Endoplasmic Reticulum Stress Drives VEGF Gene Expression in Monocytic Cells
Abstract
1. Introduction
2. Materialsand Methods
2.1. Cell Culture
2.2. Real-Time Quantitative Reverse Transcription
2.3. Enzyme-Linked Immunosorbent Assays
2.4. ROS Detection Assay
2.5. Statistical Analysis
3. Results
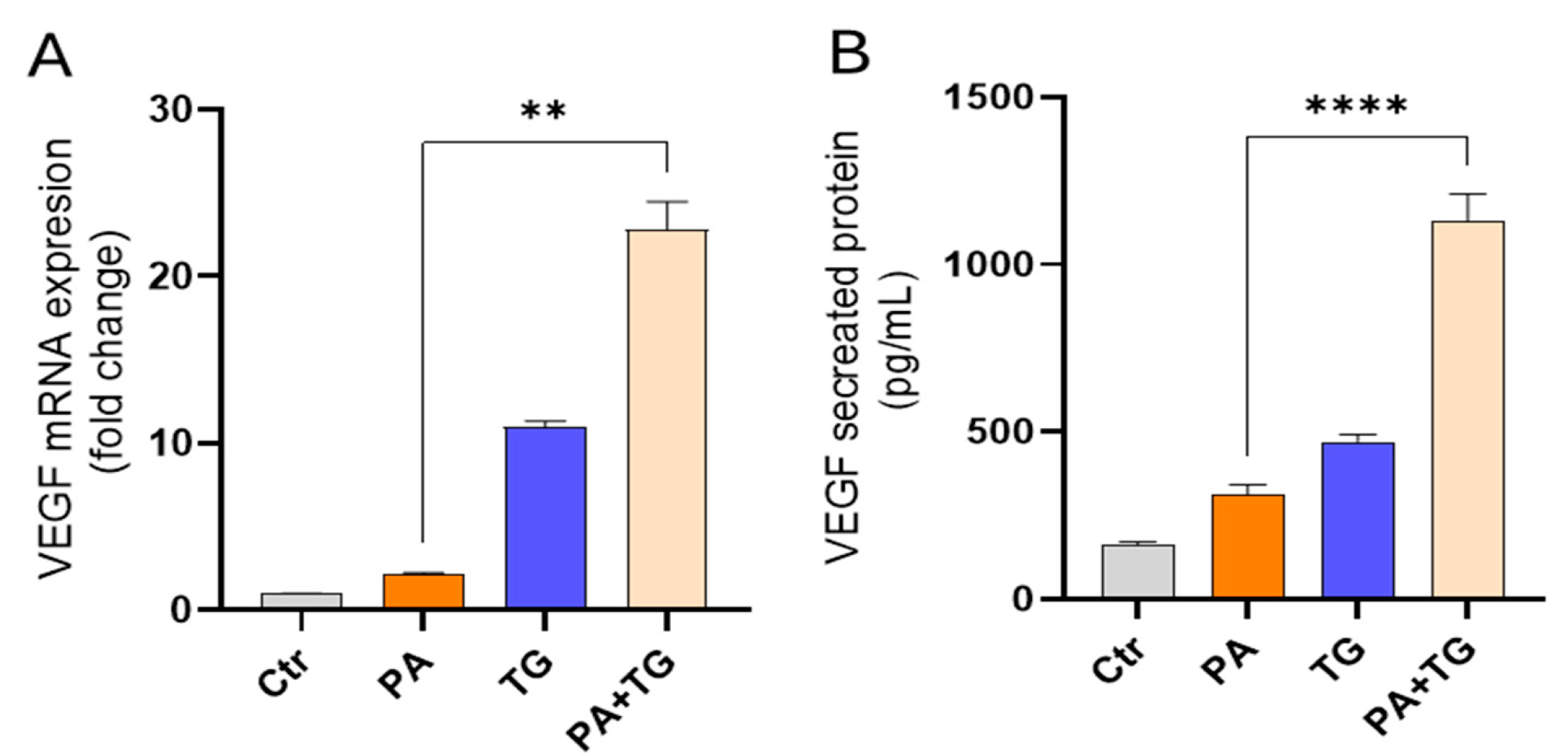
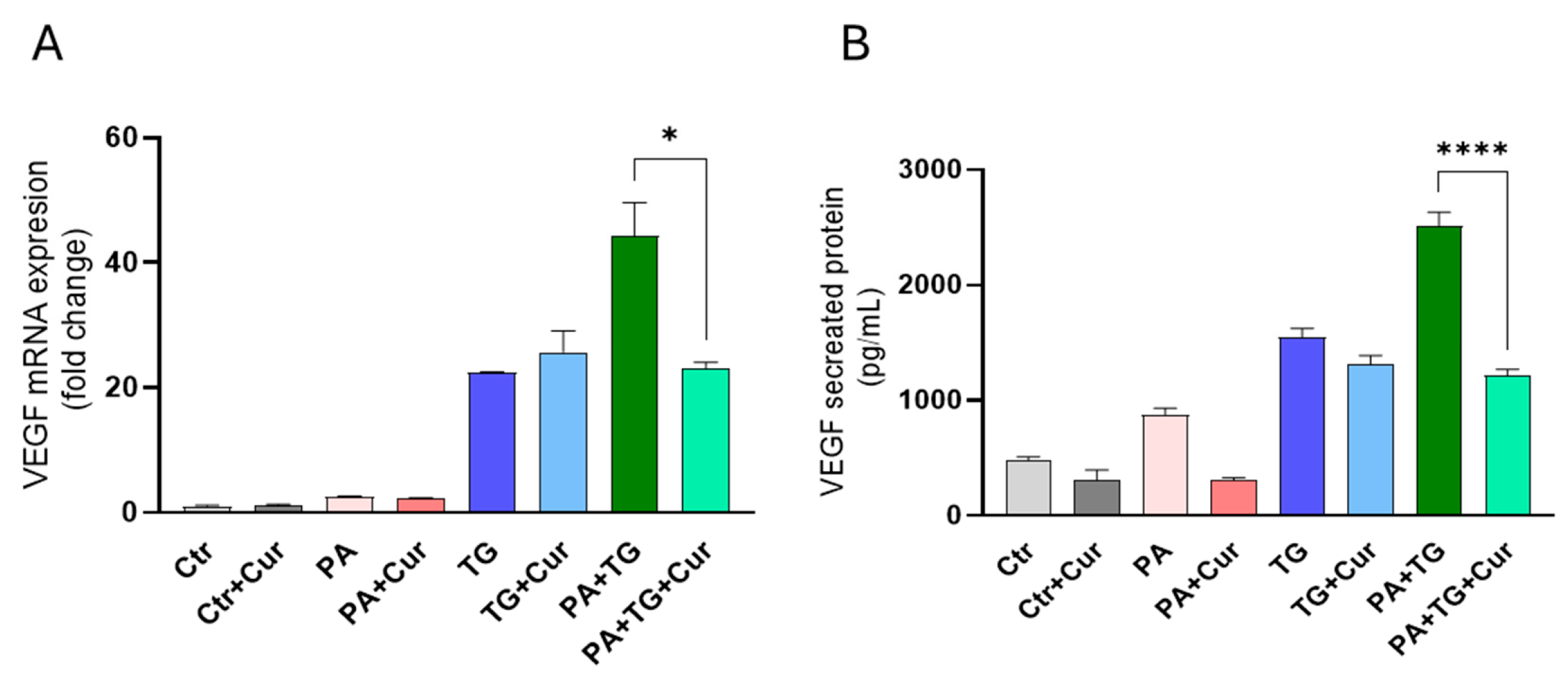
3.1. The ER and Metabolic Stresses Promote the Expression of VEFG
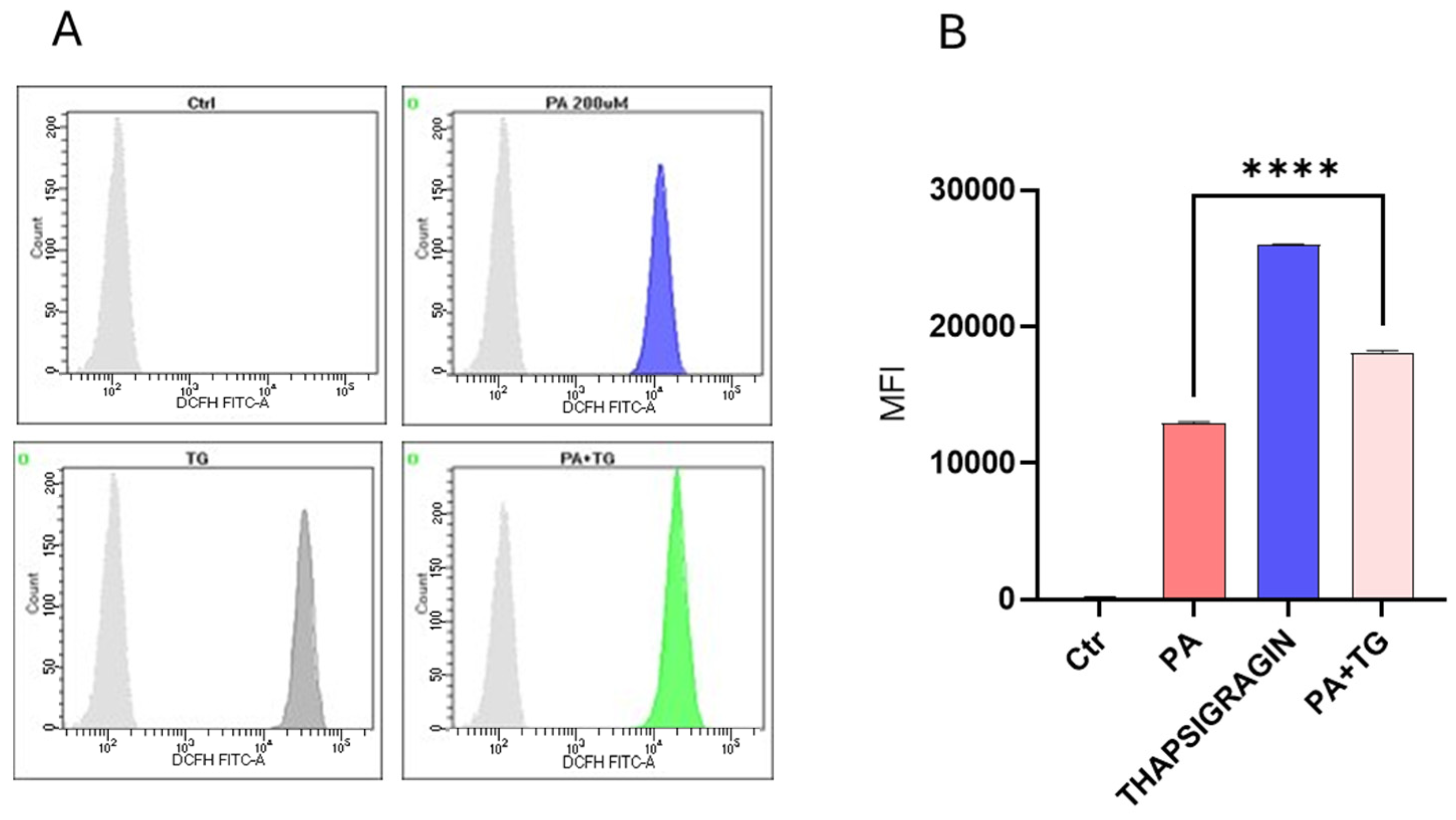
3.2. Metabolic and/or ER Stress(es) Induce(s) Reactive Oxygen Species (ROS)
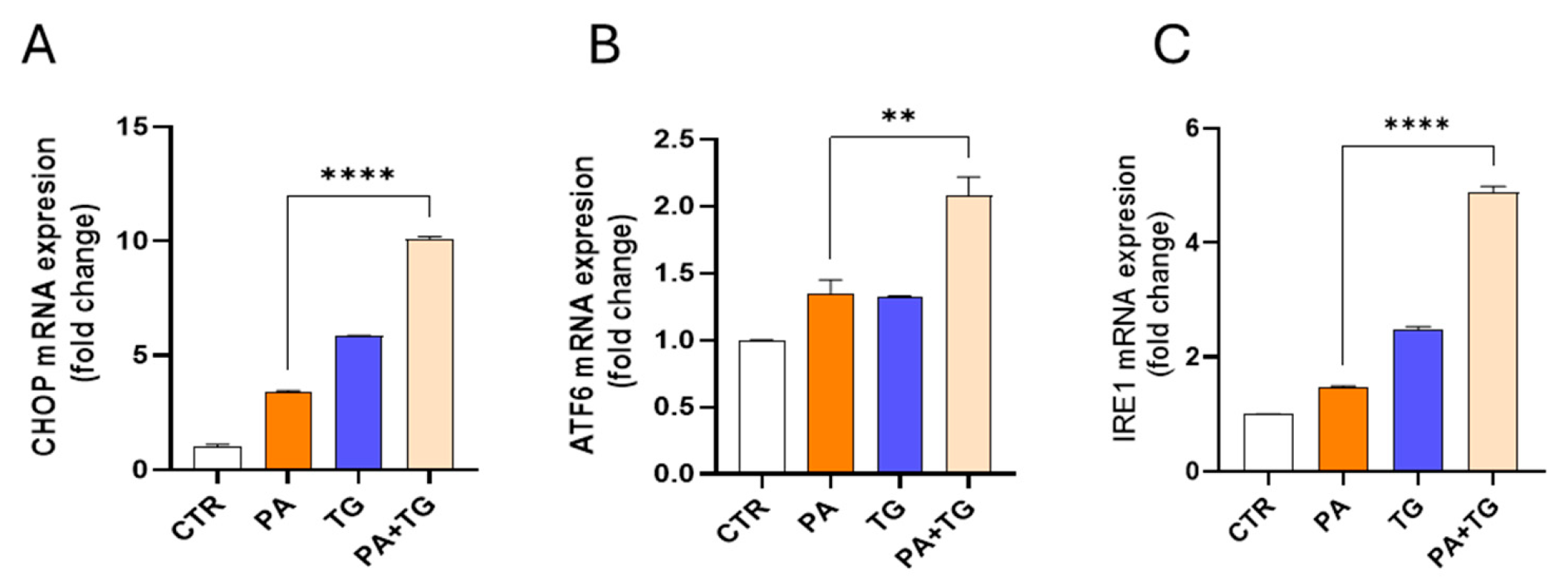
3.3. Lipotoxic Treatments Induce ER Stress in THP-1 Cells
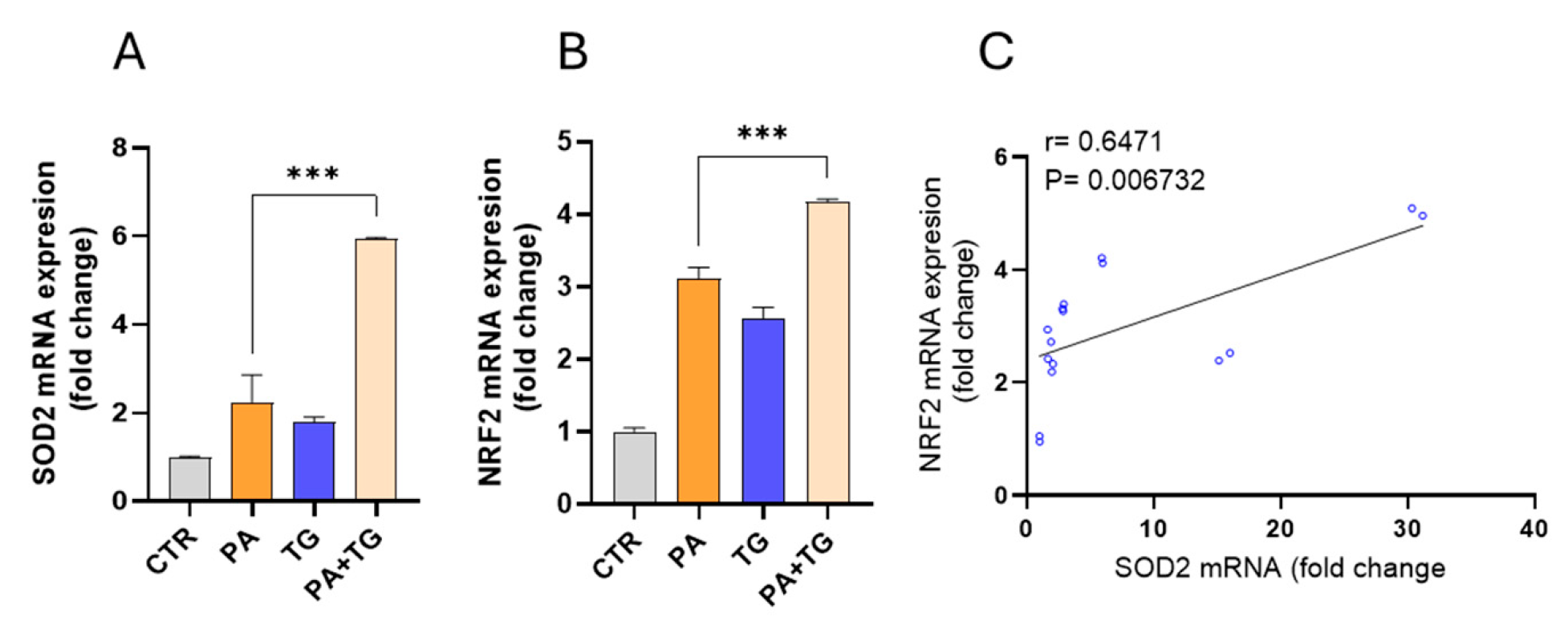
3.4. Metabolic and ER Stresses Trigger Cellular Antioxidant Defense Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzaid, F.; Fagherazzi, G.; Riveline, J.P.; Bahman, F.; Al-Rashed, F.; Al-Mulla, F.; Ahmad, R. Immune cell-adipose tissue crosstalk in metabolic diseases with a focus on type 1 diabetes. Diabetologia 2025, 68, 1616–1631. [Google Scholar]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Shen, H.; Eguchi, K.; Kono, N.; Fujiu, K.; Matsumoto, S.; Shibata, M.; Oishi-Tanaka, Y.; Komuro, I.; Arai, H.; Nagai, R.; et al. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arter. Thromb. Vasc. Biol. 2013, 33, 2596–2607. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, J.M.; Kim, S.J.; Seo, W.; Kim, M.H.; Choi, W.M.; Yoo, W.; Lee, J.H.; Shim, Y.R.; Yi, H.S.; et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat. Commun. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Yeop Han, C.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar]
- He, Q.; Gao, Z.; Yin, J.; Zhang, J.; Yun, Z.; Ye, J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E877–E885. [Google Scholar] [CrossRef]
- Engin, A. Adipose Tissue Hypoxia in Obesity: Clinical Reappraisal of Hypoxia Hypothesis. Adv. Exp. Med. Biol. 2024, 1460, 329–356. [Google Scholar]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, J.; Phillipson, M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat. Rev. Drug Discov. 2016, 15, 125–142. [Google Scholar]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1308. [Google Scholar] [CrossRef]
- Ståhlman, M.; Pham, H.T.; Adiels, M.; Mitchell, T.W.; Blanksby, S.J.; Fagerberg, B.; Ekroos, K.; Borén, J. Clinical dyslipidaemia is associated with changes in the lipid composition and inflammatory properties of apolipoprotein-B-containing lipoproteins from women with type 2 diabetes. Diabetologia 2012, 55, 1156–1166. [Google Scholar] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef]
- Grill, V.; Qvigstad, E. Fatty acids and insulin secretion. Br. J. Nutr. 2000, 83 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef]
- Bhandary, B.; Marahatta, A.; Kim, H.R.; Chae, H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Bahman, F.; Al-Roub, A.; Akhter, N.; Al Madhoun, A.; Wilson, A.; Almansour, N.; Al-Rashed, F.; Sindhu, S.; Al-Mulla, F.; Ahmad, R. TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes. Int. J. Mol. Sci. 2024, 25, 6776. [Google Scholar] [CrossRef]
- Bahman, F.; Kochumon, S.; Malik, M.Z.; Akther, N.; Jacob, S.; Drobiova, H.; Al-Mulla, F.; Ahmad, R. H3K9 acetylation-NF-κB-AP-1 nexus targeted by ITE limits TNF-α-induced MMP-9 expression in monocytic cells. J. Immunol. 2025, vkaf240. [Google Scholar] [CrossRef]
- Bahman, F.; Akhter, N.; Kochumon, S.; Al-Mulla, F.; Ahmad, R. Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes. Int. J. Mol. Sci. 2025, 26, 5663. [Google Scholar] [CrossRef]
- Kochumon, S.; Jacob, T.; Koshy, M.; Al-Rashed, F.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Rosen, E.D.; Ahmad, R. Palmitate Potentiates Lipopolysaccharide-Induced IL-6 Production via Coordinated Acetylation of H3K9/H3K18, p300, and RNA Polymerase II. J. Immunol. 2022, 209, 731–741. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Adipose Tissue Hypoxia in Obesity and Its Impact on Preadipocytes and Macrophages: Hypoxia Hypothesis. Adv. Exp. Med. Biol. 2017, 960, 305–326. [Google Scholar]
- Stafeev, I.S.; Michurina, S.S.; Podkuychenko, N.V.; Menshikov, M.Y.; Parfyonova, Y.V.; Vorotnikov, A.V. Chemical Inducers of Obesity-Associated Metabolic Stress Activate Inflammation and Reduce Insulin Sensitivity in 3T3-L1 Adipocytes. Biochem. Biokhimiia 2019, 84, 553–561. [Google Scholar]
- Binet, F.; Sapieha, P. ER Stress and Angiogenesis. Cell Metab. 2015, 22, 560–575. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Dong, N.; Lu, D.; Jiang, X.; Xu, J.; Wu, Z.; Zheng, D.; Wechsler, D.S. A positive feedback loop between ROS and Mxi1-0 promotes hypoxia-induced VEGF expression in human hepatocellular carcinoma cells. Cell. Signal. 2017, 31, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef] [PubMed]
- Karali, E.; Bellou, S.; Stellas, D.; Klinakis, A.; Murphy, C.; Fotsis, T. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol. Cell 2014, 54, 559–572. [Google Scholar] [CrossRef]
- Schneider, K.S.; Chan, J.Y. Emerging role of Nrf2 in adipocytes and adipose biology. Adv. Nutr. 2013, 4, 62–66. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahman, F.; Nadeem, T.; Alayyaf, A.; Al Madhoun, A.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Endoplasmic Reticulum Stress Drives VEGF Gene Expression in Monocytic Cells. Curr. Issues Mol. Biol. 2025, 47, 839. https://doi.org/10.3390/cimb47100839
Bahman F, Nadeem T, Alayyaf A, Al Madhoun A, Al-Mulla F, Sindhu S, Ahmad R. Endoplasmic Reticulum Stress Drives VEGF Gene Expression in Monocytic Cells. Current Issues in Molecular Biology. 2025; 47(10):839. https://doi.org/10.3390/cimb47100839
Chicago/Turabian StyleBahman, Fatemah, Taha Nadeem, Abdulrahman Alayyaf, Ashraf Al Madhoun, Fahd Al-Mulla, Sardar Sindhu, and Rasheed Ahmad. 2025. "Endoplasmic Reticulum Stress Drives VEGF Gene Expression in Monocytic Cells" Current Issues in Molecular Biology 47, no. 10: 839. https://doi.org/10.3390/cimb47100839
APA StyleBahman, F., Nadeem, T., Alayyaf, A., Al Madhoun, A., Al-Mulla, F., Sindhu, S., & Ahmad, R. (2025). Endoplasmic Reticulum Stress Drives VEGF Gene Expression in Monocytic Cells. Current Issues in Molecular Biology, 47(10), 839. https://doi.org/10.3390/cimb47100839










