Stachydrine Ameliorates Uterine Hypercontractility in Primary Dysmenorrhea by Targeting the COX-2/PGF2α Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.1.1. Chemicals and Reagents
2.1.2. Animal Treatment
2.2. Uterine Strip Preparation and Incubation
2.3. Spontaneous Uterine Contraction Assays
2.4. OT-Induced Uterine Contraction Assays
2.5. Effect of Pretreatment with Indo or L-NAME on OT-Induced Uterine Contractions
2.6. OT-Induced Writhing Assessment
2.7. Histopathological and Immunohistochemical Analyses
2.8. Biochemical Analysis of Serum and Uterine Tissue
2.9. Statistical Analysis
3. Results
3.1. Sta Reduced the Spontaneous Contraction Frequency in Isolated Uteri
3.2. Sta Reduced the Oxytocin (OT)-Induced Contraction Frequency in Isolated Uteri
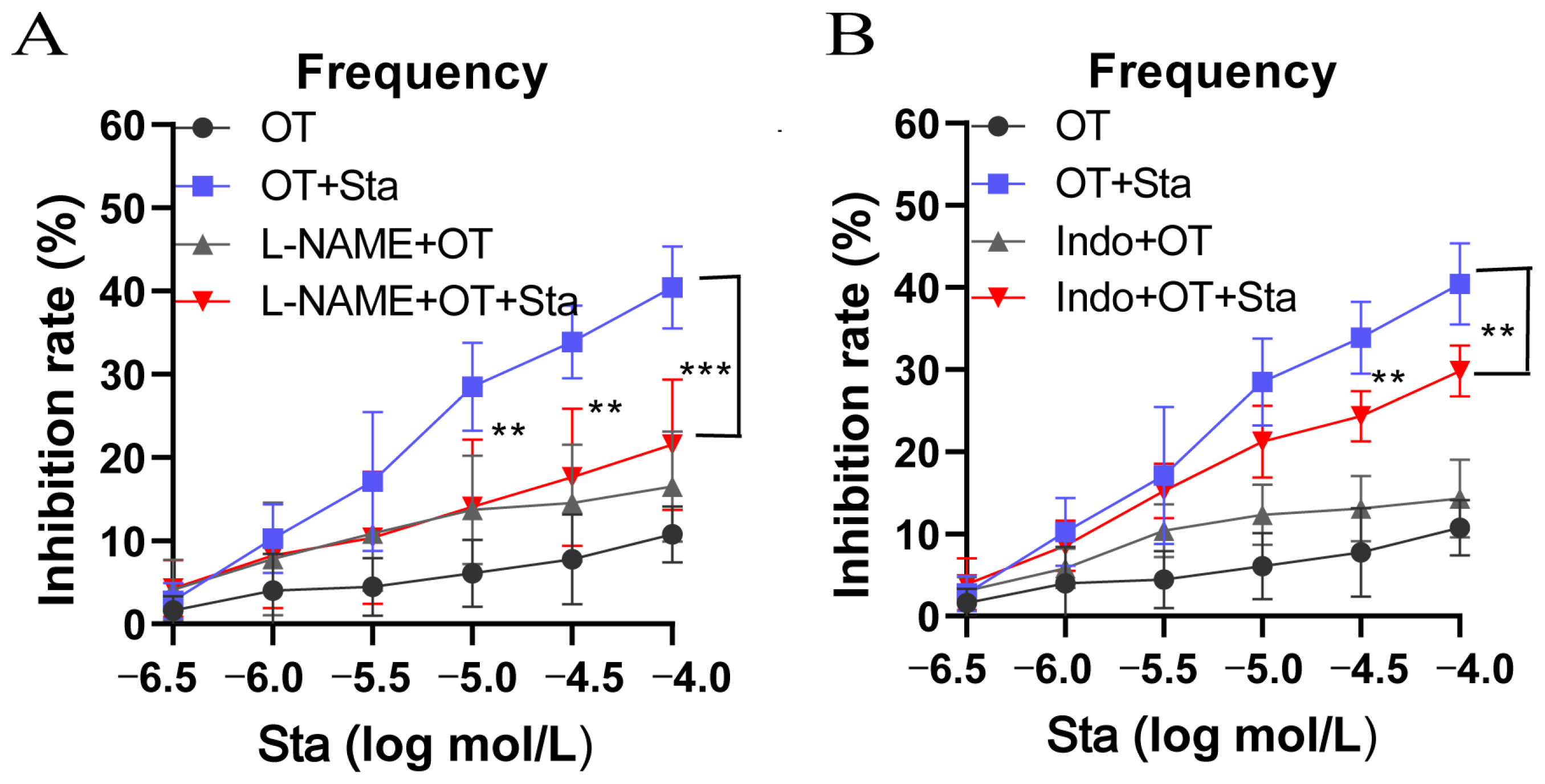
3.3. Both L-NAME and Indo Attenuated the Inhibition of OT-Induced Uterine Contractions by Sta
3.4. Effect of Sta on OT-Induced Writhing
3.5. Impact of Sta on Uterus Gross Morphology and Histopathology
3.6. Effect of Sta on COX-2 Expression in Uterine Tissues
3.7. Effects of Sta on MDA, SOD, and PGF2α Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Liu, L.; Li, J.; Lv, Y.; Zong, S.; Zhou, J.; Wang, Z.; Kou, J.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl inhibits oxytocin-induced uterine contraction in vitro and in vivo. J. Ethnopharmacol. 2017, 206, 107–114. [Google Scholar] [CrossRef]
- Dawood, M.Y. Primary Dysmenorrhea: Advances in Pathogenesis and Management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Eroglu, O.; Comertpay, E.; Vural, S.; Badem, N.D.; IşBaşaran, P.; Neşelioğlu, S.; Erel, Ö.; Deniz, T. Diagnostic value of oxidative stress markers in patients presenting with primary dysmenorrhea to the emergency department. Niger. J. Clin. Pract. 2022, 25, 636–640. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Nazıroğlu, M.; Güney, M.; Aykur, M. Non-steroidal anti-inflammatory drug modulates oxidative stress and calcium ion levels in the neutrophils of patients with primary dysmenorrhea. J. Reprod. Immunol. 2013, 100, 87–92. [Google Scholar] [CrossRef]
- Su, S.; Hua, Y.; Wang, Y.; Gu, W.; Zhou, W.; Duan, J.-A.; Jiang, H.; Chen, T.; Tang, Y. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J. Ethnopharmacol. 2012, 139, 649–656. [Google Scholar] [CrossRef]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef]
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef]
- Abdellatif, K.R.; Abdelall, E.K.; Bakr, R.B. Nitric Oxide-NASIDS Donor Prodrugs as Hybrid Safe Anti-inflammatory Agents. Curr. Top. Med. Chem. 2017, 17, 941–955. [Google Scholar] [CrossRef]
- Miao, L.; Zhou, Q.; Peng, C.; Liu, Z.; Xiong, L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: A comprehensive overview. Biomed. Pharmacother. 2019, 117, 109060. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, Y.; Wang, M.; Guo, C.; Cao, Z.; Liu, W.; Zhang, J.; Wang, Y.; Wang, Y.; Zhang, L. A review of pharmacological and pharmacokinetic properties of stachydrine. Pharmacol. Res. 2020, 155, 104745. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Wang, X.; He, H.; Li, M. Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 152, 14–32. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Manna, P.; Borah, J.C. Stachydrine, a pyrrole alkaloid with promising therapeutic potential against metabolic syndrome and associated organ dysfunction. RSC Med. Chem. 2024, 15, 3652–3673. [Google Scholar] [CrossRef]
- Liao, L.; Tang, Y.; Li, B.; Tang, J.; Xu, H.; Zhao, K.; Zhang, X. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed. Pharmacother. 2023, 161, 114489. [Google Scholar] [CrossRef]
- He, Z.; Li, P.; Liu, P.; Xu, P. Exploring stachydrine: From natural occurrence to biological activities and metabolic pathways. Front. Plant Sci. 2024, 15, 1442879. [Google Scholar] [CrossRef]
- Liu, F.; Yu, H.; Lee, H.; Chen, C.; Liao, C. The Modulation of Phospho-Extracellular Signal-Regulated Kinase and Phospho-Protein Kinase B Signaling Pathways plus Activity of Macrophage-Stimulating Protein Contribute to the Protective Effect of Stachydrine on Acetaminophen-Induced Liver Injury. Int. J. Mol. Sci. 2024, 25, 1484. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhen, J.; Hui, Z.; Meng, X.; Guan, J.; Zhang, H.; Zhang, J. Effect of dexmedetomidine on oxytocin-induced uterine contraction during optimal caesarean section anaesthesia. Basic Clin. Pharmacol. Toxicol. 2022, 131, 53–59. [Google Scholar] [CrossRef]
- Kawamata, M.; Tonomura, Y.; Kimura, T.; Sugimoto, Y.; Yanagisawa, T.; Nishimori, K. Oxytocin-induced phasic and tonic contractions are modulated by the contractile machinery rather than the quantity of oxytocin receptor. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E992–E999. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Simionescu, A.A.; Caravia, L.; Curici, A.; Cretoiu, D.; Popescu, L.M. Complex effects of imatinib on spontaneous and oxytocin-induced contractions in human non-pregnant myometrium. Acta Physiol. Hung. 2011, 98, 329–338. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, L.; Li, F.; Chen, Z. Regulation of uterine smooth muscle contractions: Implications for dysmenorrhea treatment. Exp. Ther. Med. 2024, 27, 2345. [Google Scholar]
- Yang, X.; Tian, Y.; Liu, J.; Kou, Y.; Xie, Y.; Wang, S.; Zhao, Y. Peony pollen protects against primary dysmenorrhea in mice by inhibiting inflammatory response and regulating the COX2/PGE2 pathway. Int. J. Mol. Sci. 2023, 24, 17245. [Google Scholar] [CrossRef]
- Campbell, P.S.; Albright, C.W.; Wilson, J.H.; Bridges, R.R. Inhibition of the nuclear localization of [3H]estradiol in rat uterine tissue in vitro. J. Steroid Biochem. 1989, 32, 5. [Google Scholar] [CrossRef]
- Ichida, S. L-methionine enhances the contractile responses of rat uterine smooth muscle to acetylcholine and high KCl. Jpn. J. Pharmacol. 1986, 40, 469–471. [Google Scholar] [CrossRef]
- Wong, J.; Chiang, Y.; Shih, Y.; Chiu, C.-H.; Chen, H.-Y.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+-MLCK-MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants 2020, 9, 991. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Zong, S.; Wang, Z.; Zhou, J.; Xu, Z.; Ding, G.; Xiao, W.; Kou, J. Traditional Chinese medicine Guizhi Fuling capsule used for therapy of dysmenorrhea via attenuating uterus contraction. J. Ethnopharmacol. 2016, 191, 273–279. [Google Scholar] [CrossRef]
- Sanborn, B. Ion channels and the control of myometrial electrical activity. Semin. Perinatol. 1995, 19, 31–40. [Google Scholar] [CrossRef]
- Ni, M.; Li, Y.; Wei, J.; Song, Z.; Wang, H.; Yao, J.; Chen, Y.-X.; Belke, D.; Estillore, J.P.; Wang, R.; et al. Increased Ca2+ Transient Underlies RyR2-Related Left Ventricular Noncompaction. Circ. Res. 2023, 133, 177–192. [Google Scholar] [CrossRef]
- Chen, J.; Tong, R.; Sun, X. Establishment of a Mouse Model of Primary Dysmenorrhea Using Progynova Combined with Oxytocin. Chin. J. Exp. Anim. 2013, 21, 78. [Google Scholar]
- Chung, D.; Caruso, R. Potential role for oxidative stress in 2,2’-dichlorobiphenyl-induced inhibition of uterine contractions but notmyometrial gap junctions. Toxicol. Sci. 2006, 93, 172–179. [Google Scholar] [CrossRef][Green Version]
- Bresson, E.; Boucher-Kovalik, S.; Chapdelaine, P.; Madore, E.; Harvey, N.; Laberge, P.Y.; Leboeuf, M.; Fortier, M.A. The human aldose reductaseAKR1B1 qualifies as the primary prostaglandin F synthase in the endometrium. J. Clin. Endocrinol. Metab. 2011, 96, 210–219. [Google Scholar] [CrossRef]
- Zheng, W.; Li, M.; Wang, Y.; Lv, B.; Zhang, X.; Chen, L.; Zhu, K.; Wang, Z.; Li, B.; Xiao, W. Guizhi Fuling capsule exhibits antidysmenorrhea activity by inhibition of cyclooxygenase activity. Evid.-Based Complement. Alternat. Med. 2020, 23, 8607931. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Chen, S.; Cao, D.; Cheng, H.; Chen, S.; Shu, Y.; Wang, Y.; Chen, Z. Stachydrine Ameliorates Uterine Hypercontractility in Primary Dysmenorrhea by Targeting the COX-2/PGF2α Pathway. Curr. Issues Mol. Biol. 2025, 47, 961. https://doi.org/10.3390/cimb47110961
Cheng Y, Chen S, Cao D, Cheng H, Chen S, Shu Y, Wang Y, Chen Z. Stachydrine Ameliorates Uterine Hypercontractility in Primary Dysmenorrhea by Targeting the COX-2/PGF2α Pathway. Current Issues in Molecular Biology. 2025; 47(11):961. https://doi.org/10.3390/cimb47110961
Chicago/Turabian StyleCheng, Yongfeng, Shuo Chen, Dianjie Cao, Hairu Cheng, Siyuan Chen, Yi Shu, Yue Wang, and Zhiwu Chen. 2025. "Stachydrine Ameliorates Uterine Hypercontractility in Primary Dysmenorrhea by Targeting the COX-2/PGF2α Pathway" Current Issues in Molecular Biology 47, no. 11: 961. https://doi.org/10.3390/cimb47110961
APA StyleCheng, Y., Chen, S., Cao, D., Cheng, H., Chen, S., Shu, Y., Wang, Y., & Chen, Z. (2025). Stachydrine Ameliorates Uterine Hypercontractility in Primary Dysmenorrhea by Targeting the COX-2/PGF2α Pathway. Current Issues in Molecular Biology, 47(11), 961. https://doi.org/10.3390/cimb47110961




