MiR-10a as a Potential Biomarker and Therapeutic Target in Localized and Metastatic Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. TCGA Cohort
2.2. Prostate Cancer Cell Lines
2.3. Cell Transfection with miR-10a
2.4. Extraction of Total RNA and miRNA
2.5. Reverse Transcription (RT-qPCR)
2.6. Matrigel Invasion Assay
2.7. Migration Assay
2.8. Proliferation Test
2.9. Collection and Processing of Samples
2.10. Statistical Analysis
3. Results
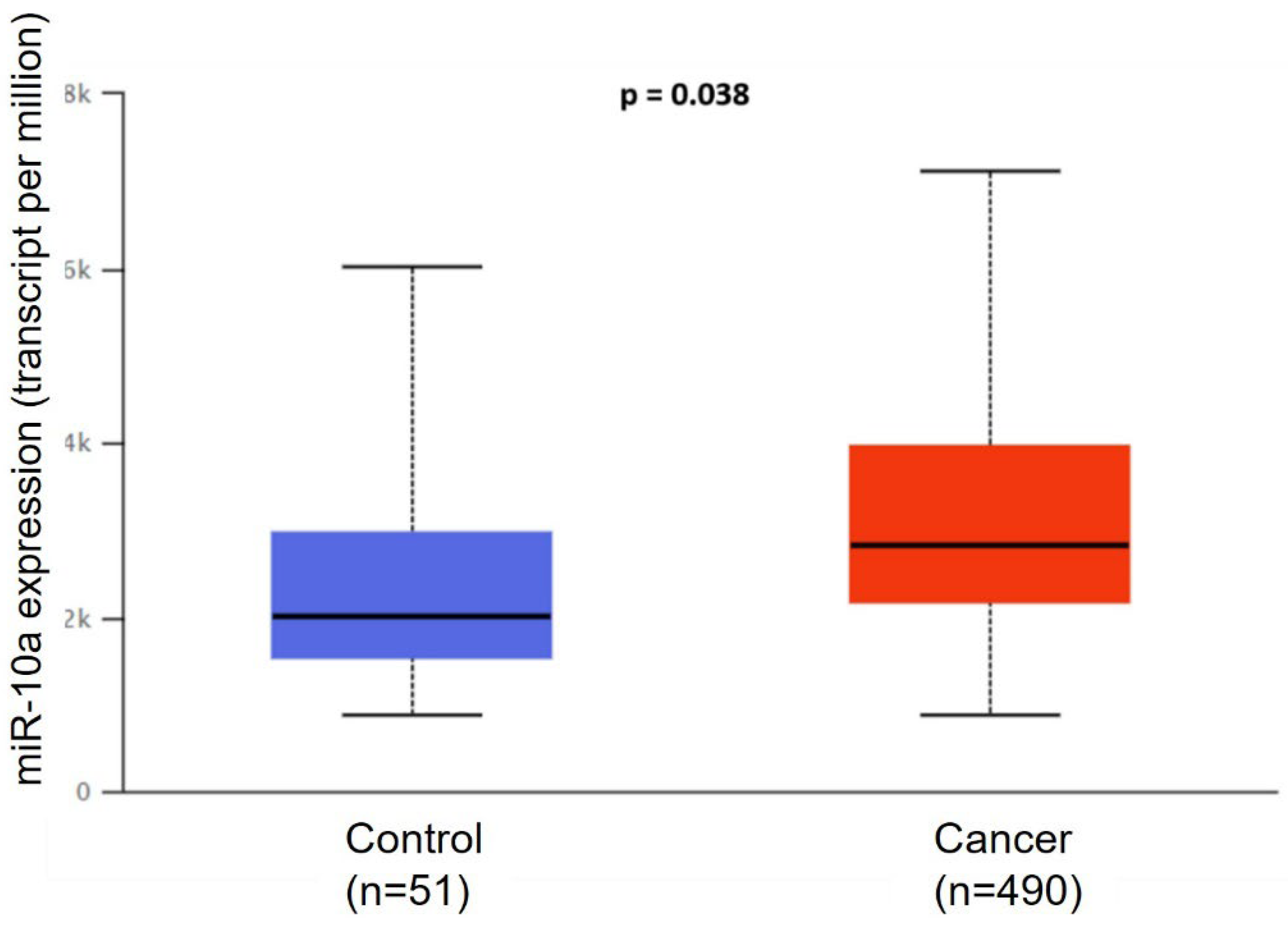
3.1. Bioinformatics Analysis
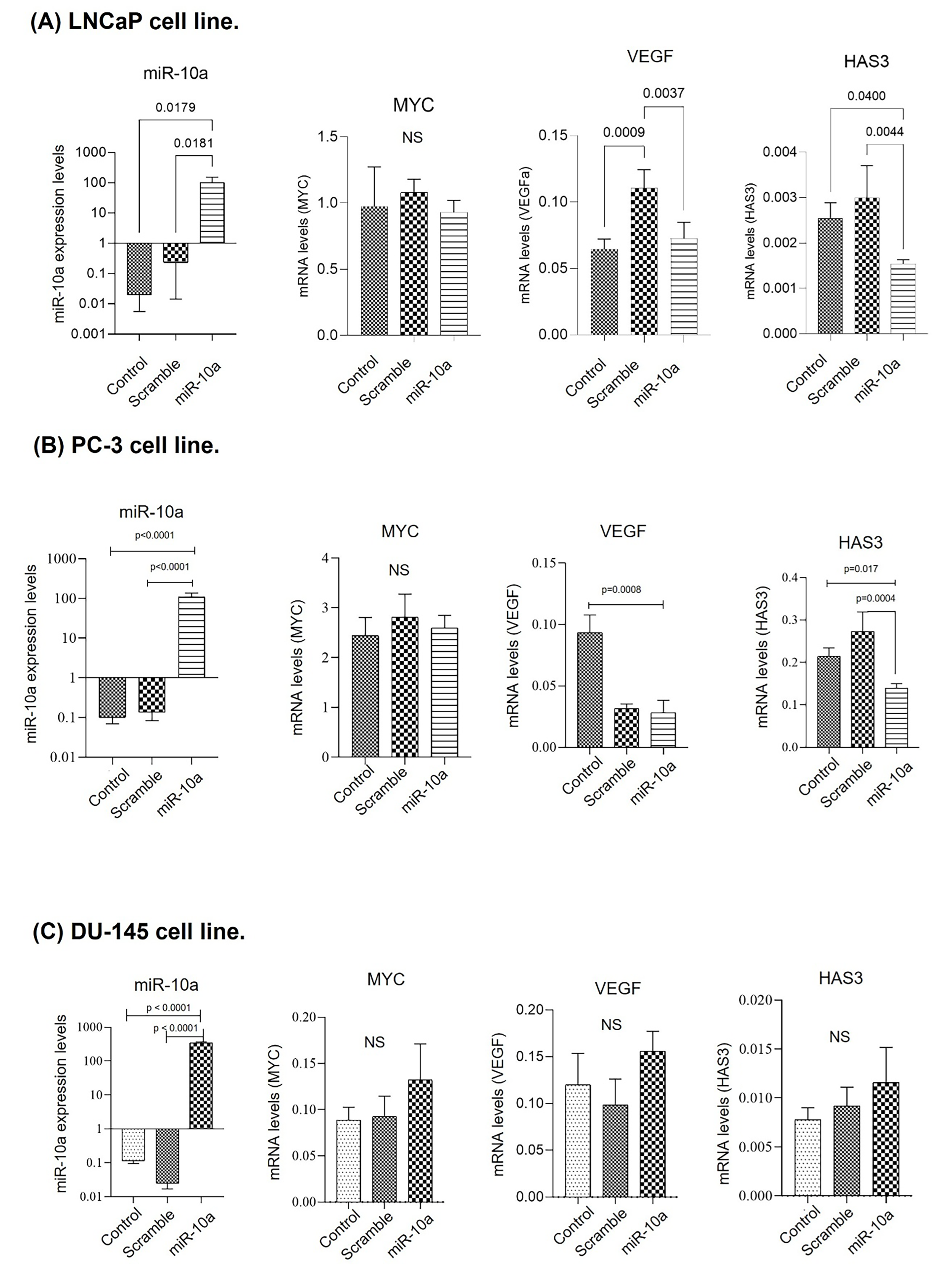
3.2. Cell Transfection in Prostate Cancer Lines
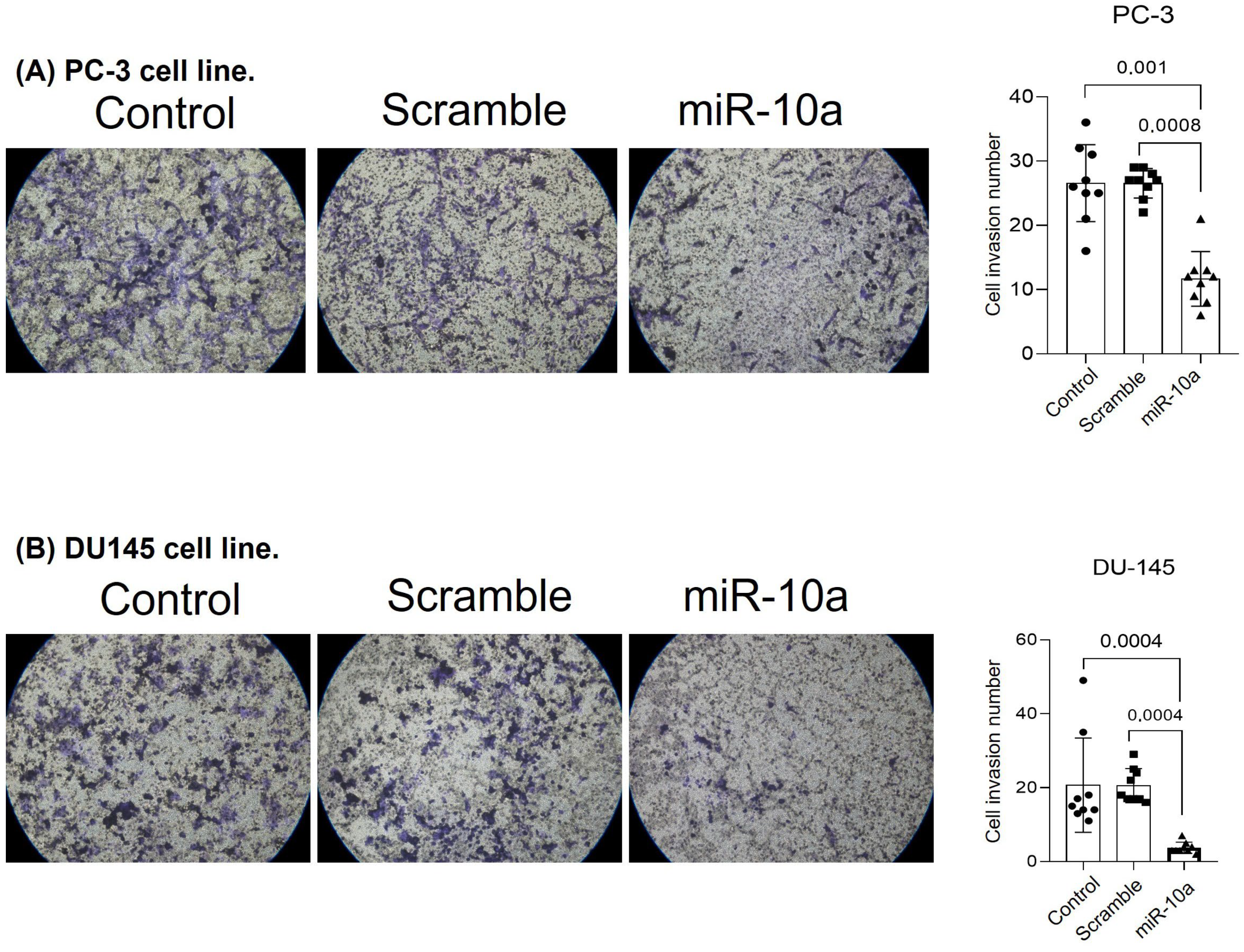
3.3. Cell Invasion Experiment (Matrigel)
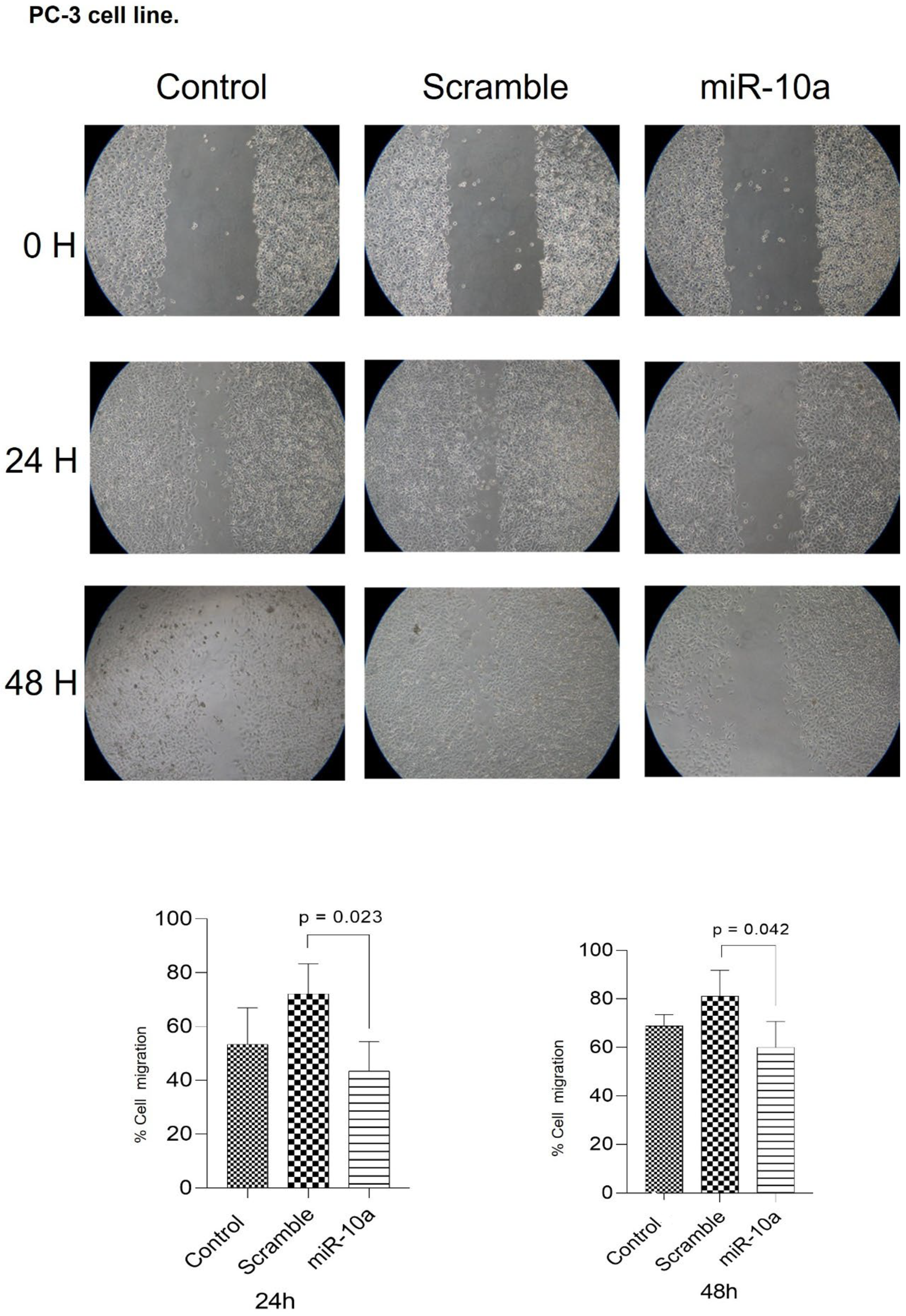
3.4. Migration Experiment
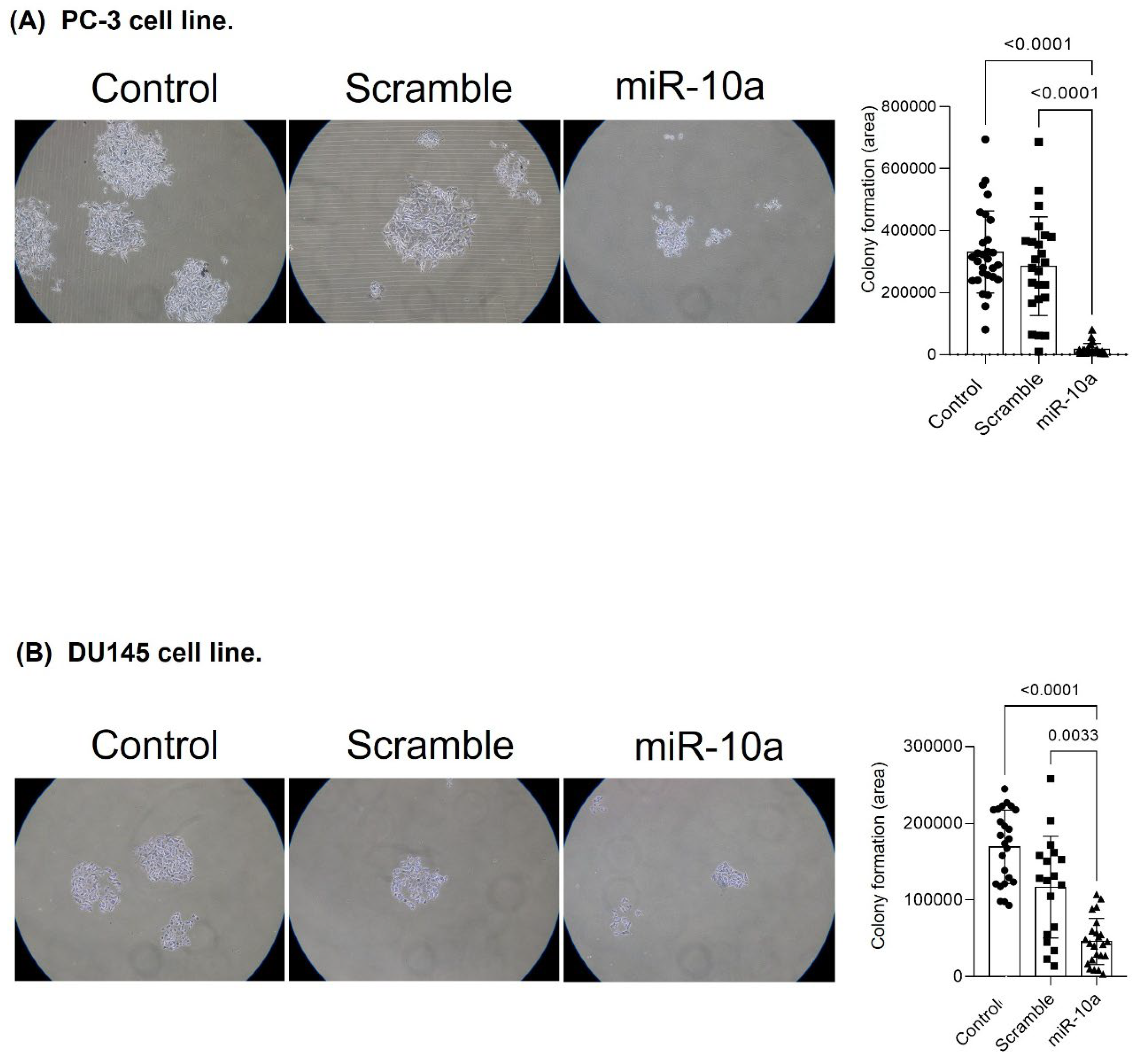
3.5. Cell Proliferation Experiment (Colony Formation)
3.6. Expression of miR-10a and Target Genes in Surgical Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory: Cancer today. Lyon 2020, 20182020. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Oliveira Santos, M. 2018 estimate: Cancer incidence in Brazil. Rev. Bras. Cancerol. 2018, 64, 119–120. [Google Scholar] [CrossRef]
- SILVAINDCJAGD Online Mortality Atlas. Available online: https://www.inca.gov.br/MortalidadeWeb 2022 (accessed on 20 September 2025).
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA 2012, 62, 10–29. [Google Scholar] [CrossRef]
- SeerCancer. Seer Cancer 2015 [Surveillance, Epidemiology, and End Results Program]. Available online: http://seer.cancer.gov/statfacts/html/prost.html (accessed on 20 September 2025).
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Gann, P.H.; Hennekens, C.H.; Stampfer, M.J. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995, 273, 289–294. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L.; Committee, I.G. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Brawer, M.K.; Stamey, T.A.; Fowler, J.; Droller, M.; Messing, E.; Fair, W.R. Perspectives on prostate cancer diagnosis and treatment: A roundtable. Urology 2001, 58, 135–140. [Google Scholar] [CrossRef]
- Wang, Y.; Navin, N.E. Advances and applications of single-cell sequencing technologies. Mol. Cell. 2015, 58, 598–609. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yarrow, J.C.; Perlman, Z.E.; Westwood, N.J.; Mitchison, T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004, 4, 21. [Google Scholar] [CrossRef]
- Xiaoli, Z.; Yawei, W.; Lianna, L.; Haifeng, L.; Hui, Z. Screening of Target Genes and Regulatory Function of miRNAs as Prognostic Indicators for Prostate Cancer. Med. Sci. Monit. 2015, 21, 3748–3759. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Yu, T.; Liu, L.; Li, J.; Yan, M.; Lin, H.; Liu, Y.; Chu, D.; Tu, H.; Gu, A.; Yao, M. MiRNA-10a is upregulated in NSCLC and may promote cancer by targeting PTEN. Oncotarget 2015, 6, 30239–30250. [Google Scholar] [CrossRef]
- Zeng, T.; Li, G. MicroRNA-10a enhances the metastatic potential of cervical cancer cells by targeting phosphatase and tensin homologue. Mol. Med. Rep. 2014, 10, 1377–1382. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Q.; Yan, X.-L.; Zhang, Y.; Li, W.; Tang, F.; Li, X.; Yang, P. miR-10a controls glioma migration and invasion through regulating epithelial-mesenchymal transition via EphA8. FEBS Lett. 2015, 589, 756–765. [Google Scholar] [CrossRef]
- Gao, F.; Wu, Q.; Lu, D. MicroRNA-10a-5p-mediated downregulation of GATA6 inhibits tumor progression in ovarian cancer. Hum. Cell 2024, 37, 271–284. [Google Scholar] [CrossRef]
- Fan, Q.; Meng, X.; Liang, H.; Zhang, H.; Liu, X.; Li, L.; Li, W.; Sun, W.; Zhang, H.; Zen, K.; et al. miR-10a inhibits cell proliferation and promotes cell apoptosis by targeting BCL6 in diffuse large B-cell lymphoma. Protein Cell 2016, 7, 899–912. [Google Scholar] [CrossRef]
- Nesbit, C.E.; Tersak, J.M.; Prochownik, E.V. MYC oncogenes and human neoplastic disease. Oncogene 1999, 18, 3004–3016. [Google Scholar] [CrossRef]
- Liu, W.; Xie, C.C.; Zhu, Y.; Li, T.; Sun, J.; Cheng, Y.; Ewing, C.M.; Dalrymple, S.; Turner, A.R.; Sun, J.; et al. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 2008, 10, 897–907. [Google Scholar] [CrossRef]
- Lapointe, J.; Li, C.; Giacomini, C.P.; Salari, K.; Huang, S.; Wang, P.; Ferrari, M.; Hernandez-Boussard, T.; Brooks, J.D.; Pollack, J.R. Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer Res. 2007, 67, 8504–8510. [Google Scholar] [CrossRef]
- Zafarana, G.; Ishkanian, A.S.; Malloff, C.A.; Locke, J.A.; Sykes, J.; Thoms, J.; Lam, W.L.; Squire, J.A.; Yoshimoto, M.; Ramnarine, V.R.; et al. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer 2012, 118, 4053–4062, Erratum in Cancer 2014, 120, 2380. [Google Scholar] [CrossRef]
- Fromont, G.; Godet, J.; Peyret, A.; Irani, J.; Celhay, O.; Rozet, F.; Cathelineau, X.; Cussenot, O. 8q24 amplification is associated with Myc expression and prostate cancer progression and independent predictor of recurrence after radical prostatectomy. Hum. Pathol. 2013, 44, 1617–1623. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Xiang, L.; Li, S.; Rao, D.; Wang, S.; Yu, K. MiR-10a functions as a tumor suppressor in prostate cancer via targeting KDM4A. J. Cell. Biochem. 2019, 120, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhao, Q.; Wang, J.; He, X. MicroRNA-10a influences osteoblast differentiation and angiogenesis by regulating β-catenin expression. Cell. Physiol. Biochem. 2015, 37, 2194–2208. [Google Scholar] [CrossRef]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wu, H.; Li, Y.; Zhang, Y.; Liu, M.; Li, X.; Tang, H. miR-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis. 2017, 8, e2739. [Google Scholar] [CrossRef]
- Moura, C.M. The expression profile of CD44 regulatory miRNAs and hyaluronic acid synthases in prostate cancer. Int. J. Biol. Markers 2015, 30, e49–e55. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E.; Vacca, A. Macrophages and tumor angiogenesis. Leukemia 2007, 21, 2085–2089. [Google Scholar] [CrossRef]
- Simpson, M.A. Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am. J. Pathol. 2006, 169, 247–257. [Google Scholar] [CrossRef]
- Liu, N.; Gao, F.; Han, Z.; Xu, X.; Underhill, C.B.; Zhang, L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001, 61, 5207–5214. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borelli Bovo, T.J.; de Camargo, J.A.; Pimenta, R.; Ribeiro Guimarães, V.; Candido, P.; Leite, K.R.M.; Camargo Passerotti, C.; Nahas, W.C.; Reis, S.T. MiR-10a as a Potential Biomarker and Therapeutic Target in Localized and Metastatic Prostate Cancer. Curr. Issues Mol. Biol. 2025, 47, 913. https://doi.org/10.3390/cimb47110913
Borelli Bovo TJ, de Camargo JA, Pimenta R, Ribeiro Guimarães V, Candido P, Leite KRM, Camargo Passerotti C, Nahas WC, Reis ST. MiR-10a as a Potential Biomarker and Therapeutic Target in Localized and Metastatic Prostate Cancer. Current Issues in Molecular Biology. 2025; 47(11):913. https://doi.org/10.3390/cimb47110913
Chicago/Turabian StyleBorelli Bovo, Tiago José, Juliana Alves de Camargo, Ruan Pimenta, Vanessa Ribeiro Guimarães, Patrícia Candido, Katia Ramos Moreira Leite, Carlo Camargo Passerotti, William Carlos Nahas, and Sabrina T. Reis. 2025. "MiR-10a as a Potential Biomarker and Therapeutic Target in Localized and Metastatic Prostate Cancer" Current Issues in Molecular Biology 47, no. 11: 913. https://doi.org/10.3390/cimb47110913
APA StyleBorelli Bovo, T. J., de Camargo, J. A., Pimenta, R., Ribeiro Guimarães, V., Candido, P., Leite, K. R. M., Camargo Passerotti, C., Nahas, W. C., & Reis, S. T. (2025). MiR-10a as a Potential Biomarker and Therapeutic Target in Localized and Metastatic Prostate Cancer. Current Issues in Molecular Biology, 47(11), 913. https://doi.org/10.3390/cimb47110913






