Lights and Shadows of Essential Oil-Derived Compounds: Antimicrobial and Anti-Inflammatory Properties of Eugenol, Thymol, Cinnamaldehyde, and Carvacrol
Abstract
1. Introduction
2. Materials and Methods
Search Strategy
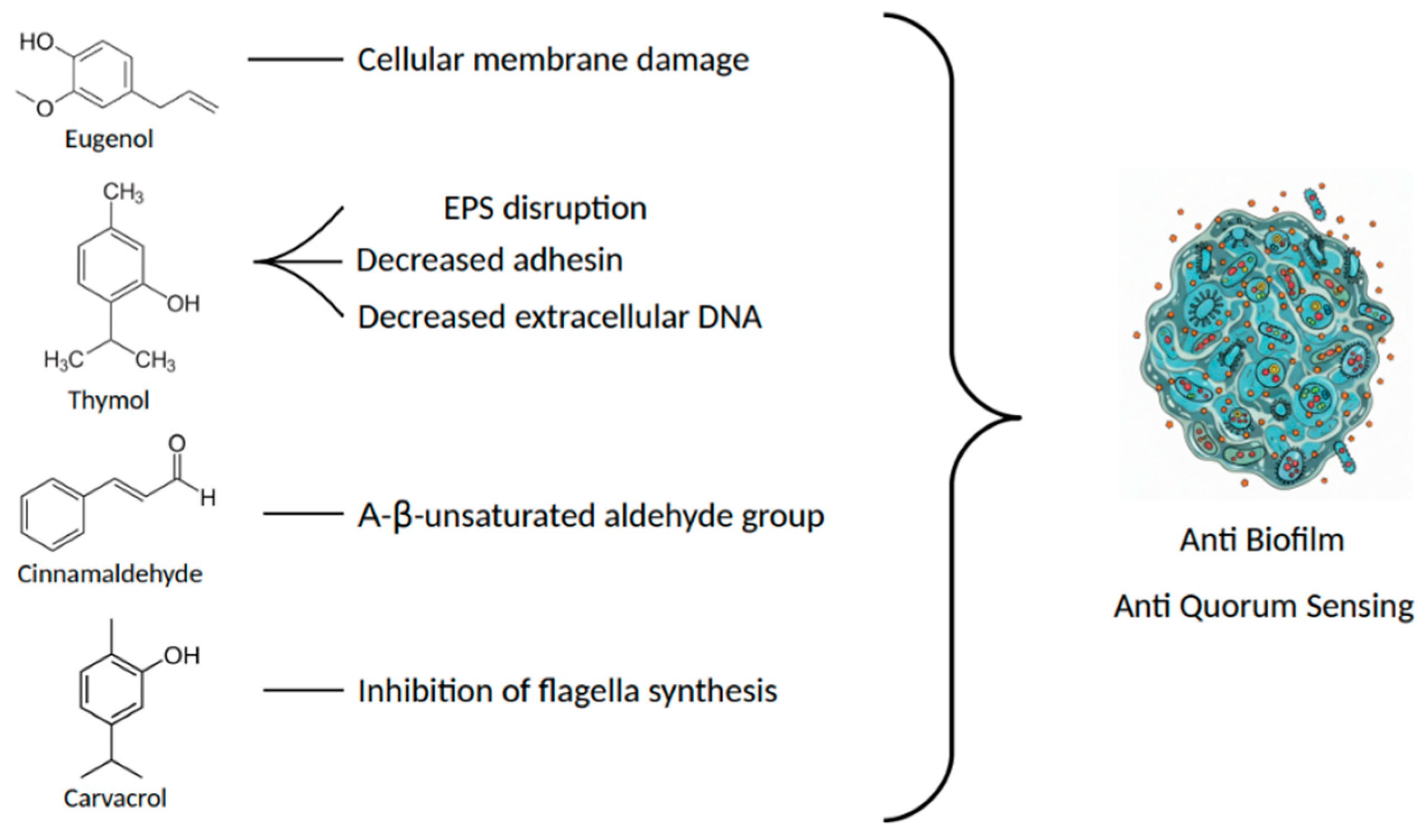
3. Narrative Description
3.1. Eugenol
3.2. Thymol
3.3. Cinnamaldehyde
3.4. Carvacrol
3.5. Therapeutic Potential and Clinical Considerations
3.6. Synergistic Activity of Essential Oils with Antimicrobials Molecules
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of Quorum Sensing Regulated Bacterial Functions by Plant Essential Oils with Special Reference to Clove Oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef]
- Olasupo, N.A.; Fitzgerald, D.J.; Gasson, M.J.; Narbad, A. Activity of Natural Antimicrobial Compounds against Escherichia Coli and Salmonella Enterica Serovar Typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D. de B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus Vulgaris, L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Huang, Z.; Li, Q.; Wang, E.; Liao, S.; Li, E.; Zou, Y.; Wang, W. Antibacterial Mechanism of Cinnamaldehyde: Modulation of Biosynthesis of Phosphatidylethanolamine and Phosphatidylglycerol in Staphylococcus aureus and Escherichia coli. J. Agric. Food Chem. 2021, 69, 13628–13636. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.C.; Whittall, J.J.; Polyak, S.W.; Foo, K.; Li, X.; Dutschke, C.J.; Ogunniyi, A.D.; Ma, S.; Sykes, M.J.; Semple, S.J.; et al. Cinnamaldehyde derivatives act as antimicrobial agents against Acinetobacter baumannii through the inhibition of cell division. Front. Microbiol. 2022, 13, 967949. [Google Scholar] [CrossRef]
- Jaramillo Jimenez, B.A.; Awwad, F.; Desgagné-Penix, I. Cinnamaldehyde in Focus: Antimicrobial Properties, Biosynthetic Pathway, and Industrial Applications. Antibiotics 2024, 13, 1095. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia Coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Chemical Composition, Antimicrobial and Antibiotic Potentiating Activity of Essential Oils from 10 Tropical Medicinal Plants from Mauritius. J. Herb. Med. 2016, 6, 88–95. [Google Scholar] [CrossRef]
- Maahury, M.F. Molecular Structure and Electronic Properties of Eugenol and Its Analogues Using DFT. J. Kim. Mul. 2022, 19, 58–62. [Google Scholar] [CrossRef]
- Ferrando, N.; Pino-Otín, M.R.; Terrado, E.; Ballestero, D.; Langa, E. Bioactivity of Eugenol: A Potential Antibiotic Adjuvant with Minimal Ecotoxicological Impact. Int. J. Mol. Sci. 2024, 25, 7069. [Google Scholar] [CrossRef]
- Aljuwayd, M.; Olson, E.G.; Abbasi, A.Z.; Rothrock, M.J.; Ricke, S.C.; Kwon, Y.M. Potential Involvement of Reactive Oxygen Species in the Bactericidal Activity of Eugenol against Salmonella Typhimurium. Pathogens 2024, 13, 899. [Google Scholar] [CrossRef]
- Pathirana, H.N.K.S.; Wimalasena, S.H.M.P.; De Silva, B.C.J.; Hossain, S.; Gang-Joon, H. Antibacterial activity of clove essential oil and eugenol against fish pathogenic bacteria isolated from cultured olive flounder (Paralichthys olivaceus) Slov. Vet. Res. 2019, 56, 31–38. [Google Scholar] [CrossRef]
- Suzuki, É.Y.; Baptista, E.B.; Resende Do Carmo, A.M.; Miranda Chaves, M.D.G.A.; Chicourel, E.L.; Barbosa Raposo, N.R. Potential of the Essential Oil from Pimenta Pseudocaryophyllus as an Antimicrobial Agent. Acta Pharm. 2014, 64, 379–385. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; Cao, J.; Chen, L.; McLandsborough, L.; Weiss, J. Inactivation of Listeria Monocytogenes and Escherichia Coli O157:H7 Biofilms by Micelle-Encapsulated Eugenol and Carvacrol. J. Food Prot. 2011, 74, 55–62. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Im, G.J.; Chung, J.-W.; Song, J.-J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Dehkordi, N.H.; Tajik, H.; Moradi, M.; Kousheh, S.A.; Molaei, R. Antibacterial Interactions of Colloid Nanosilver with Eugenol and Food Ingredients. J. Food Prot. 2019, 82, 1783–1792. [Google Scholar] [CrossRef]
- Albano, M.; Alves, F.C.B.; Andrade, B.F.M.T.; Barbosa, L.N.; Pereira, A.F.M.; de Lourdes Ribeiro de Souza da Cunha, M.; Rall, V.L.M.; Fernandes Júnior, A. Antibacterial and Anti-Staphylococcal Enterotoxin Activities of Phenolic Compounds. Innov. Food Sci. Emerg. Technol. 2016, 38, 83–90. [Google Scholar] [CrossRef]
- Kong, J.; Wang, Y.; Yao, Z.; Lin, Y.; Zhang, Y.; Han, Y.; Zhou, T.; Ye, J.; Cao, J. Eugenol Works Synergistically with Colistin against Colistin-Resistant Pseudomonas Aeruginosa and Klebsiella Pneumoniae Isolates by Enhancing Membrane Permeability. Microbiol. Spectr. 2023, 11, e03666-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, G.; Dou, K.; Yi, B.; Wang, D.; Zhou, Q.; Sun, Y. Eugenol Eliminates Carbapenem-Resistant Klebsiella Pneumoniae via Reactive Oxygen Species Mechanism. Front. Microbiol. 2023, 14, 1090787. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Baig, M.H. Eugenol Inhibits Quorum Sensing and Biofilm of Toxigenic MRSA Strains Isolated from Food Handlers Employed in Saudi Arabia. Biotechnol. Biotechnol. Equip. 2017, 31, 387–396. [Google Scholar] [CrossRef]
- He, M.; Du, M.; Fan, M.; Bian, Z. In Vitro Activity of Eugenol against Candida Albicans Biofilms. Mycopathologia 2007, 163, 137–143. [Google Scholar] [CrossRef]
- Bakshi, J.; Singh, S.; Mishra, K. Coumarin and Eugenol Ameliorate LPS-Induced Inflammation in RAW 264.7 Cells via Modulating the NLRP3 Inflammasome Pathway. Asian Pac. J. Trop. Biomed. 2024, 14, 40–46. [Google Scholar] [CrossRef]
- Permatananda, P.A.N.K.; Yasa, I.W.P.S.; Sumardika, I.W.; Saraswati, M.R.; Pandit, I.G.S. From Tradition to Translation: A Systematic Review on the Pharmacological Actions of Eugenol Extracted from Ocimum Plants in Oxidative Stress, Inflammation, and Diabetes Mellitus. Bioscmed 2025, 9, 7648–7663. [Google Scholar] [CrossRef]
- Zhu, J.; Park, S.; Kim, C.H.; Jeong, K.H.; Kim, W.-J. Eugenol Alleviates Neuronal Damage via Inhibiting Inflammatory Process against Pilocarpine-Induced Status Epilepticus. Exp. Biol. Med. 2023, 248, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.I.; Gerasymchuk, M.; Zanikov, T.; Gojani, E.G.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cruz, C.; et al. LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice. Pharmaceuticals 2025, 18, 451. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Murakami, Y. Eugenol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 45. [Google Scholar] [CrossRef]
- Guénette, S.A.; Ross, A.; Marier, J.-F.; Beaudry, F.; Vachon, P. Pharmacokinetics of Eugenol and Its Effects on Thermal Hypersensitivity in Rats. Eur. J. Pharmacol. 2007, 562, 60–67. [Google Scholar] [CrossRef]
- Tithy, S.A.; Hossain, M.M.; Shill, D.K.; Hossain, M.S.; Islam, A.; Molla, M.I.; Jahan, S. Effects of Syzygium Aromaticum (Clove) Extract on the Acquisition and Expression of Morphine-Induced Conditioned Place Preference in Swiss Albino Mice: An In Vivo, In Silico Approach. Dhaka Univ. J. Pharm. Sci. 2024, 23, 77–92. [Google Scholar] [CrossRef]
- Kashi, M.; Noei, M.; Chegini, Z.; Shariati, A. Natural Compounds in the Fight against Staphylococcus Aureus Biofilms: A Review of Antibiofilm Strategies. Front. Pharmacol. 2024, 15, 1491363. [Google Scholar] [CrossRef]
- Wan, J.; Li, H.; Fu, S.; Chen, Y.; Li, H. Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation 2018, 41, 643–653. [Google Scholar] [CrossRef]
- Pavan, B.; Bianchi, A.; Botti, G.; Ferraro, L.; Valerii, M.C.; Spisni, E.; Dalpiaz, A. Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells. Int. J. Mol. Sci. 2023, 24, 1800. [Google Scholar] [CrossRef]
- Panda, N.; Panda, A. Piper Betel—Not Just a Mouth Freshener But Also a Miraculous Herb for Healing: A Review. Asian J. Biol. 2023, 18, 20–33. [Google Scholar] [CrossRef]
- Nagoor Mee\ran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-Vitro Antimicrobial Activity and Chemical Composition of Sardinian Thymus Essential Oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.G.; Di Stefano, V.; La Giglia, M.; Di Marco Lo Presti, V.; Schillaci, D.; Pomilio, F.; Vitale, M. Control of Growth and Persistence of Listeria Monocytogenes and β-Lactam-Resistant Escherichia Coli by Thymol in Food Processing Settings. Molecules 2020, 25, 383. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Mo, H.; Zhao, Y.; Li, H.; Zhang, H.; Hu, L.; Zhou, X. Thymol Mediates Bactericidal Activity against Staphylococcus Aureus by Targeting an Aldo–Keto Reductase and Consequent Depletion of NADPH. J. Agric. Food Chem. 2019, 67, 8382–8392. [Google Scholar] [CrossRef]
- Ngome, M.T.; Alves, J.G.L.F.; De Oliveira, A.C.F.; Da Silva Machado, P.; Mondragón-Bernal, O.L.; Piccoli, R.H. Linalool, Citral, Eugenol and Thymol: Control of Planktonic and Sessile Cells of Shigella Flexneri. AMB Express 2018, 8, 105. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the Proteome of Salmonella Enterica Serovar Thompson as Stress Adaptation to Sublethal Concentrations of Thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Samaha, I.; Aiad, A.; Eskander, M.; Radwan, M. Occurrence of Staphylococcus Aureus in Various Retail Meat Products and Evaluation of Thymol-Based Decontamination Trials. Alex. J. Vet. Sci. 2025, 84, 94–103. [Google Scholar] [CrossRef]
- Marei, G.; Rabea, E.; Badawy, M. In Vitro Antimicrobial and Antioxidant Activities of Monoterpenes against Some Food-Borne Pathogens. J. Plant Prot. Pathol. 2019, 10, 87–94. [Google Scholar] [CrossRef]
- Nunes, J.; Farias, I.; Vieira, C.; Ribeiro, T.; Sampaio, F.; Menezes, V. Antimicrobial Activity and Toxicity of Glass Ionomer Cement Containing an Essential Oil. Braz. J. Med Biol. Res. 2020, 53, e9468. [Google Scholar] [CrossRef]
- Ammar, M.A.M.; Heneter, A.M.; El-Khateib, T.S.A.; Abd El-Malek, A.M.; Abo Markeb, A.M.A. Nanoparticles-Phenolics as Anti Salmonella Typhimurium. Assiut Vet. Med. J. 2021, 67, 59–80. [Google Scholar] [CrossRef]
- Farhadi, K.; Rajabi, E.; Varpaei, H.A.; Iranzadasl, M.; Khodaparast, S.; Salehi, M. Thymol and Carvacrol against Klebsiella: Anti-Bacterial, Anti-Biofilm, and Synergistic Activities—A Systematic Review. Front. Pharmacol. 2024, 15, 1487083. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-R.; Oh, J.; Cho, S.I. Inhibitory Effect of Thymol on Tympanostomy Tube Biofilms of Methicillin-Resistant Staphylococcus Aureus and Ciprofloxacin-Resistant Pseudomonas Aeruginosa. Microorganisms 2022, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, H.; Tunjung Pratiwi, S.U.; Hertiani, T. Efficacy of Thymol and Eugenol Against Polymicrobial Biofilm. Indonesian J. Pharm. 2018, 29, 214. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus Aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Bazzazan, S.; Sorourian, G.; Sorourian, M.; Akhavanzanjani, Y.; Noorbazargan, H.; Ren, Q. Encapsulation of Thymol in Gelatin Methacryloyl (GelMa)-Based Nanoniosome Enables Enhanced Antibiofilm Activity and Wound Healing. Pharmaceutics 2023, 15, 1699. [Google Scholar] [CrossRef]
- Al-Khrashi, L.A.; Badr, A.M.; Al-Amin, M.A.; Mahran, Y.F. Thymol Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis: Evidence of down-Regulatory Effect on TGF-β/MAPK Pathways through NF-κB. J. Biochem. Mol. Toxicol. 2022, 36, e22932. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, J.; Zhang, R.; Li, X.; Lv, R.; Nan, H.; Liu, J.; Zhao, Z.; He, W.; Wang, L. Huoxiang Zhengqi Oral Liquid Attenuates LPS-Induced Acute Lung Injury by Modulating Short-Chain Fatty Acid Levels and TLR4/NF- κ B P65 Pathway. BioMed Res. Int. 2023, 2023, 6183551. [Google Scholar] [CrossRef]
- Erzurumlu, Y.; Dogan, H.K.; Catakli, D. Thymol Reduces the Lipopolysaccharide-Induced Acute Kidney Inflammation by Modulating Lysosomal Stress. J. Res. Pharm. 2023, 27, 722–732. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Liu, S.; Gao, L.; Shan, A. Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway. Nutrients 2022, 14, 2809. [Google Scholar] [CrossRef]
- Bacova, K.; Eglseer, K.Z.; Räuber, G.K.; Chrastinova, L.; Laukova, A.; Takacsova, M.; Simonova, M.P.; Placha, I. Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits. Animals 2021, 11, 2595. [Google Scholar] [CrossRef]
- Placha, I.; Bacova, K.; Plachy, L. Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes. Animals 2022, 12, 1131. [Google Scholar] [CrossRef]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.; Veit, M. Systemic Availability and Pharmacokinetics of Thymol in Humans. J. Clin. Pharma 2002, 42, 731–737. [Google Scholar] [CrossRef]
- Yao, L.; Hou, G.; Wang, L.; Zuo, X.; Liu, Z. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb. Pathog. 2018, 116, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zinn, S.; Betz, T.; Medcraft, C.; Schnell, M. Structure Determination of Trans-Cinnamaldehyde by Broadband Microwave Spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 16080–16085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Chhabra, R.; Rani, J.; Chauhan, A.; Kaur, I.; Kapoor, G. Oil/Water (O/W) Nanoemulsions Developed from Essential Oil Extracted from Wildly Growing Calotropis Gigantea (Linn.) Aiton F.: Synthesis, Characterization, Stability and Evaluation of Anti-Cancerous, Anti-Oxidant, Anti-Inflammatory and Anti-Diabetic Activities. J. Biomater. Sci. Polym. Ed. 2024, 35, 2506–2527. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial Activity of Cinnamon Essential Oils and Their Synergistic Potential with Antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Braga Paiano, R.; Bonilla, J.; Moro De Sousa, R.L.; Micke Moreno, A.; Sampaio Baruselli, P. Chemical Composition and Antibacterial Activity of Essential Oils against Pathogens Often Related to Cattle Endometritis. J. Infect. Dev. Ctries. 2020, 14, 177–183. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Matar, M.A.; Alkersh, B.M. Evaluation of the antibacterial activity of cinnamon essential oil and its individual compounds on Aggregatibacter actinomycetemcomitans isolated from black extrinsic tooth stain: An in vitro study. Eur. Arch. Paediatr. Dent. 2023, 24, 661. [Google Scholar] [CrossRef]
- Yin, L.; Gou, Y.; Dai, Y.; Wang, T.; Gu, K.; Tang, T.; Hussain, S.; Huang, X.; He, C.; Liang, X.; et al. Cinnamaldehyde Restores Ceftriaxone Susceptibility against Multidrug-Resistant Salmonella. Int. J. Mol. Sci. 2023, 24, 9288. [Google Scholar] [CrossRef]
- Mireles, N.A.; Malla, C.F.; Tavío, M.M. Cinnamaldehyde and Baicalin Reverse Colistin Resistance in Enterobacterales and Acinetobacter Baumannii Strains. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1899–1908. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of Cinnamon Bark Oil and Cinnamaldehyde on Anti-Multidrug Resistant Pseudomonas Aeruginosa and the Synergistic Effects in Combination with Other Antimicrobial Agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda Jr, F.E.A. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, R.; Golus, J.; Przekora, A.; Ludwiczuk, A.; Sieniawska, E.; Ginalska, G. Antimycobacterial Activity of Cinnamaldehyde in a Mycobacterium Tuberculosis(H37Ra) Model. Molecules 2018, 23, 2381. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Cho, K.-H.; Lee, J.-H.; Lee, J. Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia Coli and Staphylococcus Aureus. Int. J. Mol. Sci. 2022, 23, 7225. [Google Scholar] [CrossRef]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of Cinnamaldehyde on Biofilm Formation and sarA Expression by Methicillin-Resistant Staphylococcus Aureus: Effect of Cinnamaldehyde against MRSA Biofilms. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef]
- Subathra, L.; Varsha, M.; Visali, K.; Rubavathi, A.; Murugan, A. Synergistic Quorum Quenching Activity of Cinnamaldehyde against Biofilm Forming Pseudomonas Aeruginosa. Res. J. Biotech. 2024, 19, 116–126. [Google Scholar] [CrossRef]
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial Activity of Cinnamaldehyde on Streptococcus Mutans Biofilms. Front. Microbiol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Lee, S.-C.; Wang, S.-Y.; Li, C.-C.; Liu, C.-T. Anti-Inflammatory Effect of Cinnamaldehyde and Linalool from the Leaf Essential Oil of Cinnamomum Osmophloeum Kanehira in Endotoxin-Induced Mice. J. Food Drug Anal. 2018, 26, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, E.; Lee, K.; Lee, D.-C. Cinnamaldehyde Derivatives Inhibit Degranulation and Inflammatory Mediator Production in Rat Basophilic Leukemia Cells. Int. Immunopharmacol. 2016, 38, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Azouz, A.A.; Saleh, E.; Abo-Saif, A.A. Aliskiren, Tadalafil, and Cinnamaldehyde Alleviate Joint Destruction Biomarkers; MMP-3 and RANKL; in Complete Freund’s Adjuvant Arthritis Model: Downregulation of IL-6/JAK2/STAT3 Signaling Pathway. Saudi Pharm. J. 2020, 28, 1101–1111. [Google Scholar] [CrossRef]
- Cheng, W.-X.; Zhong, S.; Meng, X.-B.; Zheng, N.-Y.; Zhang, P.; Wang, Y.; Qin, L.; Wang, X.-L. Cinnamaldehyde Inhibits Inflammation of Human Synoviocyte Cells Through Regulation of Jak/Stat Pathway and Ameliorates Collagen-Induced Arthritis in Rats. J. Pharmacol. Exp. Ther. 2020, 373, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos Santos, V.L.; De Assis Gonsalves, A.; Souza Silva, M.F.; Evangelista De Oliveira, F.D.C.; Pinheiro Da Costa, M.; Pessoa, C.O.; Araújo, C.R.M. Hydrazones Derived from Natural Aldehydes: In Vitro Cytotoxic Evaluation and in Silico Pharmacokinetic Predictions. Rev. Colomb. Cienc. Quím. Farm. 2021, 50, 217–235. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, B.K.; Singh, A.; Shekhar Azad, C.; K. Yadav, M.; Kumar, A.; Kumar, R.; Kumar, A.; Chaudhary, P. Pharmacokinetic Screening to Estimate the Drug Likeliness Characteristics of Selected Herbal Anticancer Drugs. Res. J. Pharm. Technol. 2023, 16, 3422–3426. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Kirimer, N.; Başer, K.H.C.; Tümen, G. Carvacrol-Rich Plants in Turkey. Chem. Nat. Compd. 1995, 31, 37–41. [Google Scholar] [CrossRef]
- Gochev, V.K.; Girova, T.D. Antimicrobial Activity of Various Essential Oils Against Spoilage and Pathogenic Microorganisms Isolated from Meat Products. Biotechnol. Biotechnol. Equip. 2009, 23, 900–904. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic Application of Carvacrol: A Comprehensive Review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Alagawany, M. Biological Effects and Modes of Action of Carvacrol in Animal and Poultry Production and Health—A Review. Adv. Anim. Vet. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of Methicillin-Resistant Staphylococci to Oregano Essential Oil, Carvacrol and Thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Ravishankar, S.; Zhu, L.; Reyna-Granados, J.; Law, B.; Joens, L.; Friedman, M. Carvacrol and Cinnamaldehyde Inactivate Antibiotic-Resistant Salmonella Enterica in Buffer and on Celery and Oysters. J. Food Prot. 2010, 73, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Shariati, A.; Noei, M.; Askarinia, M.; Khoshbayan, A.; Farahani, A.; Chegini, Z. Inhibitory Effect of Natural Compounds on Quorum Sensing System in Pseudomonas Aeruginosa: A Helpful Promise for Managing Biofilm Community. Front. Pharmacol. 2024, 15, 1350391. [Google Scholar] [CrossRef]
- Ismail, S.; Gaglione, R.; Masi, M.; Padhi, S.; Rai, A.K.; Omar, G.; Cimmino, A.; Arciello, A. Ephedra Foeminea as a Novel Source of Antimicrobial and Anti-Biofilm Compounds to Fight Multidrug Resistance Phenotype. Int. J. Mol. Sci. 2023, 24, 3284. [Google Scholar] [CrossRef]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and Anti-Biofilm Properties of Carvacrol Alone and in Combination with Cefixime against Escherichia Coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Casalini, S.; Giacinti Baschetti, M. The Use of Essential Oils in Chitosan or Cellulose-based Materials for the Production of Active Food Packaging Solutions: A Review. J. Sci. Food Agric. 2023, 103, 1021. [Google Scholar] [CrossRef]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial Action of Carvacrol at Different Stages of Dual-Species Biofilm Development by Staphylococcus Aureus and Salmonella Enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.C.S.; Santos, P.R.; Da Silva, A.F.; Dos Santos, A.R.; Machinski, M.; Mikcha, J.M.G. Effect of Carvacrol and Thymol on Salmonella Spp. Biofilms on Polypropylene. Int. J. Food Sci. Technol. 2015, 50, 2639. [Google Scholar] [CrossRef]
- Alves Coelho Trevisan, D.; Aline Zanetti Campanerut-Sá, P.; Da Silva, A.F.; Farias Pereira Batista, A.; Seixas, F.A.V.; Peralta, R.M.; De Sá-Nakanishi, A.B.; De Abreu Filho, B.A.; Machinski Junior, M.; Graton Mikcha, J.M. Action of Carvacrol in Salmonella Typhimurium Biofilm: A Proteomic Study. J. Appl. Biomed. 2020, 18, 106. [Google Scholar] [CrossRef]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Majeed, H.; Bian, Y.-Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential Oil Encapsulations: Uses, Procedures, and Trends. RSC Adv. 2015, 5, 58449. [Google Scholar] [CrossRef]
- Peng, X.; Xu, L.; Zeng, M.; Dang, H. Application and Development Prospect of Nanoscale Iron Based Metal-Organic Frameworks in Biomedicine. Int. J. Nanomed. 2023, 18, 4907–4931. [Google Scholar] [CrossRef]
- López−Gómez, A.; Navarro-Martínez, A.; Garre, A.; Iguaz, A.; Maldonado-Guzmán, P.; Martínez-Hernández, G.B. Kinetics of Carvacrol Release from Active Paper Packaging for Fresh Fruits and Vegetables Under Conditions of Open and Closed Package. Food Packag. Shelf Life 2023, 37, 101081. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Cruxen, C.E.d.S.; Bruni, G.P.; Fiorentini, Â.M.; Zavareze, E.d.R.; Lim, L.-T.; Dias, A.R.G. Development of Antimicrobial and Antioxidant Electrospun Soluble Potato Starch Nanofibers Loaded with Carvacrol. Int. J. Biol. Macromol. 2019, 139, 1182–1190. [Google Scholar] [CrossRef]
- Oliveira, F.; Andrade, L.; De Sousa, É.; De Sousa, D. Anti-Ulcer Activity of Essential Oil Constituents. Molecules 2014, 19, 5717–5747. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Xavier, M.A.; De Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.H.; Antoniolli, Â.R.; Oliveira, R.C.M.; et al. Carvacrol Attenuates Mechanical Hypernociception and Inflammatory Response. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 253–263. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Quintans-Júnior, L.J.; De Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-Inflammatory Effects of Carvacrol: Evidence for a Key Role of Interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Dal Sasso, M.; Culici, M.; Bianchi, T.; Bordoni, L.; Marabini, L. Anti-Inflammatory Activity of Thymol: Inhibitory Effect on the Release of Human Neutrophil Elastase. Pharmacology 2006, 77, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.D.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and Essential Oils Containing Such Monoterpenes on Wound Healing: A Systematic Review. J. Pharm. Pharmacol. 2019, 71, 141. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Ghorani, V.; Boskabady, M.H. Experimental and Clinical Evidence on the Effect of Carvacrol on Respiratory, Allergic, and Immunologic Disorders: A Comprehensive Review. BioFactors 2022, 48, 779. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; El Awdan, S.A.; Hassan, A.; Abdallah, H.M.I. Cardioprotective Effect of Thymol against Adrenaline-Induced Myocardial Injury in Rats. Heliyon 2020, 6, e04431. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Said, M.B. Plant Bioactive Ingredients in Delivery Systems and Nanocarriers for the Treatment of Leishmaniasis: An Evidence-Based Review. Iran. J. Parasitol. 2022, 17, 458–472. [Google Scholar] [CrossRef]
- Austgulen, L.; Solheim, E.; Scheline, R.R. Metabolism in Rats of p-Cymene Derivatives: Carvacrol and Thymol. Pharmacol. Toxicol. 1987, 61, 98. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In Vitro Degradation and in Vivo Passage Kinetics of Carvacrol, Thymol, Eugenol and Trans-cinnamaldehyde along the Gastrointestinal Tract of Piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446. [Google Scholar] [CrossRef]
- JECFA. Eugenol. In Safety Evaluation of Certain Food Additives and Contaminants; WHO Food Additives Series 44; World Health Organization: Geneva, Switzerland, 2020. Available online: https://inchem.org/documents/jecfa/jecmono/v44jec08.htm (accessed on 22 October 2025).
- EFSA CEF Panel. Scientific Opinion on Flavouring Group Evaluation 60 (FGE.60): Consideration of eugenol and related hydroxyallylbenzenes evaluated by JECFA (65th meeting). EFSA J. 2010, 8, 1632. [Google Scholar] [CrossRef]
- National Institutes of Health. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551727 (accessed on 22 October 2025).
- EFSA FEEDAP Panel. Scientific Opinion on the safety and efficacy of thymol as a feed additive for all animal species. EFSA J. 2012, 10, 2607. [Google Scholar] [CrossRef]
- EFSA FEEDAP Panel. Safety and efficacy of a feed additive consisting of thymol and related essential oils for all animal species. EFSA J. 2017, 15, 5052. [Google Scholar] [CrossRef]
- JECFA. Cinnamaldehyde. In Safety Evaluation of Certain Food Additives and Contaminants; WHO Food Additives Series 46; World Health Organization: Geneva, Switzerland, 2001. Available online: https://inchem.org/documents/jecfa/jecmono/v46je10.htm (accessed on 22 October 2025).
- Posadzki, P.; Alotaibi, A.; Ernst, E. Adverse Effects of Aromatherapy: A Systematic Review of Case Reports and Case Series. Int. J. Risk Saf. Med. 2012, 24, 147–161. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs on Fragrance Raw Materials. Food Cosmet. Toxicol. 1979, 17, 357–390. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- van Swaaij, B.W.M.; Van der Weijden, G.A.; Smith, R.J.; Timmerman, M.F.; Slot, D.E. Essential oils mouthwash with or without alcohol in relation to effect on parameters of plaque and gingivitis: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2025, 23, 186–202. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429. [Google Scholar] [CrossRef] [PubMed]
- Drioiche, A.; Baammi, S.; Zibouh, K.; Al Kamaly, O.; Alnakhli, A.M.; Remok, F.; Saidi, S.; Amaiach, R.; El Makhoukhi, F.; Elomri, A.; et al. A Study of the Synergistic Effects of Essential Oils from Origanum compactumandOriganum elongatum with Commercial Antibiotics against Highly Prioritized Multidrug-Resistant Bacteria for the World Health Organization. Metabolites 2024, 14, 210. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic activity of thymol with commercial antibiotics against critical and high WHO priority pathogenic bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Soulaimani, B. Comprehensive Review of the Combined Antimicrobial Activity of Essential Oil Mixtures and Synergism with Conventional Antimicrobials. Nat. Prod. Commun. 2025, 20, 1934578X251328241. [Google Scholar] [CrossRef]
- Abdel-Halim, M.S.; Askoura, M.; Mansour, B.; Yahya, G.; El-Ganiny, A.M. In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates. J. Antibiot. 2022, 75, 679–690. [Google Scholar] [CrossRef]
- Köse, E.O. In vitro activity of carvacrol in combination with meropenem against carbapenem-resistant Klebsiella pneumoniae. Folia Microbiol. 2022, 67, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bhando, T.; Casius, A.; Gupta, R.; Pathania, R. Deciphering meropenem persistence in Acinetobacter baumannii facilitates discovery of anti- persister activity of thymol. Antimicrob. Agents Chemother. 2025, 69, e0138124. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Wang, L.; Ammeter, E.; Lahaye, L.; Liu, S.; Nyachoti, M.; Yang, C. Evaluation of lipid matrix microencapsulation for intestinal delivery of thymol in weaned pigs. Transl. Anim. Sci. 2019, 4, 411–422. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Maeng, H.J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]

| Gram-Positive | MIC | References |
|---|---|---|
| Listeria monocytogenes | 0.5–1.0 mg/mL | [23] |
| Staphylococcus aureus | 1.25–4.0 mg/mL | [19,24] |
| Gram-Negative | ||
| Escherichia coli | 1–3.0 mg/mL | [25,26] |
| Salmonella spp. | 0.2–0.8 mg/mL | [26] |
| Gram-Positive | MIC | References |
|---|---|---|
| Staphylococcus aureus | 400 µg/mL–135 mg/L | [49,50] |
| Streptococcus mutans | 100 µg/mL | [51] |
| Gram-Negative | ||
| Salmonella typhimurium | 80 µg/mL | [52] |
| Escherichia coli | 45 mg/L | [50] |
| Klebsiella pneumoniae | 475 µg/mL | [53] |
| Gram-Positive | MIC | Reference |
|---|---|---|
| Bacillus cereus | 3.12 µg/mL | [70] |
| Enterococcus faecalis | 0.78 µg/mL | [70] |
| Listeria monocytogenes | 6.25 µg/mL | [70] |
| Micrococcus luteus | 6.25 µg/mL | [70] |
| Staphylococcus aureus | 1.56 µg/mL | [70] |
| Gram-Negative | ||
| Aeromonas hydrophila | 0.78 µg/mL | [70] |
| Escherichia coli | 12.5 µg/mL | [70] |
| Escherichia coli O157:H7 | 6.25 µg/mL | [70] |
| Pseudomonas aeruginosa | 12.5 µg/mL | [70] |
| Salmonella enteritis | 6.25 µg/mL | [70] |
| Aggregatibacter actinomycetemcomitans | 209 µg/mL | [71] |
| Gram-Positive | MIC | Reference |
|---|---|---|
| Staphylococcus aureus ATCC 29213 | 100 µg/mL | [89] |
| S. aureus MRSA WKZ-1 | 50 µg/mL | [89] |
| Enterococcus faecalis ATCC 29212 | 100 µg/mL | [89] |
| Staphylococcus aureus | 0.2% (v/v) | [92] |
| Gram-Negative | ||
| Escherichia coli ATCC 25922 | 200 µg/mL | [89] |
| Salmonella typhimurium ATCC 14028 | 100 µg/mL | [89] |
| Acinetobacter baumannii ATCC 17878 | 100 µg/mL | [89] |
| Citrobacter diversus | 0.2% (v/v) | [92] |
| Enterobacter amnigenus | 0.8% (v/v) | [92] |
| Proteus mirabilis | 0.8% (v/v) | [92] |
| Providencia rettgeri | 0.8% (v/v) | [92] |
| Pseudomonas aeruginosa | >1% (v/v) | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latorre, R.; Valerii, M.C.; Benati, M.; Lewis, R.E.; Spigarelli, R.; Bernacchi, A.; Lippi, G.; Spisni, E.; Gaibani, P. Lights and Shadows of Essential Oil-Derived Compounds: Antimicrobial and Anti-Inflammatory Properties of Eugenol, Thymol, Cinnamaldehyde, and Carvacrol. Curr. Issues Mol. Biol. 2025, 47, 915. https://doi.org/10.3390/cimb47110915
Latorre R, Valerii MC, Benati M, Lewis RE, Spigarelli R, Bernacchi A, Lippi G, Spisni E, Gaibani P. Lights and Shadows of Essential Oil-Derived Compounds: Antimicrobial and Anti-Inflammatory Properties of Eugenol, Thymol, Cinnamaldehyde, and Carvacrol. Current Issues in Molecular Biology. 2025; 47(11):915. https://doi.org/10.3390/cimb47110915
Chicago/Turabian StyleLatorre, Rocco, Maria Chiara Valerii, Marco Benati, Russell Edward Lewis, Renato Spigarelli, Alberto Bernacchi, Giuseppe Lippi, Enzo Spisni, and Paolo Gaibani. 2025. "Lights and Shadows of Essential Oil-Derived Compounds: Antimicrobial and Anti-Inflammatory Properties of Eugenol, Thymol, Cinnamaldehyde, and Carvacrol" Current Issues in Molecular Biology 47, no. 11: 915. https://doi.org/10.3390/cimb47110915
APA StyleLatorre, R., Valerii, M. C., Benati, M., Lewis, R. E., Spigarelli, R., Bernacchi, A., Lippi, G., Spisni, E., & Gaibani, P. (2025). Lights and Shadows of Essential Oil-Derived Compounds: Antimicrobial and Anti-Inflammatory Properties of Eugenol, Thymol, Cinnamaldehyde, and Carvacrol. Current Issues in Molecular Biology, 47(11), 915. https://doi.org/10.3390/cimb47110915









