Assessment of Cervical IL-6 Levels and Neonatal Inflammatory Response in Preterm Birth Following Preterm Premature Rupture of Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection and Processing
2.3. Statistical Analysis
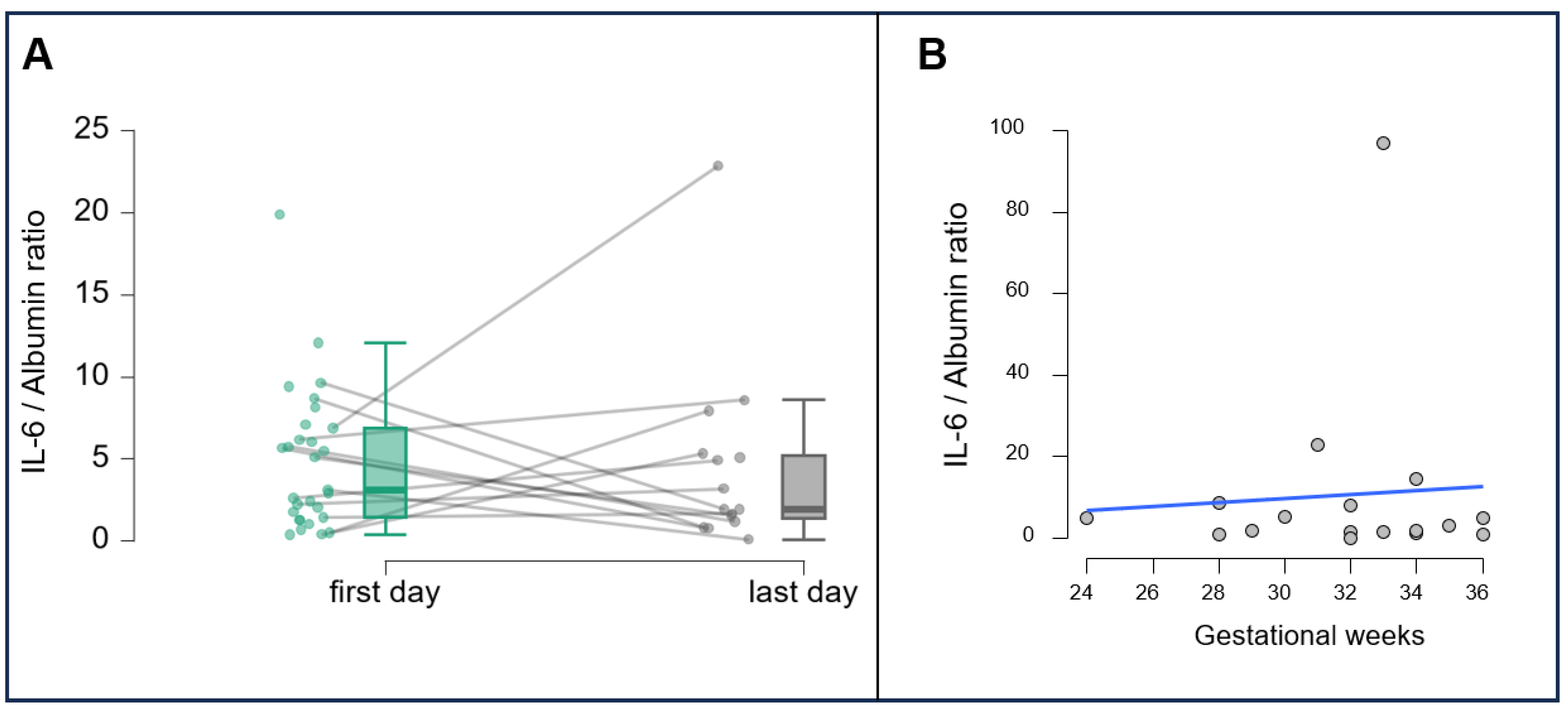
3. Results
3.1. Demographic and Obstetric Characteristics
3.2. Correlations and Dynamics of IL-6 Levels
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, E37–E46. [Google Scholar] [CrossRef]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: An updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, 103–114. [Google Scholar] [CrossRef] [PubMed]
- de Gamarra-Oca, L.F.; Ojeda, N.; Gómez-Gastiasoro, A.; Peña, J.; Ibarretxe-Bilbao, N.; García-Guerrero, M.A.; Loureiro, B.; Zubiaurre-Elorza, L. Long-term neurodevelopmental outcomes after moderate and late preterm birth: A systematic review. J. Pediatr. 2021, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Rehal, A.; Brizot, M.L.; Serra, V.; Da Fonseca, E.; Cetingoz, E.; Syngelaki, A.; Perales, A.; Hassan, S.S.; et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in twin gestations: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 15, 17–24. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Osmundson, S.S.; McCarthy, A.M.; Louis, J.M. SMFM Consult Series #71: Preterm prelabor rupture of membranes (PPROM)—contemporary guidance with pathophysiology updates. Am. J. Obstet. Gynecol. 2024, 231, B2–B15. [Google Scholar] [PubMed]
- Naeye, R.L.; Peters, E.C. Causes and consequences of premature rupture of fetal membranes. Lancet 1980, 1, 192–194. [Google Scholar] [CrossRef]
- Mercer, B.M.; Crouse, D.T.; Goldenberg, R.L.; Miodovnik, M.; Mapp, D.C.; Meis, P.J.; Dombrowski, M.P. The antibiotic treatment of PPROM study: Systemic maternal and fetal markers and perinatal outcomes. Am. J. Obstet. Gynecol. 2012, 206, 145.e1–145.e9. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin. Perinatol. 2017, 41, 409–419. [Google Scholar] [CrossRef]
- Ye, C.-X.; Chen, S.-B.; Wang, T.-T.; Zhang, S.-M.; Qin, J.-B.; Chen, L.-Z. Risk factors for preterm birth: A prospective cohort study. Chin. J. Contemp. Pediatr. 2021, 23, 1242–1249. [Google Scholar]
- DiGiulio, D.B.; Romero, R.; Kusanovic, J.P.; Gómez, R.; Kim, C.J.; Seok, K.S.; Gotsch, F.; Mazaki-Tovi, S.; Vaisbuch, E.; Sanders, K.; et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 2010, 64, 38–57. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Park, J.S.; Chang, J.W.; Kim, Y.A.; Kim, J.C.; Kim, K.S. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am. J. Obstet. Gynecol. 1998, 179, 1254–1260. [Google Scholar] [CrossRef]
- Romero, R.; Ghidini, A.; Mazor, M.; Behnke, E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin. Obstet. Gynecol. 1991, 34, 769–778. [Google Scholar] [CrossRef]
- Bond, D.M.; Middleton, P.; Levett, K.M.; van der Ham, D.P.; Crowther, C.A.; Buchanan, S.L.; Morris, J. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 2017, 3, CD004735. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Schlabritz-Loutsevitch, N.; Maher, J.; Buchmann, J.; Naberezhnev, Y.; Winarno, S.A.; Seliger, G. Mid-trimester preterm premature rupture of membranes (PPROM): Etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018, 46, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Musilova, I.; Andrys, C.; Drahosova, M.; Soucek, O.; Pliskova, L.; Jacobsson, B.; Kacerovsky, M. Cervical fluid interleukin 6 and intra-amniotic complications of preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2018, 31, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Guido, M.; Taccaliti, C.; Latorre, G.; Gallini, F.; Forziati, V.; Caringella, D.; Giocolano, A.; Fantasia, I. Conservative management of preterm premature rupture of membranes < 30 weeks of gestational age: Effectiveness of clinical guidelines implementation strategies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 19, 100209. [Google Scholar]
- Musilova, I.; Andrys, C.; Holeckova, M.; Kolarova, V.; Pliskova, L.; Drahosova, M.; Bolehovska, R.; Pilka, R. Interleukin-6 measured using the automated electrochemiluminescence immunoassay method for the identification of intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2020, 33, 1919–1926. [Google Scholar] [CrossRef]
- Kacerovský, M.I.; Musilová, L.; Plisková, I.; Kostál, L.; Hornychová, R. Jacobsson Cervical and vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2015, 28, 1546–1553. [Google Scholar]
- Manning, R.; James, C.P.; Smith, M.C.; Innes, B.A.; Stamp, E.; Peebles, D.; Bajaj-Elliott, M.; Klein, N.; Bulmer, J.N.; Robson, S.C.; et al. Predictive value of cervical cytokine, antimicrobial and microflora levels for pre-term birth in high-risk women. Sci. Rep. 2019, 9, 11246. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Moscicki, A.B.; Sumerel, J.L.; Ma, Y.; Scott, M.E. Determination of Cytokine Protein Levels in Cervical Mucus Samples from Young Women by a Multiplex Immunoassay Method and Assessment of Correlates. Clin. Vaccine Immunol. 2008, 15, 49–54.20. [Google Scholar] [CrossRef]
- Salnikova, D.I.; Nikiforov, N.G.; Postnov, A.Y.; Orekhov, A.N. Target Role of Monocytes as Key Cells of Innate Immunity in Rheumatoid Arthritis. Diseases 2024, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Kunicki, M.; Rzewuska, N.; Gross-Kępińska, K. Immunophenotypic profiles and inflammatory markers in Premature Ovarian Insufficiency. J. Reprod. Immunol. 2024, 164, 104253. [Google Scholar] [CrossRef] [PubMed]
- Etyang, A.K.; Omuse, G.; Mukaindo, A.M.; Temmerman, M. Maternal inflammatory markers for chorioamnionitis in preterm prelabour rupture of membranes: A systematic review and meta-analysis of diagnostic test accuracy studies. Syst. Rev. 2020, 91, 141. [Google Scholar] [CrossRef] [PubMed]
- Bohilțea, R.E.; Cioca, A.M.; Dima, V.; Ducu, I.; Grigoriu, C.; Varlas, V.; Furtunescu, F. Expectant Management of PPROM Improves Neonatal Outcome-A Retrospective Study of 562 Patients. J. Clin. Med. 2021, 11, 214. [Google Scholar] [CrossRef]
- Tsiartas, P.; Kacerovsky, M.; Musilova, I.; Hornychova, H.; Cobo, T.; Sävman, K.; Jacobsson, B. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2013, 26, 1332–1336. [Google Scholar] [CrossRef]
- Zanardo, V.; Vedovato, S.; Cosmi, E.; Litta, P.; Cavallin, F.; Trevisanuto, D.; Chiarelli, S. Preterm premature rupture of membranes, chorioamnion inflammatory scores and neonatal respiratory outcome. BJOG 2010, 117, 94–98. [Google Scholar] [CrossRef]
- McElrath, T. Prelabor Rupture of Membranes Before and at the Limit of Viability. UpToDate June 2025. Available online: https://www.uptodate.com/contents/prelabor-rupture-of-membranes-before-and-at-the-limit-of-viability (accessed on 12 July 2025).
- Charles, J. Lockwood Spontaneous Preterm Birth: Pathogenesis. UpToDate, June 2025. Available online: https://www.uptodate.com/contents/spontaneous-preterm-birth-pathogenesis (accessed on 12 July 2025).
- Lungu, N.; Popescu, D.-E.; Jura, A.M.C.; Zaharie, M.; Jura, M.-A.; Roșca, I.; Boia, M. Enhancing Early Detection of Sepsis in Neonates through Multimodal Biosignal Integration: A Study of Pulse Oximetry, Near-Infrared Spectroscopy (NIRS), and Skin temperature Monitoring. Bioengineering 2024, 11, 681. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Urban (n/%) | Rural (n/%) | Anemia (HGB < 120 g/L; n/%) | UTI (n/%) | GTI (n/%) | TA-AC (n/%) | Cerclage (n/%) | Spontaneous Delivery (n/%) | Cesarean Delivery (n/%) |
|---|---|---|---|---|---|---|---|---|---|
| Environment of origin | 30/59 | 21/41 | |||||||
| Pregnancy-related complications | 41/80 | 0 | 12/24 | ||||||
| Invasive interventions during pregnancy | 3/6 | 1/2 | |||||||
| Way of delivery | 15/29 | 36/71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labossa, G.; Koszegi, T.; Farkas, B.; Nagy, B.; Jakabfi-Csepregi, R.; Farkas, N.; Kovacs, K. Assessment of Cervical IL-6 Levels and Neonatal Inflammatory Response in Preterm Birth Following Preterm Premature Rupture of Membranes. Curr. Issues Mol. Biol. 2025, 47, 838. https://doi.org/10.3390/cimb47100838
Labossa G, Koszegi T, Farkas B, Nagy B, Jakabfi-Csepregi R, Farkas N, Kovacs K. Assessment of Cervical IL-6 Levels and Neonatal Inflammatory Response in Preterm Birth Following Preterm Premature Rupture of Membranes. Current Issues in Molecular Biology. 2025; 47(10):838. https://doi.org/10.3390/cimb47100838
Chicago/Turabian StyleLabossa, Gusztav, Tamas Koszegi, Balint Farkas, Bernadett Nagy, Rita Jakabfi-Csepregi, Nelli Farkas, and Kalman Kovacs. 2025. "Assessment of Cervical IL-6 Levels and Neonatal Inflammatory Response in Preterm Birth Following Preterm Premature Rupture of Membranes" Current Issues in Molecular Biology 47, no. 10: 838. https://doi.org/10.3390/cimb47100838
APA StyleLabossa, G., Koszegi, T., Farkas, B., Nagy, B., Jakabfi-Csepregi, R., Farkas, N., & Kovacs, K. (2025). Assessment of Cervical IL-6 Levels and Neonatal Inflammatory Response in Preterm Birth Following Preterm Premature Rupture of Membranes. Current Issues in Molecular Biology, 47(10), 838. https://doi.org/10.3390/cimb47100838






