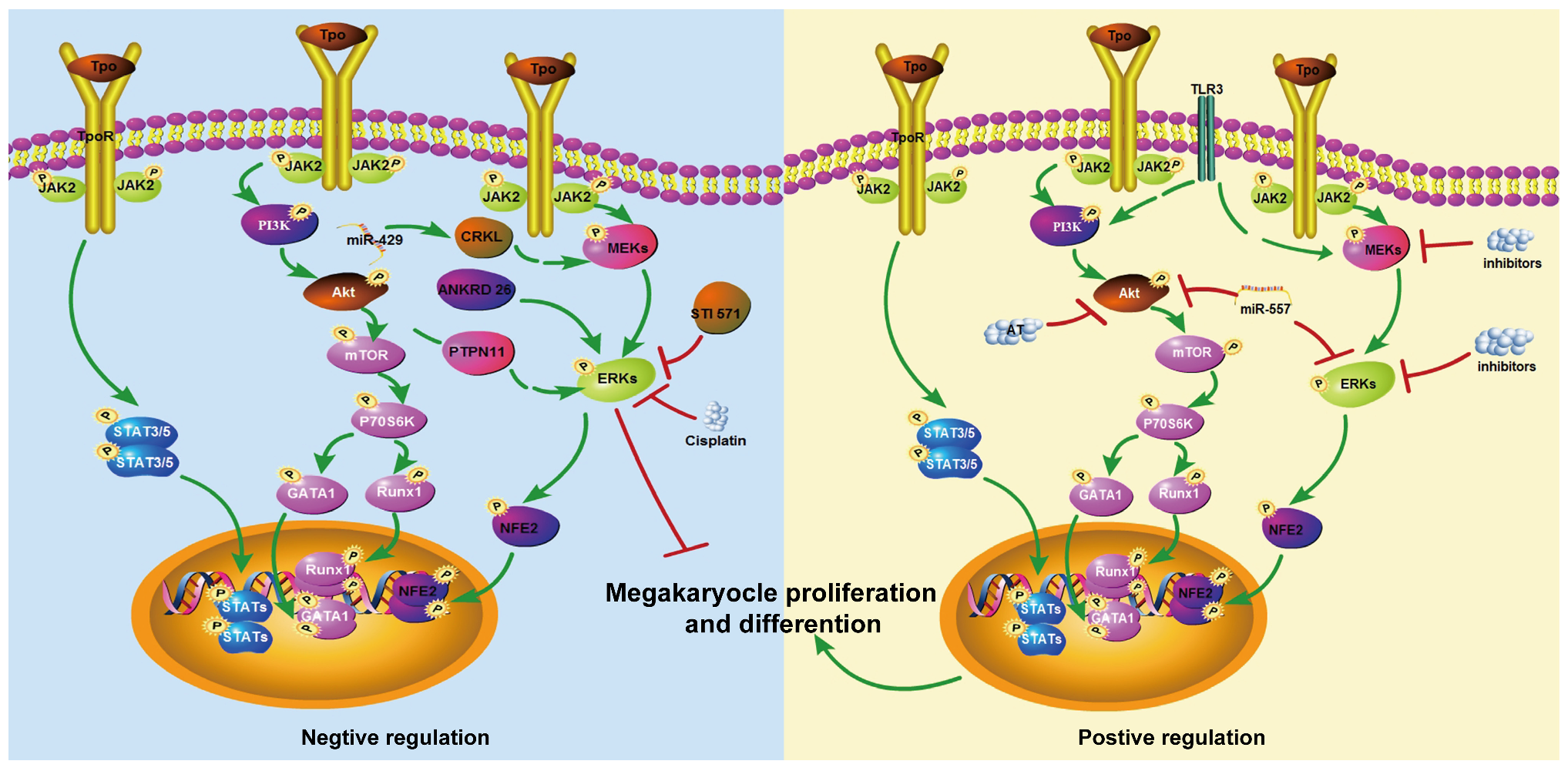
2. The Role of MAPK/ERK Signaling Pathway in the Formation of MK-Platelet Differentiation
2.1. Evidence Supporting a Positive Role of ERK/MAPK in Megakaryopoiesis
2.2. Studies Reporting Neutral or Inhibitory Roles
2.3. Possible Mechanistic Explanations for These Differences
| Signal Pathway | Cell Function | Correlation | References |
|---|---|---|---|
| ERK sustained activation | Megakaryocyte differentiation | + | [15] |
| MAPK pathway activation | Megakaryocyte differentiation | + | [16] |
| p-ERK increasing | Differentiation and ploidization of K562 and HEL cells | + | [18,20] |
| ERK activation | Cells differentiate to become erythroid or megakaryocytic | + | [19] |
| MAPK/ERK pathway activation | Mouse platelet levels | + | [21] |
| MEK and ERK activation | Megakaryocytes ploidy and platelets formation | + | [22,23,24,25] |
| ERK activation | Megakaryocyte differentiation | + | [26] |
| ERK activation | Human cells megakaryopoiesis | + | [27] |
| ERK 1/2 activation | Immature megakaryocytes proliferation | − | [28] |
| MAPK pathway was not regulated | Megakaryocyte differentiation of K562 | − | [29] |
| Phosphorylation of MAPK pathway was not elevated | Myelodys-plastic syndromes | − | [30] |
| ERK inhibited | MK polyploidy formation enhanced | − | [35] |
| ERK sustained activation | MK polyploidy and proplatelet formation decreasing | − | [32] |
| ERK dephosphorylation | K562 megakaryocyte markers increasing | − | [33] |
3. Aberrant MAPK/ERK Signaling Pathway and Thrombocytopenia
3.1. Immune Thrombocytopenia
3.2. Inherited Thrombocytopenia
3.3. Tumor and Tumor Therapy-Induced Thrombocytopenia
3.3.1. Hematologic Malignancies Induced Thrombocytopenia
3.3.2. Solid Tumors Therapy-Induced Thrombocytopenia
3.4. Thrombosis-Induced Thrombocytopenia
3.5. Inflammation and Infectious-Induced Thrombocytopenia
4. Drug Basis on ERK/MAPK Signaling Pathway for the Treatment of Thrombocytopenia Disease
4.1. Approved Therapies
4.2. Experimental or Preclinical Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| aPL | antiphospholipid antibodies |
| APS | antiphospholipid syndrome |
| AT | atorvastatin |
| BTK | bruton tyrosine kinase |
| BVDV | the bovine viral diarrhea virus |
| c-MPL | c-mannosylation of thrombopoietin receptor |
| CIC | circulating immune complexes |
| CP | cytopathic |
| CPM | Caulis Polygoni Multiflori |
| DMAG | 3,8-di-O-methylellagic acid 2-O-glucoside |
| ERK | extracellular signal-regulated kinase |
| GC | carboplatin |
| IL-2 | interleukin-2 |
| I-MFA | the inhibitor of MyoD Family A |
| JMML | juvenile myelomonocytic leukemia |
| MAPK | mitogen-activated protein kinase |
| MKs | megakaryocytes |
| mTOR | mammalian target of rapamycin |
| MVEC | microvascular endothelial cell |
| NCP | non-cytopathic |
| ITP | immune thrombocytopenia |
| p38 | anti-P38 MAPK |
| PI3K | phosphatidylinositol 3-kinase |
| PMA | phorbol ester |
| RAS | activation of rat sarcoma virus |
| rhTPO | recombinant human thrombopoietin |
| (R)-TEMOSPho | (R)-3-methoxy-3-oxo-2-stearamidopropyl phosphate |
| SLE | systemic lupus erythematosus |
| STAT | signal transducer and activator of transcription |
| THC2 | thrombocytopenia 2 |
| TNFα | tumor necrosis factor α |
| TPOR | thrombopoietin receptor |
| TTP | thrombotic thrombocytopenic purpura |
| XAT | xanthotoxin |
References
- Smock, K.J.; Perkins, S.L. Thrombocytopenia: An update. Int. J. Lab. Hematol. 2014, 36, 269–278. [Google Scholar] [CrossRef]
- George, J.N. Platelets. Lancet 2000, 355, 1531–1539. [Google Scholar] [CrossRef]
- Pene, F.; Russell, L.; Aubron, C. Thrombocytopenia in the intensive care unit: Diagnosis and management. Ann. Intensive Care 2025, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Neal, M.D.; Herman, J.H. Bacterial contamination of platelets for transfusion: Strategies for prevention. Crit. Care 2018, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Ghanima, W.; Cooper, N.; Rodeghiero, F.; Godeau, B.; Bussel, J.B. Thrombopoietin receptor agonists: Ten years later. Haematologica 2019, 104, 1112–1123. [Google Scholar] [CrossRef]
- Vianelli, N.; Auteri, G.; Buccisano, F.; Carrai, V.; Baldacci, E.; Clissa, C.; Bartoletti, D.; Giuffrida, G.; Magro, D.; Rivolti, E.; et al. Refractory primary immune thrombocytopenia (ITP): Current clinical challenges and therapeutic perspectives. Ann. Hematol. 2022, 101, 963–978. [Google Scholar] [CrossRef]
- Poston, J.N.; Gernsheimer, T.B. Glucocorticoids promote response to thrombopoietin-receptor agonists in refractory ITP: A case series. Int. J. Hematol. 2019, 110, 255–259. [Google Scholar] [CrossRef]
- van den Oudenrijn, S.; Bruin, M.; Folman, C.C.; Peters, M.; Faulkner, L.B.; de Haas, M.; von dem Borne, A.E. Mutations in the thrombopoietin receptor, Mpl, in children with congenital amegakaryocytic thrombocytopenia. Br. J. Haematol. 2000, 110, 441–448. [Google Scholar] [CrossRef]
- Ballmaier, M.; Germeshausen, M. Congenital amegakaryocytic thrombocytopenia: Clinical presentation, diagnosis, and treatment. Semin. Thromb. Hemost. 2011, 37, 673–681. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirouchi, T.; Yoshioka, H.; Watanabe, J.; Kashiwakura, I. Diverse functions of the thrombopoietin receptor agonist romiplostim rescue individuals exposed to lethal radiation. Free Radic. Biol. Med. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Zeng, J.; Wu, A.; Qin, M.; Wen, M.; Zhang, T.; Chen, W.; Mei, Q.; Qin, D.; et al. Caulis Polygoni Multiflori Accelerates Megakaryopoiesis and Thrombopoiesis via Activating PI3K/Akt and MEK/ERK Signaling Pathways. Pharmaceuticals 2022, 15, 1204. [Google Scholar] [CrossRef]
- Moroi, A.J.; Watson, S.P. Akt and mitogen-activated protein kinase enhance C-type lectin-like receptor 2-mediated platelet activation by inhibition of glycogen synthase kinase 3α/β. J. Thromb. Haemost. 2015, 13, 1139–1150. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83, Erratum in Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Masoumi, Z.; James, S.C.; Tucker, J.A.; Winkelmann, H.; Grey, W.; Picton, L.K.; Moss, L.; Wilson, S.C.; Caveney, N.A.; et al. Structure of the thrombopoietin-MPL receptor complex is a blueprint for biasing hematopoiesis. Cell 2023, 186, 4189–4203.e22. [Google Scholar] [CrossRef]
- Racke, F.K.; Lewandowska, K.; Goueli, S.; Goldfarb, A.N. Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J. Biol. Chem. 1997, 272, 23366–23370. [Google Scholar] [CrossRef]
- Melemed, A.S.; Ryder, J.W.; Vik, T.A. Activation of the mitogen-activated protein kinase pathway is involved in and sufficient for megakaryocytic differentiation of CMK cells. Blood 1997, 90, 3462–3470. [Google Scholar] [CrossRef] [PubMed]
- Ezumi, Y.; Uchiyama, T.; Takayama, H. Thrombopoietin potentiates the protein-kinase-C-mediated activation of mitogen-activated protein kinase/ERK kinases and extracellular signal-regulated kinases in human platelets. Eur. J. Biochem. 1998, 258, 976–985. [Google Scholar] [CrossRef]
- Levay, K.; Slepak, V.Z. Tescalcin is an essential factor in megakaryocytic differentiation associated with Ets family gene expression. J. Clin. Investig. 2007, 117, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kirito, K.; Shimizu, R.; Miura, Y.; Ozawa, K.; Komatsu, N. A functional role of mitogen-activated protein kinases, erk1 and erk2, in the differentiation of a human leukemia cell line, UT-7/GM: A possible key factor for cell fate determination toward erythroid and megakaryocytic lineages. Int. J. Hematol. 2001, 73, 78–83. [Google Scholar] [CrossRef]
- fPettiford, S.M.; Herbst, R. The protein tyrosine phosphatase HePTP regulates nuclear translocation of ERK2 and can modulate megakaryocytic differentiation of K562 cells. Leukemia 2003, 17, 366–378. [Google Scholar] [CrossRef]
- Hakak, Y.; Lehmann-Bruinsma, K.; Phillips, S.; Le, T.; Liaw, C.; Connolly, D.T.; Behan, D.P. The role of the GPR91 ligand succinate in hematopoiesis. J. Leukoc. Biol. 2009, 85, 837–843. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Drachman, J.G.; Kaushansky, K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: Role in endomitosis. Blood 1999, 94, 1273–1282. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Tan, P.; Xu, P.; Zhang, Q. Overexpression of nuclear distribution protein (hNUDC) causes pro-apoptosis and differentiation in Dami megakaryocytes. Cell Prolif. 2013, 46, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Limb, J.K.; Song, D.; Jeon, M.; Han, S.Y.; Han, G.; Jhon, G.J.; Bae, Y.S.; Kim, J. 2-(trimethylammonium)ethyl (R)-3-methoxy-3-oxo-2-stearamidopropyl phosphate promotes megakaryocytic differentiation of myeloid leukaemia cells and primary human CD34+ haematopoietic stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 435–446. [Google Scholar] [CrossRef]
- Liu, J.; Song, Q.; Huang, Y.; Sun, W.; Lu, D.; Zhou, B. R-lipoic acid overdosing affects platelet life span via ROS mediated autophagy. Platelets 2018, 29, 695–701. [Google Scholar] [CrossRef]
- Ha, S.H.; Kwak, C.H.; Park, J.Y.; Abekura, F.; Lee, Y.C.; Kim, J.S.; Chung, T.W.; Kim, C.H. 3′-sialyllactose targets cell surface protein, SIGLEC-3, and induces megakaryocyte differentiation and apoptosis by lipid raft-dependent endocytosis. Glycoconj. J. 2020, 37, 187–200. [Google Scholar] [CrossRef]
- Di Buduo, C.A.; Currao, M.; Pecci, A.; Kaplan, D.L.; Balduini, C.L.; Balduini, A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica 2016, 101, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Mazharian, A.; Watson, S.P.; Séverin, S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp. Hematol. 2009, 37, 1238–1249.e5. [Google Scholar] [CrossRef]
- Shelly, C.; Petruzzelli, L.; Herrera, R. PMA-induced phenotypic changes in K562 cells: MAPK-dependent and -independent events. Leukemia 1998, 12, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Kalina, U.; Hofmann, W.K.; Koschmieder, S.; Wagner, S.; Kauschat, D.; Hoelzer, D.; Ottmann, O.G. Alteration of c-mpl-mediated signal transduction in CD34(+) cells from patients with myelodysplastic syndromes. Exp. Hematol. 2000, 28, 1158–1163. [Google Scholar] [CrossRef]
- Ito, T.; Fujihara, M.; Oda, A.; Wakamoto, S.; Yamaguchi, M.; Komatsu, N.; Miyazaki, H.; Azuma, H.; Ikeda, H.; Ikebuchi, K. Thrombopoietin upregulates nucleolin mRNA and protein in thrombopoietin-dependent megakaryocytic cell line, UT-7/TPO. Mol. Cell Biochem. 2003, 247, 75–82. [Google Scholar] [CrossRef]
- Bluteau, D.; Balduini, A.; Balayn, N.; Currao, M.; Nurden, P.; Deswarte, C.; Leverger, G.; Noris, P.; Perrotta, S.; Solary, E.; et al. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J. Clin. Investig. 2014, 124, 580–591. [Google Scholar] [CrossRef]
- Kohmura, K.; Miyakawa, Y.; Kawai, Y.; Ikeda, Y.; Kizaki, M. Different roles of p38 MAPK and ERK in STI571-induced multi-lineage differentiation of K562 cells. J. Cell Physiol. 2004, 198, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Séverin, S.; Ghevaert, C.; Mazharian, A. The mitogen-activated protein kinase signaling pathways: Role in megakaryocyte differentiation. J. Thromb. Haemost. 2010, 8, 17–26. [Google Scholar] [CrossRef]
- Gibellini, D.; Re, M.C.; Bassini, A.; Guidotti, L.; Catani, L.; La Placa, M.; Zauli, G. HIV-1 gp120 induces the activation of both c-fos and c-jun immediate-early genes in HEL megakaryocytic cells. Br. J. Haematol. 1999, 104, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Zhang, X.; Zhao, H.; Zhang, B.; Wu, Y.; Zhang, J. The role and mechanism of miR-557 in inhibiting the differentiation and maturation of megakaryocytes in immune thrombocytopenia. RNA Biol. 2021, 18, 1953–1968. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.Y.; Kang, Y.H.; Zhang, Y.; Chen, X.Y.; Zhou, J.L.; Ma, W. Modified Sijunzi Granules Exhibit Hemostatic Effect by Activating Akt and Erk Signal Pathways via Regulating 5-HT and Its Receptors Levels. Chin. J. Integr. Med. 2024, 30, 1121–1127. [Google Scholar] [CrossRef]
- D’Atri, L.P.; Etulain, J.; Rivadeneyra, L.; Lapponi, M.J.; Centurion, M.; Cheng, K.; Yin, H.; Schattner, M. Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J. Thromb. Haemost. 2015, 13, 839–850. [Google Scholar] [CrossRef]
- Kostyak, J.C.; Liverani, E.; Kunapuli, S.P. PKC-epsilon deficiency alters progenitor cell populations in favor of megakaryopoiesis. PLoS ONE 2017, 12, e0182867. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Y.; Yu, T.; Yu, Y.; Ni, X.; Wang, H.; Sun, L.; Han, P.; Wang, L.; Sun, T.; et al. Atorvastatin restores imbalance of cluster of differentiation 4 (CD4)(+) T cells in immune thrombocytopenia in vivo and in vitro. Br. J. Haematol. 2023, 201, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Zlatopolskiy, A.; Raife, T.J.; Laurence, J. Thienopyridine-linked thrombotic microangiopathy: Association with endothelial cell apoptosis and activation of MAP kinase signalling cascades. Br. J. Haematol. 2004, 124, 200–210. [Google Scholar] [CrossRef]
- Kyrmizi, I.; Ioannou, M.; Hatziapostolou, M.; Tsichlis, P.N.; Boumpas, D.T.; Tassiulas, I. Tpl2 kinase regulates FcγR signaling and immune thrombocytopenia in mice. J. Leukoc. Biol. 2013, 94, 751–757. [Google Scholar] [CrossRef]
- Sprague, D.L.; Elzey, B.D.; Crist, S.A.; Waldschmidt, T.J.; Jensen, R.J.; Ratliff, T.L. Platelet-mediated modulation of adaptive immunity: Unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood 2008, 111, 5028–5036. [Google Scholar] [CrossRef]
- Lentaigne, C.; Freson, K.; Laffan, M.A.; Turro, E.; Ouwehand, W.H. Inherited platelet disorders: Toward DNA-based diagnosis. Blood 2016, 127, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Palma-Barqueros, V.; Revilla, N.; Zaninetti, C.; Galera, A.M.; Sánchez-Fuentes, A.; Zámora-Cánovas, A.; Bohdan, N.; Padilla, J.; Marín-Quilez, A.; Rodriguez-Alen, A.; et al. Src-related thrombocytopenia: A fine line between a megakaryocyte dysfunction and an immune-mediated disease. Blood Adv. 2022, 6, 5244–5255. [Google Scholar] [CrossRef]
- Marconi, C.; Canobbio, I.; Bozzi, V.; Pippucci, T.; Simonetti, G.; Melazzini, F.; Angori, S.; Martinelli, G.; Saglio, G.; Torti, M.; et al. 5′UTR point substitutions and N-terminal truncating mutations of ANKRD26 in acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 18. [Google Scholar] [CrossRef]
- Matsumura, I.; Kanakura, Y. Molecular control of megakaryopoiesis and thrombopoiesis. Int. J. Hematol. 2002, 75, 473–483. [Google Scholar] [CrossRef]
- Liebman, H.A. Thrombocytopenia in cancer patients. Thromb. Res. 2014, 133 (Suppl. 2), S63–S69. [Google Scholar] [CrossRef]
- Rahul, E.; Ningombam, A.; Acharya, S.; Tanwar, P.; Ranjan, A.; Chopra, A. Large granular lymphocytic leukemia: A brief review. Am. J. Blood Res. 2022, 12, 17–32. [Google Scholar] [PubMed]
- Guo, C.; Lv, X.; Zhang, Q.; Yi, L.; Ren, Y.; Li, Z.; Yan, J.; Zheng, S.; Sun, M.Z.; Liu, S. CRKL but not CRKII contributes to hemin-induced erythroid differentiation of CML. J. Cell Mol. Med. 2024, 28, e18308. [Google Scholar] [CrossRef] [PubMed]
- Houser, J.S.; Patel, M.; Wright, K.; Onopiuk, M.; Tsiokas, L.; Humphrey, M.B. The inhibitor of MyoD Family A (I-MFA) regulates megakaryocyte lineage commitment and terminal differentiation. Blood Cells Mol. Dis. 2023, 102, 102760. [Google Scholar] [CrossRef]
- Ramdas, B.; Yuen, L.D.; Palam, L.R.; Patel, R.; Pasupuleti, S.K.; Jideonwo, V.; Zhang, J.; Maguire, C.; Wong, E.; Kanumuri, R.; et al. Inhibition of BTK and PI3Kδ impairs the development of human JMML stem and progenitor cells. Mol. Ther. 2022, 30, 2505–2521. [Google Scholar] [CrossRef]
- Wintering, A.; Dvorak, C.C.; Stieglitz, E.; Loh, M.L. Juvenile myelomonocytic leukemia in the molecular era: A clinician’s guide to diagnosis, risk stratification, and treatment. Blood Adv. 2021, 5, 4783–4793. [Google Scholar] [CrossRef]
- Tarnawsky, S.P.; Kobayashi, M.; Chan, R.J.; Yoder, M.C. Mice expressing KrasG12D in hematopoietic multipotent progenitor cells develop neonatal myeloid leukemia. J. Clin. Investig. 2017, 127, 3652–3656. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, Y.; Zhou, Z. Recombinant human thrombopoietin combined with interleukin-2 improves the effects of chemosensitivity and thrombocytopenia on a basic gemcitabine and carboplatin combination therapy for non-small cell lung cancer in a nude mouse model. J. Thorac. Dis. 2019, 11, 4671–4681. [Google Scholar] [CrossRef]
- Korcheva, V.; Wong, J.; Corless, C.; Iordanov, M.; Magun, B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am. J. Pathol. 2005, 166, 323–339. [Google Scholar] [CrossRef]
- Davies, M.A.; Fox, P.S.; Papadopoulos, N.E.; Bedikian, A.Y.; Hwu, W.J.; Lazar, A.J.; Prieto, V.G.; Culotta, K.S.; Madden, T.L.; Xu, Q.; et al. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin. Cancer Res. 2012, 18, 1120–1128. [Google Scholar] [CrossRef]
- Joly, F.; Fabbro, M.; Follana, P.; Lequesne, J.; Medioni, J.; Lesoin, A.; Frenel, J.S.; Abadie-Lacourtoisie, S.; Floquet, A.; Gladieff, L.; et al. A phase II study of Navitoclax (ABT-263) as single agent in women heavily pretreated for recurrent epithelial ovarian cancer: The MONAVI-GINECO study. Gynecol. Oncol. 2022, 165, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, L.; Liu, J.; Du, J.; Wang, Z.; Ruan, C.; Dai, K. Cisplatin induces platelet apoptosis through the ERK signaling pathway. Thromb. Res. 2012, 130, 81–91. [Google Scholar] [CrossRef]
- Brusch, A. The Significance of Anti-Beta-2-Glycoprotein I Antibodies in Antiphospholipid Syndrome. Antibodies 2016, 5, 16. [Google Scholar] [CrossRef]
- Naranjo, L.; Stojanovich, L.; Djokovic, A.; Andreoli, L.; Tincani, A.; Maślińska, M.; Sciascia, S.; Infantino, M.; Garcinuño, S.; Kostyra-Grabczak, K.; et al. Circulating immune-complexes of IgG/IgM bound to B2-glycoprotein-I associated with complement consumption and thrombocytopenia in antiphospholipid syndrome. Front. Immunol. 2022, 13, 957201. [Google Scholar] [CrossRef]
- Vega-Ostertag, M.; Harris, E.N.; Pierangeli, S.S. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004, 50, 2911–2919. [Google Scholar] [CrossRef]
- Chu, T.; Hu, S.; Qi, J.; Li, X.; Zhang, X.; Tang, Y.; Yang, M.; Xu, Y.; Ruan, C.G.; Han, Y.; et al. Bifunctional effect of the inflammatory cytokine tumor necrosis factor α on megakaryopoiesis and platelet production. J. Thromb. Haemost. 2022, 20, 2998–3010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ni, X.; Xu, P.; Liu, Q.; Sun, T.; Liu, X.; Ji, X.; Qiu, J.; Li, J.; Wang, S.; et al. Interleukin-37 reduces inflammation and impairs phagocytosis of platelets in immune thrombocytopenia (ITP). Cytokine 2020, 125, 154853, Corrigendum to Cytokine 2022, 155, 155900. [Google Scholar] [CrossRef]
- Whiteheart, S.W. Platelet-HIV: Interactions and their implications. Platelets 2022, 33, 208–211. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Chen, N.; Li, Y.; Fan, C.; Zhao, S.; Bai, T.; Zhao, Z.; Chen, J.; Su, S.; et al. PD-1 Blockade Restores the Proliferation of Peripheral Blood Lymphocyte and Inhibits Lymphocyte Apoptosis in a BALB/c Mouse Model of CP BVDV Acute Infection. Front. Immunol. 2021, 12, 727254. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Song, X.; Zhang, Y.; Lin, N.; Wang, J.; Dai, Q. Investigation of the mechanism of action of Shengxuexiaoban Capsules against primary immune thrombocytopenia using network pharmacology and experimental validation. Phytomedicine 2022, 106, 154413. [Google Scholar] [CrossRef]
- Carrara, C.; Mataj, B.; Gastoldi, S.; Ruggenenti, P.; Sciascia, S.; Roccatello, D. Case report: Timing of eculizumab treatment in catastrophic antiphospholipid syndrome. Front. Immunol. 2024, 15, 1460317. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhao, H.; Bao, X.; Fu, H.; Lou, L. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist. J. Cell. Mol. Med. 2018, 22, 5367–5377. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Shen, X.; Sun, Y.; Jiang, N.; Zeng, J.; Lin, J.; Yue, L.; Lai, J.; Li, Y.; et al. TMEA, a Polyphenol in Sanguisorba officinalis, Promotes Thrombocytopoiesis by Upregulating PI3K/Akt Signaling. Front. Cell Dev. Biol. 2021, 9, 708331. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Luo, J.; Mo, Q.; Ran, M.; Zhang, T.; Li, X.; Zou, W.; Mei, Q.; Chen, J.; et al. Targeting a thrombopoietin-independent strategy in the discovery of a novel inducer of megakaryocytopoiesis, DMAG, for the treatment of thrombocytopenia. Haematologica 2023, 108, 1394–1411. [Google Scholar] [CrossRef]
- Lai, J.; Li, Y.; Ran, M.; Huang, Q.; Huang, F.; Zhu, L.; Wu, Y.; Zou, W.; Xie, X.; Tang, Y.; et al. Xanthotoxin, a novel inducer of platelet formation, promotes thrombocytopoiesis via IL-1R1 and MEK/ERK signaling. Biomed. Pharmacother. 2023, 163, 114811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, J.; Ran, M.; Yi, T.; Zhou, L.; Luo, J.; Liu, X.; Tang, X.; Huang, M.; Xie, X.; et al. Alnustone promotes megakaryocyte differentiation and platelet production via the interleukin-17A/interleukin-17A receptor/Src/RAC1/MEK/ERK signaling pathway. Eur. J. Pharmacol. 2024, 971, 176548. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Wong, M.; Zimlichman, E.; Tsur, A. Novel machine learning applications in peripartum care: A scoping review. Am. J. Obstet. Gynecol. MFM 2025, 7, 101612. [Google Scholar] [CrossRef] [PubMed]
| Disease Patterns | Signal Pathway | Correlation | References |
|---|---|---|---|
| Large granular lymphocyte leukemia-induced thrombocytopenia | MEK1/ERK pathway was activated | − | [49] |
| Chronic myeloid leukemia-induced thrombocytopenia | MEK1/ERK pathway was activated | − | [50] |
| I-MFA−/− mice carcinoma model-induced thrombocytopenia | p-ERK signaling pathways were enhanced | − | [51] |
| Juvenile myelomonocytic leukemia-induced thrombocytopenia | Hyperactivation of ERK and AKT | − | [52,53] |
| JMML model mouse-induced thrombocytopenia | Hyperactive RAS/ERK signaling | − | [54] |
| The platelet count of murine lung carcinoma model was increased | p-AKT and p-ERK were decreasing | − | [55] |
| Hemolytic uremic syndrome induced thrombocytopenia | Activation of ERK, JNK and p38 MAPK signaling | − | [56] |
| Tesirofenib and sorafenib toxicities-induced thrombocytopenia | p-ERK was not inhibited | − | [57] |
| Navitoclax side effect induced thrombocytopenia in recurrent epithelial ovarian cancer | Not related to the expression of p-ERK | − | [58] |
| Cisplatin side effects induced thrombocytopenia | ERK pathway was significantly blocked | − | [59] |
| Drug Name | Status | Disease Patterns | Signal Pathway | References |
|---|---|---|---|---|
| Shengxuexiaoban Capsules | Launched | Primary ITP | PI3K-AKT and MAPK-ERK1/2 | [67] |
| Ticlopidine | Launched | Thrombotic thrombocytopenic purpura | ERK1/2 and p38 | [41] |
| Eltrombopag | Launched | Thrombocytopenia | AKT and ERK1/2 | [27] |
| Eculizumab | Launched | Thrombocytopenia | the complement system | [68] |
| Hetrombopag | Launched | Thrombocytopenia | PI3K, ERK and STAT | [69] |
| TMEA | Preclinical research | Thrombocytopenia | mTOR and ERK signaling | [70] |
| DMAG | Preclinical research | Thrombocytopenia | ERK/HIF1/NF-E2 | [71] |
| Caulis Polygoni Multiflori | Preclinical research | Radiation-induced Thrombocytopenia | MEK/ERK (MAPK) and PI3K/Akt | [11] |
| Xanthotoxin (XAT) | Preclinical research | Thrombocytopenia | MEK/ERK | [72] |
| Alnustone | Preclinical research | Thrombocytopenia | MEK/ERK | [73] |
| MiR-557 inhibitor | Preclinical research | ITP | p-ERK, p-Akt and bcl-2 | [36] |
| BTK and PI3K dual drug | Preclinical research | Thrombocytopenia | ERK and AKT | [52] |
| (R)-TEMOSPho | Preclinical research | Thrombocytopenia | P-selectin expression and aggregated | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, P.; Chen, M. The Complex Role of MAPK/ERK Signaling Pathway in Different Types of Thrombocytopenia. Curr. Issues Mol. Biol. 2025, 47, 960. https://doi.org/10.3390/cimb47110960
Xue P, Chen M. The Complex Role of MAPK/ERK Signaling Pathway in Different Types of Thrombocytopenia. Current Issues in Molecular Biology. 2025; 47(11):960. https://doi.org/10.3390/cimb47110960
Chicago/Turabian StyleXue, Peipei, and Maoshan Chen. 2025. "The Complex Role of MAPK/ERK Signaling Pathway in Different Types of Thrombocytopenia" Current Issues in Molecular Biology 47, no. 11: 960. https://doi.org/10.3390/cimb47110960
APA StyleXue, P., & Chen, M. (2025). The Complex Role of MAPK/ERK Signaling Pathway in Different Types of Thrombocytopenia. Current Issues in Molecular Biology, 47(11), 960. https://doi.org/10.3390/cimb47110960







