Non-Coding RNAs and Their Role in Maintaining Epidermal Homeostasis
Abstract
1. Introduction
2. Methodology
3. ncRNAs: Classification and Functions
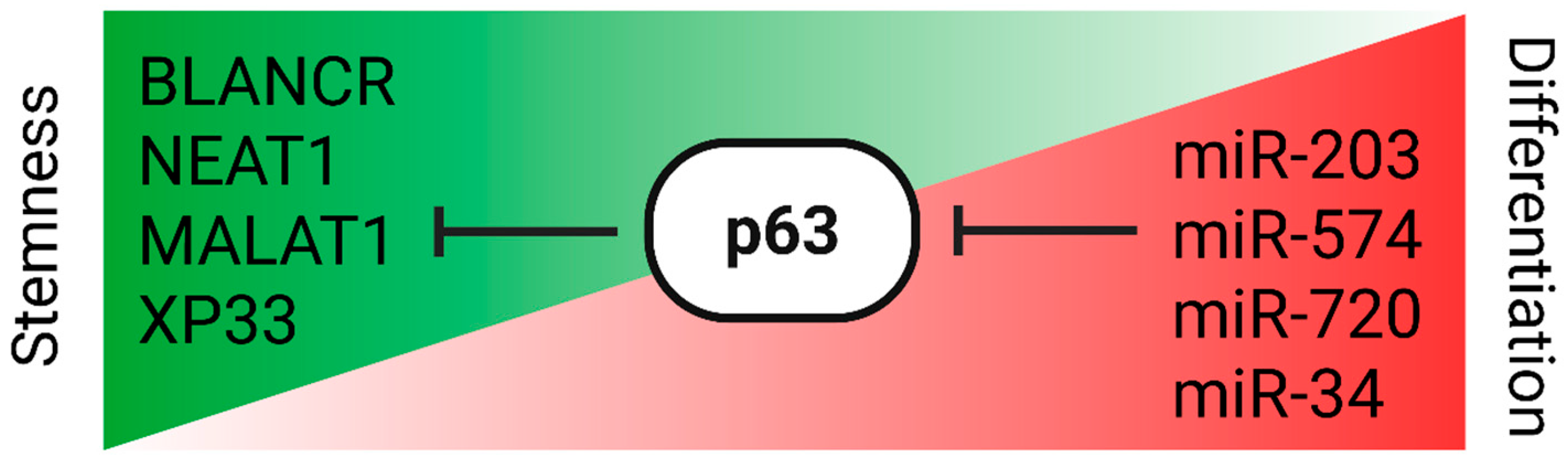
4. ncRNAs Regulating Epidermal Differentiation
p63-ncRNA Regulatory Circuitry
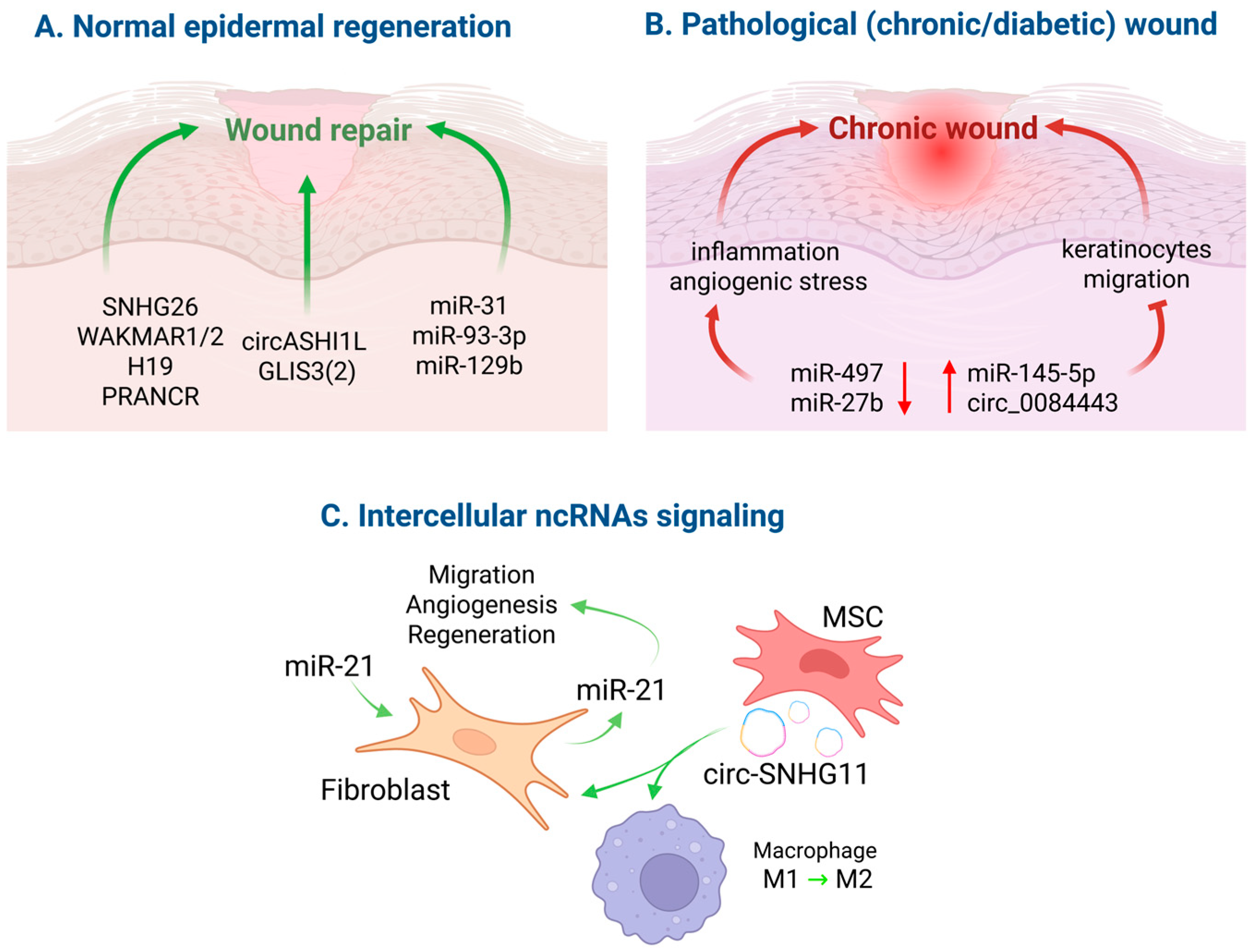
5. NcRNAs in Regenerative Repair
5.1. Normal Wound Healing
5.2. Chronic and Diabetic Wounds
5.3. ncRNA-Based Therapeutic Strategies
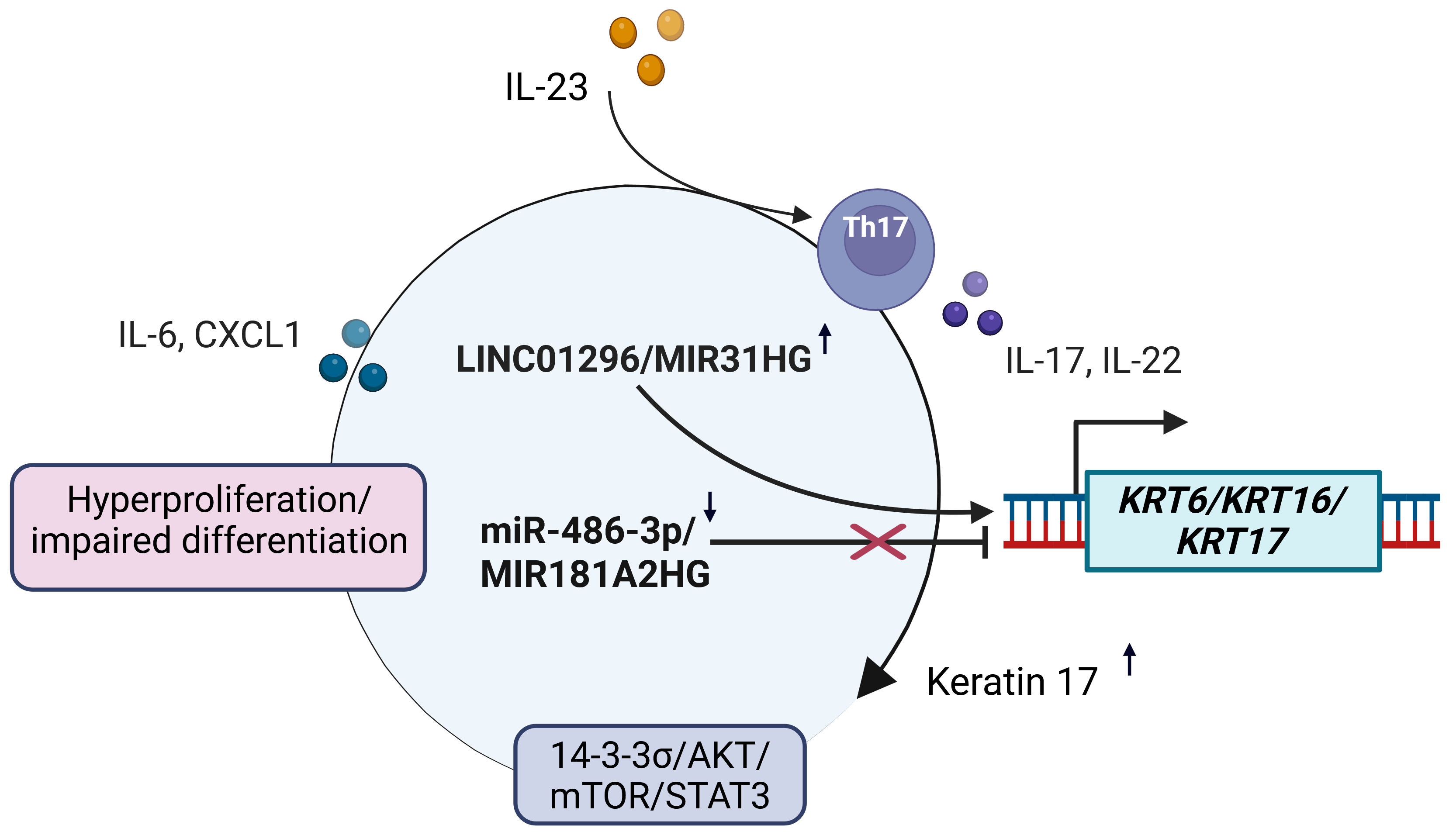
6. The Landscape of Non-Coding RNAs in Psoriatic Keratinocytes
6.1. MicroRNAs
6.2. LncRNAs
6.3. CircRNAs
7. NcRNAs as Potential Therapeutic Agents and Targets
| NcRNA | Subject/Model | Biological Effect | Confirmed Targets/Mechanism | Therapeutic Perspective | References |
|---|---|---|---|---|---|
| miR-17-3p | Psoriatic epidermis; human keratinocytes | Pro-proliferative and pro-inflammatory effects; shifts the program away from differentiation (↓ IVL/FLG as a consequence of the proliferative shift) | Targets CTR9; increases cell proliferation and cytokine secretion | Use anti-miR-17-3p ASO to inhibit the proliferation of keratinocytes | [117] |
| miR-383 | Psoriasis model; keratinocytes | The anti-inflammatory effect and stimulation of cell differentiation through suppression of JAK/STAT activity | Directly targeting LCN2 → JAK3/STAT3 signals | Simulating the action of miR-383 to help restore balance in the differentiation process | [118] |
| miR-155 | Human keratinocytes; psoriatic skin | A reduction in miR-155 levels is necessary for normal cell differentiation and barrier formation (↑ IVL, FLG, KRT1, and KRT10) | It is high in psoriatic plaque; the decrease supports the differentiation of CC | Anti-miR-155 is designed to help normalize differentiation processes and create a barrier | [119] |
| LINC01026 | Psoriatic keratinocytes | Pro-proliferative effect; inhibits the transition to differentiation | Increases EHF and promotes cell cycle progression | Anti-LINC01026 ASO to reduce hyperproliferation | [105] |
| MIR181A2HG | Psoriatic keratinocytes, HaCaT keratinocytes | Anti-proliferative; promotes the differentiation pathway | The miR-223-3p/SOX6 axis and SRSF1 binding are associated with suppression of proliferation | lncRNA replacement therapy or anti-miR-223-3p to restore the miR181A2HG axis | [8,9] |
| circARNTL2 | Psoriatic keratinocytes | Pro-proliferative; shifts the balance toward proliferation | Stimulates the progression of the keratinocyte cell cycle | ASO/Cas13 against circARNTL2 for promoting differentiation | [109] |
| PRKCQ-AS1 | Keratinocyte exosomes /CD4+ T cells (psoriasis) | Increases inflammation caused by Th17 cells, which in turn negatively affects the differentiation process | Activates STAT3 and promotes Th17 differentiation in recipient cells | Application of ASO to PRKCQ-AS1 (PRKCQ-AS1 release and capture block) | [121] |
| Anti-Fn14 siRNA (targets TNFRSF12A) | Keratinocytes; psoriatic mouse skin | ↓ hyperproliferation; improvement of PASI-like metrics → normalization of differentiation | Fn14 siRNA knockdown; transdermal ionic liquid delivery | Topical/Transdermal siRNA therapy for Fn14 nodes | [122,138] |
| miR-146a | Psoriatic keratinocytes | It inhibits the activity of the natural NF-kB response, indirectly promoting differentiation | IRAK1/TRAF6 weakens IL-17/TLR-dependent responses | Mimic miR-146a to reduce inflammation and restore epidermal barrier | [126] |
| miR-21 | Psoriatic keratinocytes, aging/wounded skin | Increased miR-21 inhibits CC apoptosis and differentiation, and is associated with the caspase-8 pathway. It also affects SATB1 | CASP8 (psoriasis) and SATB1 (skin aging) | Anti-miR-21 is used to restore the balance between apoptosis and differentiation | [131,132,133] |
| miR-10a-5p | Atopic dermatitis, keratinocytes | Modulates cell proliferation and differentiation; blood pressure is elevated in certain areas | Regulated by the inflammatory environment and affects barrier and cell cycle genes | Anti-miR-10a-5p is used to normalize differentiation in blood pressure | [125] |
| miR-939 | Atopic dermatitis, keratinocytes | Pro-inflammatory cytokines in the CC (MMP1/3/9 and ICAM1) indirectly worsen the barrier and differentiation | Increases the activity of matrix metalloproteinases (MMPs), leading to increased inflammatory processes | Anti-miR-939 is used to reduce inflammation and protect the skin barrier | [139] |
8. Challenges, Limitations, and Future Directions
- Target screening and identification are crucial for the development of ncRNA-based therapeutics. Despite the discovery of dozens of potential therapeutic agents in the last decade, only a limited number of ncRNA treatments have progressed to clinical trials. Recently, screening strategies have incorporated cutting-edge technologies such as high-throughput screening (HTS), small molecule microarrays (SMMs), and fragment-based drug discovery (FBDD) [142];
- Efficient transdermal drug delivery remains a challenge due to the barrier function of the skin and intracellular RNA degradation. Various strategies have been developed, including microneedles, electroporation, nanoparticles, hydrogel systems, and liposomes. However, each of these methods requires further optimization and validation for new drug candidates [143,144];
- Since ncRNAs can regulate multiple pathways simultaneously, off-target effects remain a significant concern for clinical applications. Therapeutic oligonucleotides can impact pathways unrelated to the intended target and influence immune responses. Chemical modifications and other strategies can help to reduce these effects, but careful safety evaluation is still essential [144].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahanty, S.; Setty, S.R.G. Epidermal Lamellar Body Biogenesis: Insight Into the Roles of Golgi and Lysosomes. Front. Cell Dev. Biol. 2021, 9, 701950. [Google Scholar] [CrossRef]
- Rice, G.; Rompolas, P. Advances in Resolving the Heterogeneity and Dynamics of Keratinocyte Differentiation. Curr. Opin. Cell Biol. 2020, 67, 92–98. [Google Scholar] [CrossRef]
- Fuchs, E. Skin Stem Cells: Rising to the Surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Flora, P.; Ezhkova, E. Regulatory Mechanisms Governing Epidermal Stem Cell Function during Development and Homeostasis. Development 2020, 147, dev194100. [Google Scholar] [CrossRef]
- Yan, S.; Ripamonti, R.; Kawabe, H.; Ben-Yehuda Greenwald, M.; Werner, S. NEDD4-1 Is a Key Regulator of Epidermal Homeostasis and Wound Repair. J. Investig. Dermatol. 2022, 142, 1703–1713.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Raymundo, J.; Daly, K.; Zhu, W.; Senapati, B.; Zhong, H.; Ahilan, A.; Marneros, A. AP-2α/AP-2β Transcription Factors Are Key Regulators of Epidermal Homeostasis. J. Investig. Dermatol. 2024, 144, 1505–1521.e12. [Google Scholar] [CrossRef] [PubMed]
- Cottle, D.L.; Kretzschmar, K.; Gollnick, H.P.; Quist, S.R. P53 Activity Contributes to Defective Interfollicular Epidermal Differentiation in Hyperproliferative Murine Skin. Br. J. Dermatol. 2016, 174, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Flores, E.R. P53/P63/P73 in the Epidermis in Health and Disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015248. [Google Scholar] [CrossRef]
- Poliseno, L.; Lanza, M.; Pandolfi, P.P. Coding, or Non-Coding, That Is the Question. Cell Res. 2024, 34, 609–629. [Google Scholar] [CrossRef]
- Jacovetti, C.; Bayazit, M.B.; Regazzi, R. Emerging Classes of Small Non-Coding RNAs With Potential Implications in Diabetes and Associated Metabolic Disorders. Front. Endocrinol. 2021, 12, 670719. [Google Scholar] [CrossRef]
- Huang, Z.; Du, Y.; Wen, J.; Lu, B.; Zhao, Y. snoRNAs: Functions and Mechanisms in Biological Processes, and Roles in Tumor Pathophysiology. Cell Death Discov. 2022, 8, 259. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhang, Y.; Zhou, L.; Xu, Q.; Li, L.; Zeng, L.; Xue, J.; Niu, H.; Zhong, J.; et al. Mammalian PIWI-piRNA-Target Complexes Reveal Features for Broad and Efficient Target Silencing. Nat. Struct. Mol. Biol. 2024, 31, 1222–1231. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P. RISC Assembly and Post-Transcriptional Gene Regulation in Hepatocellular Carcinoma. Genes. Dis. 2020, 7, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in Action: Biogenesis, Function and Regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Pérez, M.G.; Grecco, A.; Rosenzvit, M.C. Chapter 3-MicroRNA Interference. In MicroRNA; Xiao, J., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 33–52. ISBN 978-0-323-89774-7. [Google Scholar]
- Meijer, H.A.; Smith, E.M.; Bushell, M. Regulation of miRNA Strand Selection: Follow the Leader? Biochem. Soc. Trans. 2014, 42, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.; Maier, A.; Klimovich, P.; Sysoeva, V.; Romashin, D.; Semina, E.; Tkachuk, V. T-Cadherin (CDH13) and Non-Coding RNAs: The Crosstalk Between Health and Disease. Int. J. Mol. Sci. 2025, 26, 6127. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Chiang, T.-W.; Jhong, S.-E.; Chen, Y.-C.; Chen, C.-Y.; Wu, W.-S.; Chuang, T.-J. FL-circAS: An Integrative Resource and Analysis for Full-Length Sequences and Alternative Splicing of Circular RNAs with Nanopore Sequencing. Nucleic Acids Res. 2023, 52, D115–D123. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, S.; Li, X.; Feng, C.; Huang, Q.; Wang, S.; Wang, S.; Xia, W.; Yang, F.; Yin, R.; et al. Profiling Expression of Coding Genes, Long Noncoding RNA, and Circular RNA in Lung Adenocarcinoma by Ribosomal RNA-Depleted RNA Sequencing. FEBS Open Bio 2018, 8, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Misir, S.; Wu, N.; Yang, B.B. Specific Expression and Functions of Circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Fu, L.; Xu, T. Investigating the Underlying Mechanisms of Circular RNAs and Their Application in Clinical Research of Cervical Cancer. Front. Genet. 2021, 12, 653051. [Google Scholar] [CrossRef]
- Catanzaro, G. Non-Coding RNAs in Health and Disease: Editorial. Biomedicines 2022, 11, 14. [Google Scholar] [CrossRef]
- Good, D.J. Non-Coding RNAs in Human Health and Diseases. Genes 2023, 14, 1429. [Google Scholar] [CrossRef] [PubMed]
- Viticchiè, G.; Lena, A.M.; Cianfarani, F.; Odorisio, T.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. MicroRNA-203 Contributes to Skin Re-Epithelialization. Cell Death Dis. 2012, 3, e435. [Google Scholar] [CrossRef]
- Vieu, D.-L.; Golebiewski, C.; Gastaldi, C.; Foucher, A.; Mari, B.; Rezzonico, R.; Droit, A.; Dumont, M.; Bastien, P.; Bernerd, F.; et al. Identification of miR-141 as a Regulator of Epidermal Homeostasis. J. Investig. Dermatol. 2025, 145, 1670–1682.e15. [Google Scholar] [CrossRef]
- Lena, A.M.; Shalom-Feuerstein, R.; Rivetti di Val Cervo, P.; Aberdam, D.; Knight, R.A.; Melino, G.; Candi, E. miR-203 Represses “stemness” by Repressing DeltaNp63. Cell Death Differ. 2008, 15, 1187–1195. [Google Scholar] [CrossRef]
- Sonkoly, E.; Lovén, J.; Xu, N.; Meisgen, F.; Wei, T.; Brodin, P.; Jaks, V.; Kasper, M.; Shimokawa, T.; Harada, M.; et al. MicroRNA-203 Functions as a Tumor Suppressor in Basal Cell Carcinoma. Oncogenesis 2012, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; McDade, S.S.; Patel, D.; McCance, D.J. MicroRNA 203 Expression in Keratinocytes Is Dependent on Regulation of P53 Levels by E6. J. Virol. 2010, 84, 10644–10652. [Google Scholar] [CrossRef]
- Dallaglio, K.; Petrachi, T.; Marconi, A.; Truzzi, F.; Lotti, R.; Saltari, A.; Morandi, P.; Puviani, M.; Maiorana, A.; Pincelli, C. Expression of Nuclear Survivin in Normal Skin and Squamous Cell Carcinoma: A Possible Role in Tumour Invasion. Br. J. Cancer 2014, 110, 199–207. [Google Scholar] [CrossRef]
- Labarrade, F.; Botto, J.-M.; Imbert, I.M. miR-203 Represses Keratinocyte Stemness by Targeting Survivin. J. Cosmet. Dermatol. 2022, 21, 6100–6108. [Google Scholar] [CrossRef]
- Wang, J.; Dan, G.; Shangguan, T.; Hao, H.; Tang, R.; Peng, K.; Zhao, J.; Sun, H.; Zou, Z. miR-198 Represses the Proliferation of HaCaT Cells by Targeting Cyclin D2. Int. J. Mol. Sci. 2015, 16, 17018–17028. [Google Scholar] [CrossRef] [PubMed]
- Nagosa, S.; Leesch, F.; Putin, D.; Bhattacharya, S.; Altshuler, A.; Serror, L.; Amitai-Lange, A.; Nasser, W.; Aberdam, E.; Rouleau, M.; et al. microRNA-184 Induces a Commitment Switch to Epidermal Differentiation. Stem Cell Rep. 2017, 9, 1991–2004. [Google Scholar] [CrossRef]
- Pickup, M.E.; Hu, A.; Patel, H.J.; Ahmed, M.I. MicroRNA-148a Controls Epidermal and Hair Follicle Stem/Progenitor Cells by Modulating the Activities of ROCK1 and ELF5. J. Investig. Dermatol. 2023, 143, 480–491.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Huang, L.; Kong, Y.; Chen, F.; Liang, J.; Yu, H.; Yao, Z. H19 lncRNA Regulates Keratinocyte Differentiation by Targeting miR-130b-3p. Cell Death Dis. 2017, 8, e3174. [Google Scholar] [CrossRef]
- Lopez-Pajares, V.; Qu, K.; Zhang, J.; Webster, D.E.; Barajas, B.C.; Siprashvili, Z.; Zarnegar, B.J.; Boxer, L.D.; Rios, E.J.; Tao, S.; et al. A LncRNA-MAF:MAFB Transcription Factor Network Regulates Epidermal Differentiation. Dev. Cell 2015, 32, 693–706. [Google Scholar] [CrossRef]
- Piipponen, M.; Nissinen, L.; Kähäri, V.-M. Long Non-Coding RNAs in Cutaneous Biology and Keratinocyte Carcinomas. Cell. Mol. Life Sci. 2020, 77, 4601–4614. [Google Scholar] [CrossRef]
- Ziegler, C.; Graf, J.; Faderl, S.; Schedlbauer, J.; Strieder, N.; Förstl, B.; Spang, R.; Bruckmann, A.; Merkl, R.; Hombach, S.; et al. The Long Non-coding RNA LINC00941 and SPRR5 Are Novel Regulators of Human Epidermal Homeostasis. EMBO Rep. 2019, 20, e46612. [Google Scholar] [CrossRef]
- Lee, J.; Wu, Y.; Harada, B.T.; Li, Y.; Zhao, J.; He, C.; Ma, Y.; Wu, X. N6-methyladenosine Modification of lncRNA Pvt1 Governs Epidermal Stemness. EMBO J. 2021, 40, e106276. [Google Scholar] [CrossRef]
- Morgenstern, E.; Molthof, C.; Schwartz, U.; Graf, J.; Bruckmann, A.; Hombach, S.; Kretz, M. lncRNA LINC00941 Modulates MTA2/NuRD Occupancy to Suppress Premature Human Epidermal Differentiation. Life Sci. Alliance 2024, 7, e202302475. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Okholm, T.L.H.; Venø, M.T.; Kjems, J. Circular RNAs Are Abundantly Expressed and Upregulated during Human Epidermal Stem Cell Differentiation. RNA Biol. 2017, 15, 280–291. [Google Scholar] [CrossRef]
- Barbollat-Boutrand, L.; Joly-Tonetti, N.; Dos Santos, M.; Metral, E.; Boher, A.; Masse, I.; Berthier-Vergnes, O.; Bertolino, P.; Damour, O.; Lamartine, J. MicroRNA-23b-3p Regulates Human Keratinocyte Differentiation through Repression of TGIF1 and Activation of the TGF-ß-SMAD2 Signalling Pathway. Exp. Dermatol. 2017, 26, 51–57. [Google Scholar] [CrossRef]
- Novelli, F.; Ganini, C.; Melino, G.; Nucci, C.; Han, Y.; Shi, Y.; Wang, Y.; Candi, E. P63 in Corneal and Epidermal Differentiation. Biochem. Biophys. Res. Commun. 2022, 610, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Eyermann, C.E.; Chen, X.; Somuncu, O.S.; Li, J.; Joukov, A.N.; Chen, J.; Alexandrova, E.M. ΔNp63 Regulates Homeostasis, Stemness, and Suppression of Inflammation in the Adult Epidermis. J. Investig. Dermatol. 2024, 144, 73–83.e10. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Lena, A.M.; Tosetti, G.; Yang, X.; Cappello, A.; Helmer Citterich, M.; Melino, G.; Candi, E. Epigenetic Priming of an Epithelial Enhancer by P63 and CTCF Controls Expression of a Skin-Restricted Gene XP33. Cell Death Discov. 2023, 9, 446. [Google Scholar] [CrossRef]
- Fierro, C.; Gatti, V.; La Banca, V.; De Domenico, S.; Scalera, S.; Corleone, G.; Fanciulli, M.; De Nicola, F.; Mauriello, A.; Montanaro, M.; et al. The Long Non-Coding RNA NEAT1 Is a ΔNp63 Target Gene Modulating Epidermal Differentiation. Nat. Commun. 2023, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; La Banca, V.; D’Amico, S.; Nicolai, S.; Peschiaroli, A. Defining the Transcriptional Routes Controlling lncRNA NEAT1 Expression: Implications in Cellular Stress Response, Inflammation, and Differentiation. Discov. Oncol. 2025, 16, 768. [Google Scholar] [CrossRef]
- Li, Y.; Giovannini, S.; Wang, T.; Fang, J.; Li, P.; Shao, C.; Wang, Y.; TOR Centre; Shi, Y.; Candi, E.; et al. P63: A Crucial Player in Epithelial Stemness Regulation. Oncogene 2023, 42, 3371–3384. [Google Scholar] [CrossRef]
- Tanis, S.E.J.; Köksal, E.S.; van Buggenum, J.A.G.L.; Mulder, K.W. BLNCR Is a Long Non-Coding RNA Adjacent to Integrin Beta-1 That Is Rapidly Lost during Epidermal Progenitor Cell Differentiation. Sci. Rep. 2019, 9, 31. [Google Scholar] [CrossRef]
- Candi, E.; Amelio, I.; Agostini, M.; Melino, G. MicroRNAs and P63 in Epithelial Stemness. Cell Death Differ. 2015, 22, 12–21. [Google Scholar] [CrossRef]
- Woodstock, D.L.; Sammons, M.A.; Fischer, M. P63 and P53: Collaborative Partners or Dueling Rivals? Front. Cell Dev. Biol. 2021, 9, 701986. [Google Scholar] [CrossRef]
- Westfall, M.D.; Mays, D.J.; Sniezek, J.C.; Pietenpol, J.A. The Delta Np63 Alpha Phosphoprotein Binds the P21 and 14-3-3 Sigma Promoters in Vivo and Has Transcriptional Repressor Activity That Is Reduced by Hay-Wells Syndrome-Derived Mutations. Mol. Cell Biol. 2003, 23, 2264–2276. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by P53 Broadly Influences Gene Expression and Promotes Apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef]
- Antonini, D.; Russo, M.T.; De Rosa, L.; Gorrese, M.; Del Vecchio, L.; Missero, C. Transcriptional Repression of miR-34 Family Contributes to P63-Mediated Cell Cycle Progression in Epidermal Cells. J. Investig. Dermatol. 2010, 130, 1249–1257. [Google Scholar] [CrossRef]
- Domíguez-Hüttinger, E.; Flores-Garza, E.; Caldú-Primo, J.L.; Day, H.; Ramírez, A.R.; Tanaka, R.J. History-Dependent Switch-like Differentiation of Keratinocytes in Response to Skin Barrier Damage. PLoS Comput. Biol. 2025, 21, e1013162. [Google Scholar] [CrossRef] [PubMed]
- Romashin, D.D.; Tolstova, T.V.; Varshaver, A.M.; Kozhin, P.M.; Rusanov, A.L.; Luzgina, N.G. Keratins 6, 16, and 17 in Health and Disease: A Summary of Recent Findings. Curr. Issues Mol. Biol. 2024, 46, 8627–8641. [Google Scholar] [CrossRef] [PubMed]
- Sass, P.A.; Dąbrowski, M.; Charzyńska, A.; Sachadyn, P. Transcriptomic Responses to Wounding: Meta-Analysis of Gene Expression Microarray Data. BMC Genom. 2017, 18, 850. [Google Scholar] [CrossRef]
- Liu, Z.; Bian, X.; Luo, L.; Björklund, Å.K.; Li, L.; Zhang, L.; Chen, Y.; Guo, L.; Gao, J.; Cao, C.; et al. Spatiotemporal Single-Cell Roadmap of Human Skin Wound Healing. Cell Stem Cell 2025, 32, 479–498.e8. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Li, D.; Ren, X.; Li, Y.; Herter, E.K.; Qian, M.; Toma, M.-A.; Wintler, A.-M.; Sérézal, I.G.; et al. MicroRNA-34 Family Enhances Wound Inflammation by Targeting LGR4. J. Investig. Dermatol. 2020, 140, 465–476.e11. [Google Scholar] [CrossRef]
- Shi, J.; Ma, X.; Su, Y.; Song, Y.; Tian, Y.; Yuan, S.; Zhang, X.; Yang, D.; Zhang, H.; Shuai, J.; et al. MiR-31 Mediates Inflammatory Signaling to Promote Re-Epithelialization during Skin Wound Healing. J. Investig. Dermatol. 2018, 138, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human Keratinocyte-Derived Microvesicle miRNA-21 Promotes Skin Wound Healing in Diabetic Rats through Facilitating Fibroblast Function and Angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Liu, Z.; Zhuang, D.; Wang, S.; Deng, H.; Shi, Y.; Sun, J.; Guo, J.; Wei, F.; et al. miR-21 Expressed by Dermal Fibroblasts Enhances Skin Wound Healing Through the Regulation of Inflammatory Cytokine Expression. Inflammation 2024, 47, 572–590. [Google Scholar] [CrossRef]
- Zhang, L.; Hung, G.C.-C.; Meng, S.; Evans, R.; Xu, J. LncRNA MALAT1 Regulates Hyperglycemia Induced EMT in Keratinocyte via miR-205. Noncoding RNA 2023, 9, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Meshkat, B.I.; Liechty, K.W.; Xu, J. LncRNA MALAT1 Modulates TGF-Β1-Induced EMT in Keratinocyte. Int. J. Mol. Sci. 2021, 22, 11816. [Google Scholar] [CrossRef]
- Zhang, L.; Piipponen, M.; Liu, Z.; Li, D.; Bian, X.; Niu, G.; Geara, J.; Toma, M.A.; Sommar, P.; Landén, N.X. Human Skin Specific Long Noncoding RNA HOXC13-AS Regulates Epidermal Differentiation by Interfering with Golgi-ER Retrograde Transport. Cell Death Differ. 2023, 30, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Herter, E.K.; Li, D.; Toma, M.A.; Vij, M.; Li, X.; Visscher, D.; Wang, A.; Chu, T.; Sommar, P.; Blomqvist, L.; et al. WAKMAR2, a Long Noncoding RNA Downregulated in Human Chronic Wounds, Modulates Keratinocyte Motility and Production of Inflammatory Chemokines. J. Investig. Dermatol. 2019, 139, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.; Zhang, L.; Bian, X.; Wu, J.; Li, L.; Chen, Y.; Luo, L.; Pan, L.; Kong, L.; et al. The lncRNA SNHG26 Drives the Inflammatory-to-Proliferative State Transition of Keratinocyte Progenitor Cells during Wound Healing. Nat. Commun. 2024, 15, 8637. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, Q.; Sun, Z.; Cheng, Y. LncRNA H19 Inhibits Keratinocyte Cell Proliferation and Migration by Targeting miR-17-5p/RUNX1 Axis in Chronic Wounds. J. Burn. Care Res. 2024, 45, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.A.; Liu, Z.; Wang, Q.; Zhang, L.; Li, D.; Sommar, P.; Landén, N.X. Circular RNA Signatures of Human Healing and Nonhealing Wounds. J. Investig. Dermatol. 2022, 142, 2793–2804.e26. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, G.; Liu, Z.; Toma, M.A.; Geara, J.; Bian, X.; Zhang, L.; Piipponen, M.; Li, D.; Wang, A.; et al. Circular RNA circASH1L(4,5) Protects microRNA-129-5p from Target-Directed microRNA Degradation in Human Skin Wound Healing. Br. J. Dermatol. 2025, 192, 468–480. [Google Scholar] [CrossRef]
- Niu, G.; Toma, M.A.; Geara, J.; Bian, X.; Chen, Y.; Luo, L.; Wang, Q.; Xiao, Y.; Vij, M.; Piipponen, M.; et al. Collaborative Duality of CircGLIS3(2) RNA and Protein in Human Wound Repair. Adv. Sci. 2025, 12, e2416784. [Google Scholar] [CrossRef]
- Ban, E.; Jeong, S.; Park, M.; Kwon, H.; Park, J.; Song, E.J.; Kim, A. Accelerated Wound Healing in Diabetic Mice by miRNA-497 and Its Anti-Inflammatory Activity. Biomed. Pharmacother. 2020, 121, 109613. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Li, J.; Liu, D.; Wu, B. MicroRNA miR-145-5p Inhibits Cutaneous Wound Healing by Targeting PDGFD in Diabetic Foot Ulcer. Biochem. Genet. 2024, 62, 2437–2454. [Google Scholar] [CrossRef] [PubMed]
- Sakshi, S.; Jayasuriya, R.; Sathish Kumar, R.C.; Umapathy, D.; Gopinathan, A.; Balamurugan, R.; Ganesan, K.; Ramkumar, K.M. MicroRNA-27b Impairs Nrf2-Mediated Angiogenesis in the Progression of Diabetic Foot Ulcer. J. Clin. Med. 2023, 12, 4551. [Google Scholar] [CrossRef]
- Kurinna, S.; Schäfer, M.; Ostano, P.; Karouzakis, E.; Chiorino, G.; Bloch, W.; Bachmann, A.; Gay, S.; Garrod, D.; Lefort, K.; et al. A Novel Nrf2-miR-29-Desmocollin-2 Axis Regulates Desmosome Function in Keratinocytes. Nat. Commun. 2014, 5, 5099. [Google Scholar] [CrossRef]
- Thiagarajan, L.; Sanchez-Alvarez, R.; Kambara, C.; Rajasekar, P.; Wang, Y.; Halloy, F.; Hall, J.; Stark, H.; Martin, I.; Boukamp, P.; et al. miRNA-29 Regulates Epidermal and Mesenchymal Functions in Skin Repair. FEBS Lett. 2025, 599, 1795–1817. [Google Scholar] [CrossRef]
- Jie, R.; Qian, J.; Tang, Y.; Li, Y.; Xu, M.; Zhao, X.; Chen, M. Role of Increased miR-222-3p Expression in Peripheral Blood and Wound Marginal Tissues of Type 2 Diabetes Mellitus Patients with Diabetic Foot Ulcer. Diabetes Metab. Syndr. Obes. 2023, 16, 2419–2432. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Tang, Y.; Xie, D.; Wang, Y.; Chen, M. Changes in miroRNA-103 Expression in Wound Margin Tissue Are Related to Wound Healing of Diabetes Foot Ulcers. Int. Wound J. 2022, 20, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Toma, M.A.; Ma, J.; Li, D.; Vij, M.; Chu, T.; Wang, J.; Li, X.; Landén, N.X. Circular RNA Hsa_circ_0084443 Is Upregulated in Diabetic Foot Ulcer and Modulates Keratinocyte Migration and Proliferation. Adv. Wound Care 2020, 9, 145–160. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Zhao, S.; Yuan, H.; Shi, J.; Zhao, H. Hypoxic ADSC-Derived Exosomes Enhance Wound Healing in Diabetic Mice via Delivery of Circ-Snhg11 and Induction of M2-like Macrophage Polarization. Biomed. Pharmacother. 2022, 153, 113463. [Google Scholar] [CrossRef]
- Nair, P.A.; Badri, T. Psoriasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Freisenhausen, J.C.; Luo, L.; Kelemen, E.; Elton, J.; Skoog, V.; Pivarcsi, A.; Sonkoly, E. RNA Sequencing Reveals the Long Non-Coding RNA Signature in Psoriasis Keratinocytes and Identifies CYDAER as a Long Non-Coding RNA Regulating Epidermal Differentiation. Exp. Dermatol. 2025, 34, e70054. [Google Scholar] [CrossRef]
- Teo, L.T.K.; Juantuah-Kusi, N.; Subramanian, G.; Sampath, P. Psoriasis Treatments: Emerging Roles and Future Prospects of MicroRNAs. Non-Coding RNA 2025, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Meisgen, F.; Butler, L.M.; Han, G.; Wang, X.-J.; Söderberg-Nauclér, C.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-31 Is Overexpressed in Psoriasis and Modulates Inflammatory Cytokine and Chemokine Production in Keratinocytes via Targeting Serine/Threonine Kinase 40. J. Immunol. 2013, 190, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Huang, H.-J.; Xu, Y.-Y.; Vos, H.; Gulersonmez, C.; Stigter, E.; Gerritsen, J.; Gallego, M.P.; van Es, R.; Li, L.; et al. Metabolic Rewiring in Keratinocytes by miR-31-5p Identifies Therapeutic Intervention for Psoriasis. EMBO Mol. Med. 2023, 15, e15674. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, L.; An, L.; He, L. Hsa-miR-31-3p Targets CLDN8 to Compromise Skin Barrier Integrity in Psoriasis. Biochem. Biophys. Rep. 2025, 42, 101976. [Google Scholar] [CrossRef]
- Guzmán-Martín, C.A.; Jiménez-Ortega, R.F.; Ortega-Springall, M.F.; Peña-Peña, M.; Guerrero-Ponce, A.E.; Vega-Memije, M.E.; Amezcua-Guerra, L.M.; Sánchez-Muñoz, F.; Springall, R. miR-16-5p, miR-21-5p, and miR-155-5p in Circulating Vesicles as Psoriasis Biomarkers. Sci. Rep. 2025, 15, 6971. [Google Scholar] [CrossRef]
- Hoefert, J.E.; Bjerke, G.A.; Wang, D.; Yi, R. The microRNA-200 Family Coordinately Regulates Cell Adhesion and Proliferation in Hair Morphogenesis. J. Cell Biol. 2018, 217, 2185–2204. [Google Scholar] [CrossRef] [PubMed]
- Solvin, Å.Ø.; Chawla, K.; Olsen, L.C.; Hegre, S.A.; Danielsen, K.; Jenssen, M.; Furberg, A.-S.; Saunes, M.; Hveem, K.; Sætrom, P.; et al. MicroRNA Profiling of Psoriatic Skin Identifies 11 miRNAs Associated with Disease Severity. Exp. Dermatol. 2022, 31, 535–547. [Google Scholar] [CrossRef]
- Chicharro, P.; Rodríguez-Jiménez, P.; Llamas-Velasco, M.; Montes, N.; Sanz-García, A.; Cibrian, D.; Vara, A.; Gómez, M.J.; Jiménez-Fernández, M.; Martínez-Fleta, P.; et al. Expression of miR-135b in Psoriatic Skin and Its Association with Disease Improvement. Cells 2020, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lei, L.; Jiang, L.; Zeng, H.; Zhang, Y.; Fu, C.; Guo, H.; Dong, Y.; Ouyang, Y.; Zhang, X.; et al. LncRNA UCA1 Promotes Keratinocyte-Driven Inflammation via Suppressing METTL14 and Activating the HIF-1α/NF-κB Axis in Psoriasis. Cell Death Dis. 2023, 14, 279. [Google Scholar] [CrossRef]
- Ahmed Nour, Z.; Elwan, Y.; Nassar, Y.; Fathy Elmasry, M.; Rashed, L.; Salama Ashour, S. Possible Role of lncRNA MEG3-microRNA-21 and Endoplasmic Reticulum (ER) Stress Proteins in the Pathogenesis of Psoriasis Vulgaris. Rep. Biochem. Mol. Biol. 2022, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Trufin, I.I.; Ungureanu, L.; Halmágyi, S.-R.; Apostu, A.P.; Șenilă, S.C. Long Non-Coding RNAs in Psoriasis and Cutaneous Squamous Cell Carcinoma. J. Clin. Med. 2025, 14, 5081. [Google Scholar] [CrossRef]
- Wang, W.; Sun, L.; Wang, L.; Zhao, J.; She, S.; Hou, P.; He, M. Using 0.1 THz Radiation Regulates Collagen Synthesis Through TGF-β/Smad Signaling Pathway in Human Fetal Scleral Fibroblasts. Cells 2025, 14, 1512. [Google Scholar] [CrossRef]
- Hobbs, R.P.; Jacob, J.T.; Coulombe, P.A. Keratins Are Going Nuclear. Dev. Cell 2016, 38, 227–233. [Google Scholar] [CrossRef]
- Jacob, J.T.; Nair, R.R.; Poll, B.G.; Pineda, C.M.; Hobbs, R.P.; Matunis, M.J.; Coulombe, P.A. Keratin 17 Regulates Nuclear Morphology and Chromatin Organization. J. Cell Sci. 2020, 133, jcs254094. [Google Scholar] [CrossRef]
- Jin, L.; Wang, G. Keratin 17: A Critical Player in the Pathogenesis of Psoriasis. Med. Res. Rev. 2014, 34, 438–454. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, Z.; Dang, E.; Li, B.; Fang, H.; Li, J.; Gao, L.; Zhang, K.; Wang, G. TGFβ/SMAD/microRNA-486-3p Signaling Axis Mediates Keratin 17 Expression and Keratinocyte Hyperproliferation in Psoriasis. J. Investig. Dermatol. 2017, 137, 2177–2186. [Google Scholar] [CrossRef]
- Jang, T.-H.; Huang, W.-C.; Tung, S.-L.; Lin, S.-C.; Chen, P.-M.; Cho, C.-Y.; Yang, Y.-Y.; Yen, T.-C.; Lo, G.-H.; Chuang, S.-E.; et al. MicroRNA-485-5p Targets Keratin 17 to Regulate Oral Cancer Stemness and Chemoresistance via the Integrin/FAK/Src/ERK/β-Catenin Pathway. J. Biomed. Sci. 2022, 29, 42. [Google Scholar] [CrossRef]
- Chen, P.; Pan, M.; Shen, Z.; Yang, Y.; Wang, X. MicroRNA-485-5p Targets Keratin17 to Regulate Pancreatic Cancer Cell Proliferation and Invasion via the FAK/SRC/ERK Pathway. J. Cancer 2024, 15, 2033–2044. [Google Scholar] [CrossRef]
- Yuan, K.; Sun, Y.; Ji, Y. MicroRNA-485-5p Reduces Keratinocyte Proliferation and Migration by Regulating ITGA5 Expression in Skin Wound Healing. Trop. J. Pharm. Res. 2020, 19, 2553–2557. [Google Scholar] [CrossRef]
- Lin, J.; Su, H.; Zhong, Y.; Zheng, H.; Chen, Y. LncRNA LINC01026 Is Overexpressed in Psoriasis and Enhances Keratinocyte Cell Cycle Progression by Regulating the Ets Homologous Factor (EHF). J. Cell Mol. Med. 2025, 29, e70719. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Niu, M.; Fan, X.; Chen, F.; Cao, H.; Liu, Q.; Gan, S.; Yue, P.; Gao, J. LncRNA MIR181A2HG Inhibits Keratinocytes Proliferation through miR-223-3p/SOX6 Axis. Aging 2024, 16, 9846–9858. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, F.; Hua, M.; Guo, J.; Nong, Y.; Tang, Q.; Zhong, F.; Qin, L. Knockdown of lncRNA MIR31HG Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Biol. Res. 2018, 51, 30. [Google Scholar] [CrossRef]
- Liu, X.; Frost, J.; Bowcock, A.; Zhang, W. Canonical and Interior Circular RNAs Function as Competing Endogenous RNAs in Psoriatic Skin. Int. J. Mol. Sci. 2021, 22, 5182. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Li, S.; Zhang, P.; Deng, M.; Su, Y.; Wu, R.; Shen, W. Circular RNA Sequencing Identified circARNTL2 as a Pathogenic Factor in Psoriasis by Facilitating Proliferation and Cell Cycle Progression of Keratinocytes. Clin. Immunol. 2023, 255, 109766. [Google Scholar] [CrossRef]
- He, Q.; Liu, N.; Hu, F.; Shi, Q.; Pi, X.; Chen, H.; Li, J.; Zhang, B. Circ_0061012 Contributes to IL-22-Induced Proliferation, Migration and Invasion in Keratinocytes through miR-194-5p/GAB1 Axis in Psoriasis. Biosci. Rep. 2021, 41, BSR20203130. [Google Scholar] [CrossRef]
- Naguib, R.M.; El-Rifaie, A.-E.; El-Taweel, A.-E.; Dahab, M.M.F.A.A. An Insight into the Value of Circular RNA in Psoriasis Pathogenesis and Its Correlation with PASI Score. Egypt. J. Dermatol. Venerol. 2022, 42, 155. [Google Scholar] [CrossRef]
- Seeler, S.; Moldovan, L.-I.; Bertelsen, T.; Hager, H.; Iversen, L.; Johansen, C.; Kjems, J.; Kristensen, L.S. Global circRNA Expression Changes Predate Clinical and Histological Improvements of Psoriasis Patients upon Secukinumab Treatment. PLoS ONE 2022, 17, e0275219. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Zhao, X.; Liu, J.; Chang, W.; Zhou, L.; Zhang, K. circRNA: Regulatory Factors and Potential Therapeutic Targets in Inflammatory Dermatoses. J. Cell Mol. Med. 2022, 26, 4389–4400. [Google Scholar] [CrossRef]
- Juárez-Vicuña, Y.; Ruiz-Ojeda, D.; González-Ramírez, J.; Flores-Balderas, X.; Springall, R.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A. LncRNA MALAT1 in Keratinocyte Function: A Review of Recent Advances. Noncoding RNA Res. 2024, 9, 594–601. [Google Scholar] [CrossRef]
- Shi, R.; Ma, R.; Jiang, X.; Tang, X.; Gong, Y.; Yu, Z.; Shi, Y. Implications of LncRNAs and CircRNAs in Psoriasis: A Review. RNA Biol. 2023, 20, 334–347. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Liu, S.; Zhang, F.; Zhuang, J.; Chen, Y. MicroRNA-17-3p Is Upregulated in Psoriasis and Regulates Keratinocyte Hyperproliferation and pro-Inflammatory Cytokine Secretion by Targeting CTR9. Eur. J. Histochem. 2022, 66, 3275. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Jin, M.; Li, H.; Li, S. miR-383 Reduces Keratinocyte Proliferation and Induces the Apoptosis in Psoriasis via Disruption of LCN2-Dependent JAK/STAT Pathway Activation. Int. Immunopharmacol. 2021, 96, 107587. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Kalinina, P.; Köcher, M.; Laggner, M.; Jeitler, M.; Abbas Zadeh, S.; Copic, D.; Tschachler, E.; Mildner, M. miR-155 Contributes to Normal Keratinocyte Differentiation and Is Upregulated in the Epidermis of Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 9288. [Google Scholar] [CrossRef]
- Fan, X.; Li, M.; Niu, M.; Chen, F.; Mo, Z.; Yue, P.; Wang, M.; Liu, Q.; Liang, B.; Gan, S.; et al. LncRNA MIR181A2HG Negatively Regulates Human Keratinocytes Proliferation by Binding SRSF1. Cytotechnology 2024, 76, 313–327. [Google Scholar] [CrossRef]
- Gao, P.; Gao, X.; Lin, L.; Zhang, M.; Luo, D.; Chen, C.; Li, Y.; He, Y.; Liu, X.; Shi, C.; et al. Identification of PRKCQ-AS1 as a Keratinocyte-Derived Exosomal lncRNA That Promotes Th17 Differentiation and IL-17 Secretion in Psoriasis Through Bioinformatics, Machine Learning Algorithms, and Cell Experiments. J. Inflamm. Res. 2025, 18, 6557–6582. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.; Wu, J.; Zhang, X.; Miao, F.; Wang, J.; Lu, J.; Liu, J.; Chen, Z.; Tai, Z.; et al. Transdermal Delivery of Fn14 siRNA Using a Novel Composite Ionic Liquid for Treatment of Psoriasis-like Skin Lesions. J. Control. Release 2024, 365, 818–832. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, J.; Wei, K.; Jiang, P.; Shi, Y.; Chang, C.; Zheng, Y.; Shan, Y.; Li, Y.; He, B.; et al. Targeted siRNA Therapy for Psoriasis: Translating Preclinical Potential into Clinical Treatments. Immunotargets Ther. 2024, 13, 259–271. [Google Scholar] [CrossRef]
- Khosrojerdi, M.; Azad, F.J.; Yadegari, Y.; Ahanchian, H.; Azimian, A. The Role of microRNAs in Atopic Dermatitis. Noncoding RNA Res. 2024, 9, 1033–1039. [Google Scholar] [CrossRef]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p Is Increased in Atopic Dermatitis and Has Capacity to Inhibit Keratinocyte Proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Srivastava, A.; Nikamo, P.; Lohcharoenkal, W.; Li, D.; Meisgen, F.; Landén, N.X.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-146a Suppresses IL-17–Mediated Skin Inflammation and Is Genetically Associated with Psoriasis. J. Allergy Clin. Immunol. 2017, 139, 550–561. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Shen, K.; Luo, L.; Zhao, M.; Xu, C.; Jia, Y.; Xiao, D.; Li, Y.; Gao, X.; et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells 2022, 11, 2568. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Huang, Q.; Yang, J.; Li, B.; Ma, K.; Wei, Q.; Wang, Y.; Su, J.; Sun, M.; et al. MiR146a-Loaded Engineered Exosomes Released from Silk Fibroin Patch Promote Diabetic Wound Healing by Targeting IRAK1. Signal Transduct. Target. Ther. 2023, 8, 62. [Google Scholar] [CrossRef]
- Gondaliya, P.; Sayyed, A.A.; Bhat, P.; Mali, M.; Arya, N.; Khairnar, A.; Kalia, K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol. Pharm. 2022, 19, 1294–1308. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, e1902232. [Google Scholar] [CrossRef]
- Jia, H.-Y.; Zhang, K.; Lu, W.-J.; Xu, G.-W.; Zhang, J.-F.; Tang, Z.-L. LncRNA MEG3 Influences the Proliferation and Apoptosis of Psoriasis Epidermal Cells by Targeting miR-21/Caspase-8. BMC Mol. Cell Biol. 2019, 20, 46. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Pickup, M.E.; Rimmer, A.G.; Alam, M.; Mardaryev, A.N.; Poterlowicz, K.; Botchkareva, N.V.; Botchkarev, V.A. Interplay of MicroRNA-21 and SATB1 in Epidermal Keratinocytes during Skin Aging. J. Investig. Dermatol. 2019, 139, 2538–2542.e2. [Google Scholar] [CrossRef]
- Abdallah, F.; Henriet, E.; Suet, A.; Arar, A.; Clemençon, R.; Malinge, J.-M.; Lecellier, G.; Baril, P.; Pichon, C. miR-21-3p/IL-22 Axes Are Major Drivers of Psoriasis Pathogenesis by Modulating Keratinocytes Proliferation-Survival Balance and Inflammatory Response. Cells 2021, 10, 2547. [Google Scholar] [CrossRef]
- Conley, J.; Genenger, B.; Ashford, B.; Ranson, M. Micro RNA Dysregulation in Keratinocyte Carcinomas: Clinical Evidence, Functional Impact, and Future Directions. Int. J. Mol. Sci. 2024, 25, 8493. [Google Scholar] [CrossRef]
- Martinez Junior, A.M.; Ruiz, T.F.R.; Vilamaior, P.S.L.; Tiera, V.A.d.O.; Taboga, S.R.; Tiera, M.J. Topical Delivery of siRNA to Psoriatic Skin Model Using High Molecular Weight Chitosan Derivatives: In Vitro and in Vivo Studies. Drug Deliv. Transl. Res. 2025, 15, 3199–3225. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Y.; Ma, X.; Duan, Z.; Xu, H.; Li, Y.; Kong, Y.; Yang, L.; Xin, X. Topical Therapy in Psoriasis: Clinical Benefits, Advances in Novel Drug Delivery Strategies, and Gene Therapy Regimen. Pharmaceutics 2025, 17, 283. [Google Scholar] [CrossRef]
- Lin, Z.-C.; Hung, C.-F.; Aljuffali, I.A.; Lin, M.-H.; Fang, J.-Y. RNA-Based Antipsoriatic Gene Therapy: An Updated Review Focusing on Evidence from Animal Models. DDDT 2024, 18, 1277–1296. [Google Scholar] [CrossRef]
- Gao, S.; Cheng, X.; Zhang, M.; Dai, Q.; Liu, C.; Lu, Y. Design Principles and Applications of Ionic Liquids for Transdermal Drug Delivery. Adv. Sci. 2024, 11, 2405983. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Wu, X.; Li, D. MicroRNA-939 Amplifies Staphylococcus Aureus-Induced Matrix Metalloproteinase Expression in Atopic Dermatitis. Front. Immunol. 2024, 15, 1354154. [Google Scholar] [CrossRef]
- Nappi, F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 3630. [Google Scholar] [CrossRef]
- Kieser, R.E.; Khan, S.; Bejar, N.; Kiss, D.L. The Dawning of a New Enterprise: RNA Therapeutics for the Skin. J. Dermatol. Ski. Sci. 2023, 5, 4–13. [Google Scholar] [CrossRef]
- Ruzi, Z.; Han, D.; Aierken, K. Advanced Strategies for Screening and Identifying RNA-Targeted Small Molecules: Bridging Therapeutic Potential and Innovation. Results Chem. 2025, 15, 102305. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Kumar, S.; Mitragotri, S. Nucleic Acid Delivery into Skin for the Treatment of Skin Disease: Proofs-of-Concept, Potential Impact, and Remaining Challenges. J. Control. Release 2015, 219, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romashin, D.D.; Tolstova, T.V.; Rusanov, A.L.; Luzgina, N.G. Non-Coding RNAs and Their Role in Maintaining Epidermal Homeostasis. Curr. Issues Mol. Biol. 2025, 47, 924. https://doi.org/10.3390/cimb47110924
Romashin DD, Tolstova TV, Rusanov AL, Luzgina NG. Non-Coding RNAs and Their Role in Maintaining Epidermal Homeostasis. Current Issues in Molecular Biology. 2025; 47(11):924. https://doi.org/10.3390/cimb47110924
Chicago/Turabian StyleRomashin, Daniil D., Tatiana V. Tolstova, Alexander L. Rusanov, and Natalia G. Luzgina. 2025. "Non-Coding RNAs and Their Role in Maintaining Epidermal Homeostasis" Current Issues in Molecular Biology 47, no. 11: 924. https://doi.org/10.3390/cimb47110924
APA StyleRomashin, D. D., Tolstova, T. V., Rusanov, A. L., & Luzgina, N. G. (2025). Non-Coding RNAs and Their Role in Maintaining Epidermal Homeostasis. Current Issues in Molecular Biology, 47(11), 924. https://doi.org/10.3390/cimb47110924






