1. Introduction
The immune checkpoint ligand Programmed cell death ligand 1 (PD-L1), also known as CD274 and B7-H1, is a type I transmembrane glycoprotein that belongs to the immunoglobulin superfamily [
1]. PD-L1 is expressed on the surface of antigen-presenting cells, tumor cells, or non-transformed cells. Elevated PD-L1 protein levels have been detected in many different cancers, including breast, colorectal, and non-small cell lung cancer (NSCLC) [
2], and are associated with a poor prognosis. Furthermore, PD-L1 expression is regulated at the transcriptional level by several transcription factors that are involved in oncogenic and inflammatory signaling pathways [
2]. The activation or mutation of several transcription factors, including Myc, HIF1/2α, NF-κB, MAPK, PTEN/PI3K and EGFR, which are components of multiple oncogenic signaling pathways, leads to the upregulation of PD-L1 mRNA [
3]. Studies have shown that elevated levels of PD-L1 mRNA, mediated by the IFN-γ-induced JAK/STAT1/IRF1 pathway, are associated with immune evasion in melanoma cells [
1]. Post-translational modifications of PD-L1, including glycosylation, phosphorylation, and ubiquitination, are key regulators of protein stability and protein–protein interactions [
3]. PD-L1 suppresses the immune response by interacting with Programmed cell death 1 (PD-1) (CD279) in T cells, thus inhibiting their activation and allowing cancer cells to evade immune surveillance [
1,
2]. PD-L1 is heavily glycosylated, showing only N-linked glycosylation in the extracellular N-terminal domain of PD-L1, which contains the N-X-T/S motif and shows a high level of glycosylation in N35, N192, N200, and N219 [
4]. The oncogenic behavior of these N-linked glycosylated residues in PD-L1 is associated with tumorigenesis, as studies using 4NQ mutants have shown increased anti-tumor immunity [
4]. Consistently, PD-L1 glycosylation is required for the interaction between PD-L1 and PD-1 in breast cancer cells [
4]. Therefore, promoting PD-L1 degradation to prevent the PD-L1-PD-1 interaction is a feasible strategy to develop more effective cancer immunotherapies.
Glycoprotein 78 (GP78), also known as the autocrine motility factor receptor (AMFR), is an E3 ubiquitin ligase. GP78 is a resident transmembrane protein of the endoplasmic reticulum (ER) involved in the ER-associated degradation (ERAD) of misfolded proteins, which is necessary for maintaining cellular homeostasis [
5,
6]. Overexpression of autocrine motility factor (AMF) and the AMF receptor (AMFR) is often associated with cancer and poor clinical outcomes. In breast cancer, elevated GP78 expression and the formation of AMF-AMFR complexes are negatively associated with clinical outcomes and are implicated in breast cancer progression [
7]. Upregulation of GP78 expression has also been reported in bladder carcinoma [
8], while histochemical analyses in colorectal cancer patients have revealed an association between higher GP78 expression and lower survival rates and cancer recurrence [
5]. Similarly, high AMFR expression in stage I non-small cell lung cancer is associated with poor clinical outcomes [
9]. Beyond these clinical correlations, GP78 also plays a critical role in protein quality control and signaling. It binds to ERAD substrates such as the cystic fibrosis transmembrane conductance regulator (CFTR) and apolipoprotein B (APOB) and targets them for ubiquitination, followed by proteasomal degradation [
10,
11]. GP78 self-ubiquitination is mediated by the 25 kDa protein containing the tripartite motif (TRIM25) for proteasomal degradation [
12]. A recent study from our lab has reported that ERK activation is mediated by GP78 through the degradation of DUSP1, resulting in EGFR-dependent proliferation and invasion of cancer cells [
13].
In this study, we describe a previously unreported mechanism of PD-L1 degradation, preceded by GP78-mediated ubiquitination. This post-translational modification depends on a physical interaction between GP78 and PD-L1, as demonstrated by immunoprecipitation (IP) assays and molecular modeling of the GP78-PD-L1 complex. Our study reveals that the core interface residues of the GP78-PD-L1 complex overlap with those involved in the binding of PD-L1 to PD-1. These findings suggest that the downregulation of the PD-L1 protein by GP78-mediated degradation can lead to blockade of the PD-L1-PD-1 interaction, allowing the immune system to target and attack cancer cells more effectively.
2. Materials and Methods
Cell lines and cell culture. HEK293T, OV433, and MDA-MB-231 cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). These cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) and maintained at 37 °C in a humidified atmosphere of 5% CO2.
The reagents used are as follows: Bio-Rad (Hercules, CA, USA): 30% acrylamide/Bis (1610156). Calbiochem (San Diego, CA, USA): Protein G PLUS/Protein A-Agarose (IP05). Cell Signaling (Danvers, MA, USA): PD-L1 Rabbit mAb (13684), anti-AMFR antibody (9590S), DYKDDDDK (Flag) mAb (14793S), K48-linkage polyubiquitin antibody (4289), anti-mouse IgG, HRP-linked secondary antibody (7076S), anti-rabbit IgG, HRP-linked secondary antibody (7074S), and normal rabbit IgG (2729). EMD Millipore (Burlington, MA, USA): Ub-K63 monoclonal antibody (05-1308). GE Healthcare (Waukesha, WI, USA): Penicillin-streptomycin (SV30010). Gibco (Mount Laurel, NJ, USA): Trypsin-EDTA and DMEM (11995-065). Invitrogen (Carlsbad, CA, USA): Blasticidine (R210-01), Lipofectamine 2000 (11668-027), Lipofectamine RNAiMAX (13778-075), V5 monoclonal antibody (R960-25), goat anti-mouse IgG Alexa fluor 680 secondary antibody (A21058), and goat anti-rabbit IgG Alexa fluor 680 secondary antibody (A-21109). Santa Cruz (Dallas, TX, USA): GP78 (sc-293371), anti-HA antibody (F7) (sc-7392), and normal mouse IgG (c-2025). Sigma-Aldrich (St. Louis, MO, USA): FBS (F0926), Z-Leu-Leu-Leu-al (MG132) (C2211), puromycin (P9620), protease inhibitors (5892970001), phosphatase inhibitors (4906845001), β-actin antibody (A1978), and ANTI-FLAG® M2 affinity gel (A2220-1ML). ThermoFisher (Waltham, MA, USA): West Pico PLUS chemiluminescent substrate (34580), and Sample buffer (50-196-784). Dharmacon (Lafayette, CO, USA): siGenome Human AMFR siRNA-SMART pool (M-006522-01-0005) contains the following sequences: (1) GCAAGGAUCGAUUUGAAUA, (2) GGAGCUGGCUGUCAACAAU, (3) GAGGACUGCUCAUGUGAUU, and (4) CGAGCUGGCUGCCGAGUUU. siGenome non-targeting siRNA pool # 1 (D-001206-13-05) contains the following sequences: (1) UAGCGACUAAACACAUCAA, (2) UAAGGCUAUGAAGAGAUAC, (3) AUGUAUUGGCCUGUAUUAG, and (4) AUGAACGUGAAUUGCUCAA.
2.1. Plasmids and Cloning
Full-length human GP78 cDNA was inserted into pCDNA6-V5, as described [
12]. The pCMV-PD-L1 flag plasmid was purchased from Sino Biological Inc (Wayne, PA, USA). (HG10084-CF). PRK-HA-Ub, tagged with hemagglutinin-ubiquitin (HA-Ub), was purchased from Addgene (17608) (Watertown, MA, USA). OV433-L-gpva2-shRNA GP78 and OV433-PL01-con shRNA cells were prepared using shRNA constructs against hGP78 that were purchased from Open Biosystems (Lafayette, CO, USA): va1 V2LHS_11875 NM_001144 shRNAmir Hs Lentiviral pGIPZ RHS4430-98521426, va2 V2LHS_14183 NM_001144 shRNAmir Hs Lentiviral pGIPZ RHS4430-98715204, va3 V2LHS_208794 NM_001144 shRNAmir Hs Lentiviral pGIPZ RHS4430-99291935, and va4 V2LHS_206751 NM_001144 shRNAmir Hs Lentiviral pGIPZ RHS4430-99294626. To generate OV433 cells with GP78 knockdown, control and V2LHS_14183 constructs were transfected into OV433 cells and selected with puromycin. A single colony was then collected, analyzed, and confirmed by Western blotting.
2.2. Transfection
For plasmid DNA transfection, mammalian cells were 80% confluent at the time of transfection and transfected with Lipofectamine 2000 following the manufacturer’s instructions. Cells were treated with 10 μM MG132 for 4 h prior to 24 h before being harvested. For siRNA transfection, mammalian cells were grown to 50% confluency and transfected with Lipofectamine RNAiMAX following the manufacturer’s instructions. Cells were harvested after 48 h.
2.3. Generation of MDA-MB-231-pcDNA6-V5-hGP78 and MDA-MB-231-pcDNA6-V5 Vector Stable Cell Lines
MDA-MB-231 cells were transfected with pcDNA6-V5-hGP78 or pcDNA6-V5-Vector. After 24 h, cells were washed with PBS and selected with Blasticidin at 10 μg/mL. The process was repeated every 3 days until separate colonies were visible and had grown in the presence of blasticidin. The colonies were picked and the GP78 overexpression clones were verified by Western blotting.
2.4. Immunoprecipitation (IP) Assay
IP was performed as described elsewhere [
13]. Specifically, cells were lysed in 1X NP-40 lysis buffer supplemented with protease inhibitors on ice. After centrifugation at 4 °C for 12 min, cell extract supernatants were collected, and 500 μg of protein lysate was used for the IP assay. For the GP78 pull-down, 0.8 μg of mouse V5 antibody or control mouse antibody was used to bind to 20 μL of protein A/G agarose beads and 500 μg of protein lysate was added. The next day, the beads were centrifuged at 5000×
g and washed three times with 1X NP-40 wash buffer, followed by the addition of 40 μL of 2X Laemmli buffer. Then they were boiled and Western blotting was performed. For PD-L1 pull-down, M2 Flag affinity agarose gel beads (40 μL) were used, and immunoprecipitation was performed according to the manufacturer’s protocol.
2.5. Molecular Model for the GP78-PD-L1 Interaction
The structure of the PD-L1-GP78 complex was predicted using the AlphaFold server powered by AlphaFold 2 (
https://deepmind.google/technologies/alphafold/ accessed on 16 September 2025) [
14]. The full-length amino acid sequence of human PD-L1 (residues 1-290, UniProt ID: Q9NZQ7) and the sequence of human GP78 (residues 309-643, UniProt ID: Q9UKV5) were used as input in paired complex mode. Multiple structural models were generated using the default random seed settings. The top-ranked models were selected using a combination of quantitative and structural criteria: a mean per-residue pLDDT (predicted Local Distance Difference Test) ≥ 70 for structured regions and a mean PAE (predicted Aligned Error) < 5 Å across predicted interfaces to ensure confident domain placement. In addition to these numerical thresholds, we visually inspected domain compartmentalization to confirm that the predicted ER-lumen and cytosolic domains were orientated plausibly and were consistent with expected biology. Structural visualization and superposition analysis of the PD-L1-GP78 complex with the PD-1-PD-L1 crystal structure (PDB code: 3BIK) were performed using PyMOL v2.5.5 software.
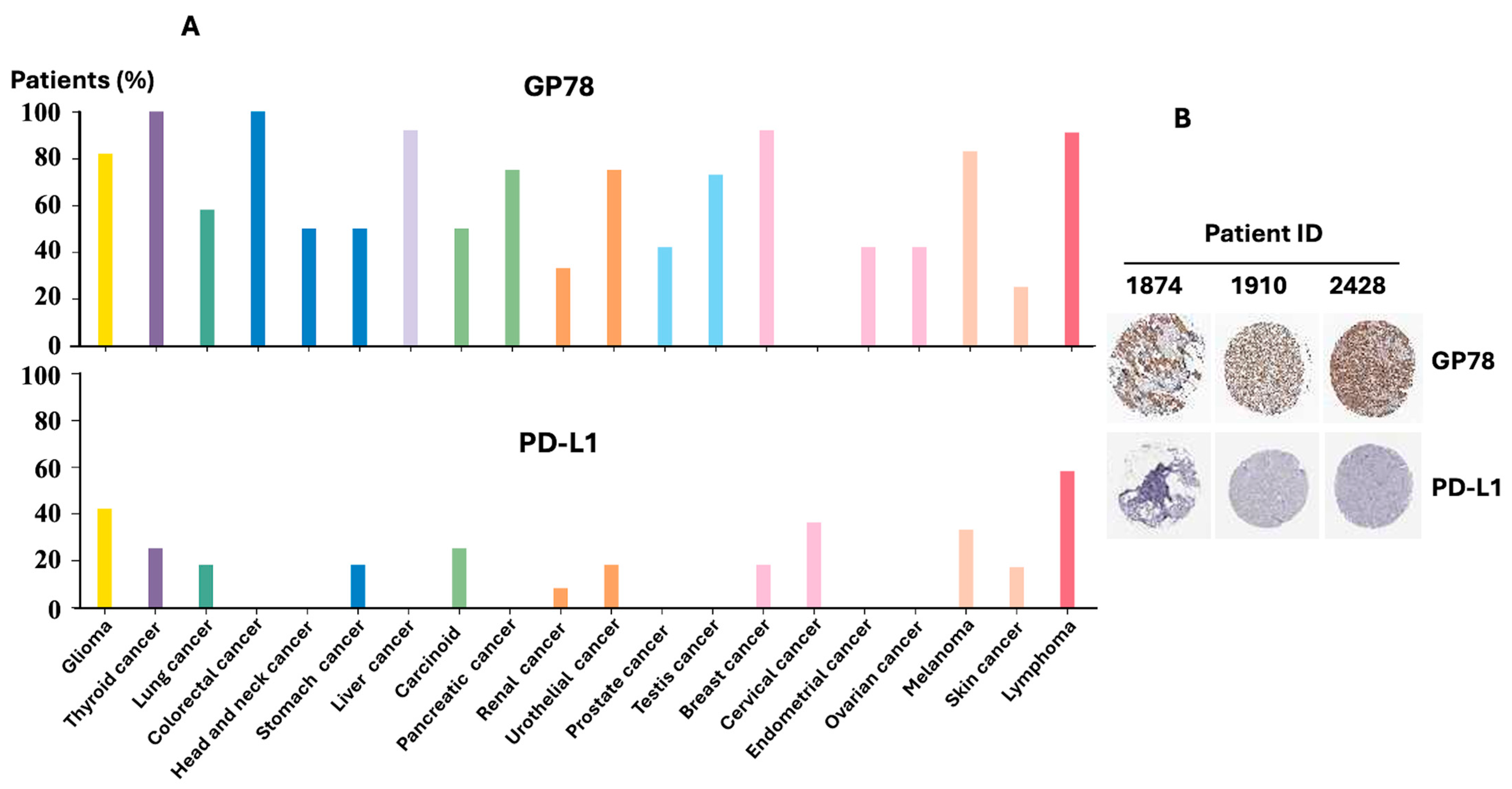
2.6. Analysis of GP78 and PD-L1 Protein Expression in the Human Protein Atlas
Publicly available immunohistochemistry (IHC) data for GP78 (CAB026381) and PD-L1 (CAB080537) were obtained from the Human Protein Atlas (
https://www.proteinatlas.org accessed on 16 September 2025). Data plots show the percentage of patient samples (a maximum of 12 per cancer type) with high, medium, or low protein expression, grouped by tissue of origin. Representative HPA breast cancer IHC images were selected to illustrate GP78 and PD-L1 expression patterns.
Western Blotting
The preparation of whole-cell lysates and Western blot analysis have been previously described [
15]. Cells were lysed in 1X NP-40 lysis buffer and centrifuged at 4 °C for 12 min. A total of 50 μg of protein lysate was used for gel electrophoresis on a 10% denaturing polyacrylamide gel. After transferring and blocking with a 2% Casein solution, the membrane was washed and then incubated with the appropriate primary antibody. It was subsequently labeled with fluorescently labeled or HRP-conjugated secondary antibodies. Antibody binding was detected using the Li-COR Odyssey Imaging system or by HRP protein detection systems. Densitometric analyses of protein bands were performed using ImageJ1.54p (NIH), with signal intensities normalized to the corresponding actin loading control for each blot.
In vivo ubiquitination. HEK293T cells were transfected with PRK-HA-Ub, pCMV-PD-L1-Flag, and either pcDNA6-V5-hGP78 or pcDNA6-V5-Vector. After 24 h, cells were lysed with 1X RIPA lysis buffer. For in vivo ubiquitination of PD-L1, we followed the procedure described previously. Briefly, the cell lysates were boiled at 100 °C for 10 min, cooled, and then sonicated. After centrifugation, the supernatants were saved, and protein concentrations were measured. For the immunoprecipitation assay, 500 μg of protein lysates were used and diluted to 1:1000 with dilution buffer [
15]. The lysates were then incubated with M2 Flag affinity gel agarose beads at 4 °C in a rotor overnight, following the manufacturer’s instructions. The antigen–antibody complex bound to the agarose beads was washed, and Western blotting was performed.
4. Discussion
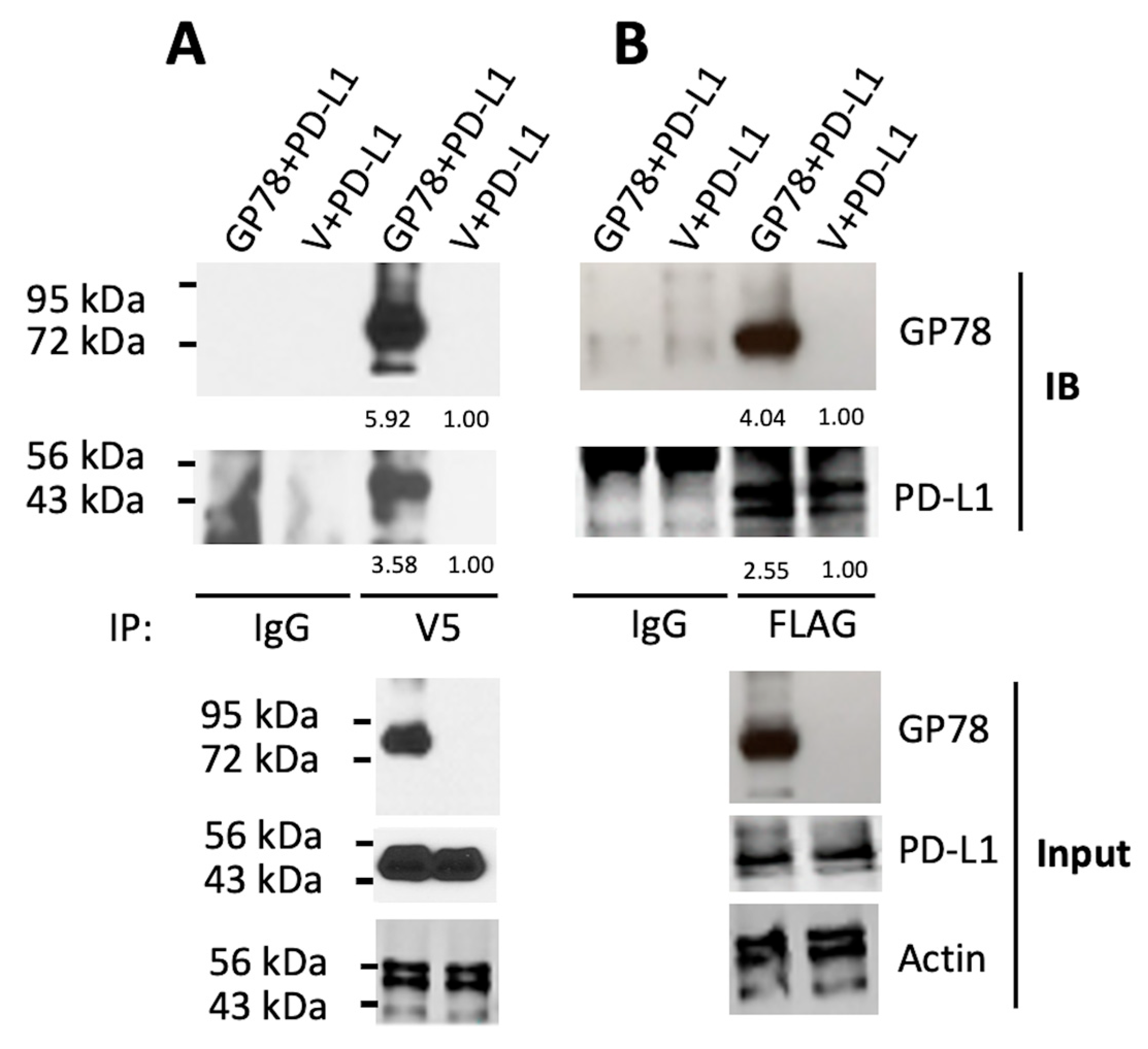
PD-L1/PD-1 immune checkpoint blockade is at the forefront of research aimed at developing clinically feasible treatments for cancer patients. Our study showed that GP78 physically binds to and targets PD-L1 for degradation. A direct interaction between GP78 and PD-L1 was observed in HEK293T cells (
Figure 3A,B), and this interaction was supported by molecular modeling experiments using AlphaFold (
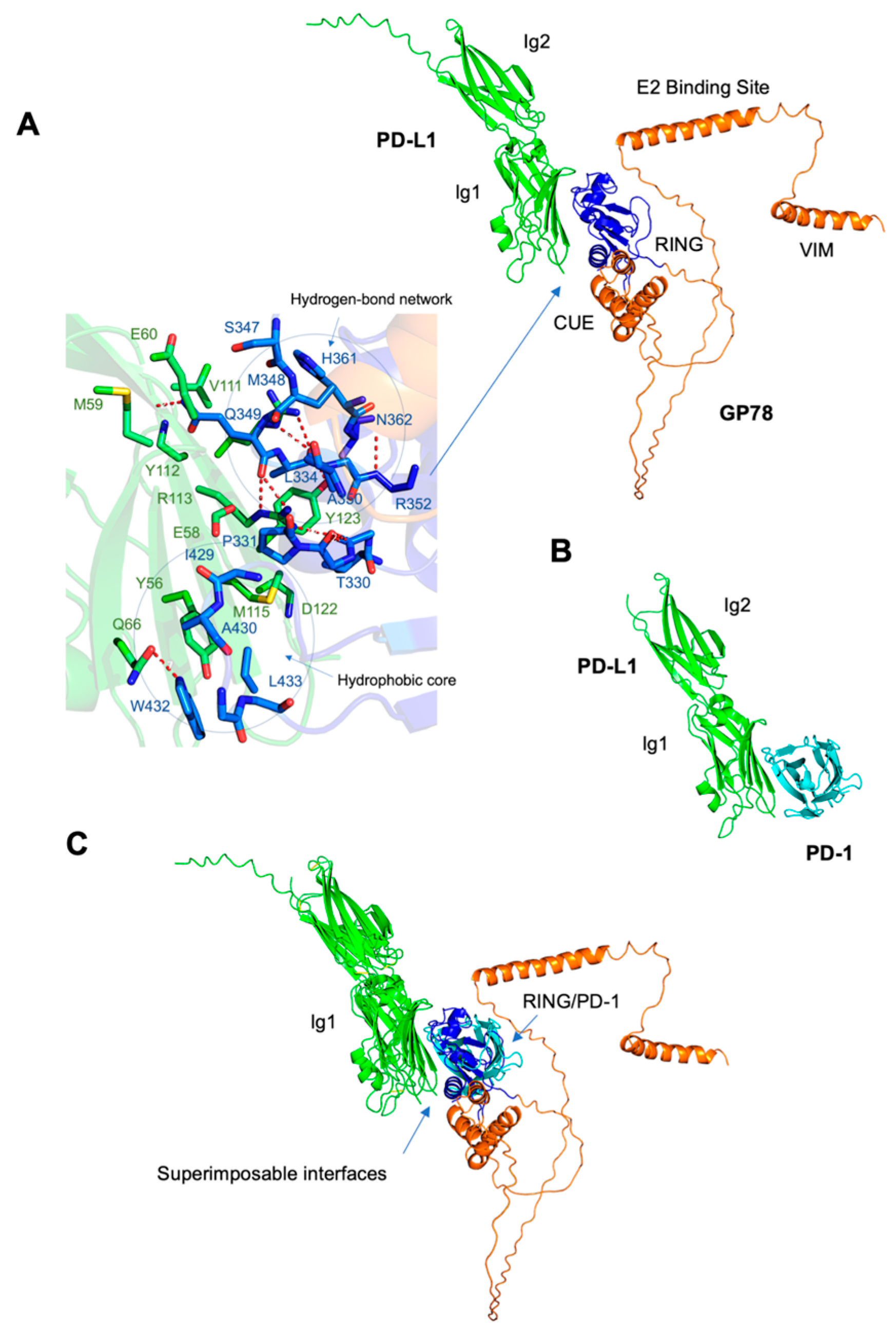
Figure 4A–C). Among the four domains of GP78-RING (residues: 341–379), CUE (residues: 456–498), E2 binding site (residues: 574–612), and VIM (residues: 622–640), the RING functions to confer E3 ubiquitin ligase activity, which facilitates the transfer of ubiquitin to the target substrates from ubiquitin-conjugating enzymes (E2s) thereby tagging the target proteins for proteasomal degradation [
16]. The region corresponding to β-hairpin loop between the RING finger and the GP78 CUE domain is responsible for its E3 ligase activity [
16]. PD-L1 contains two extracellular domains, the IgV domain (also called Ig1, residues 19–132) and the IgC domain (also called Ig2, residues 133–231) [
17]. Our study predicted an interaction between the Ig1 domain of PD-L1 and the RING domain, together with a β-hairpin preceding the CUE domain of GP78 (blue,
Figure 4A). The interface amino acids demonstrate extensive stabilization through hydrophobic interactions and hydrogen bonds. Hydrophobic interactions involve Ile54, Tyr56, Val68, Met115, and Tyr123 of PD-L1, and Val328, Pro331, Phe424, Ile429, Trp432, and Leu433 of GP78. Six hydrogen bonds are formed at the interface, including those between Glu60 and Gln349, Gln66 and Trp432, Arg113 and Gln349, Tyr123 and Ala351, and Arg125 and Ala350. Studies showed that the Ig1 domain of PD-1 (green) interacts with the Ig1 domain of PD-L1 (cyan,
Figure 4A) in the crystal structure of the PD-1/PD-L1 complex (PDB code: 3BIK). This binding is crucial because it sends inhibitory signals to suppress T lymphocyte proliferation and decrease cytokine production, thus weakening the immune system and allowing tumors to bypass immune surveillance [
18]. Our study demonstrated that the superposition of the PD-L1/PD-1 and PD-L1/GP78 complexes ensured that the binding modes of the two complexes were superimposable, suggesting that PD-L1 utilizes the same surface of the IgG1 domain to bind to PD-1 or GP78. Several core interface residues in PD-L1 are shared between the complexes, including Ile54, Tyr56, Gln66, Arg113, Met115, Tyr123, and Arg125. These residues, predicted by AlphaFold, provide useful hypotheses for potential interaction sites, and future experimental studies are needed to confirm their functional relevance. More mutation analysis will be required to assess the effects of PD-L1 interface residues on tumor growth.
PD-L1 inhibitors, such as monoclonal antibodies or small molecules, directly block PD-L1 from binding to the PD-1 protein [
19]. Li et al. reported a novel drug design strategy using PROTACs (Proteolysis Targeting Chimeras), which recruit E3 ligases to ubiquitinate a substrate of interest and target it for degradation by the proteasome. PROTAC function by forming a ternary complex that involves the PROTAC molecule, the protein of interest (ligand), and an E3 ligase, all linked through an optimized chemical linker [
20]. More studies are needed to determine whether GP78 can be recruited more efficiently to target PD-L1 for degradation, thus enhancing immunotherapy strategies for cancer treatment.
PD-L1 is known to be ubiquitinated by various E3 ligases [
21], but the underlying mechanism involving the glycosylation status of PD-L1 remains largely unknown. PD-L1 is highly N-glycosylated, a modification necessary for its stabilization and binding to PD-1, which suppresses immune responses in cancer [
22]. Recent studies have described the formation of ternary complexes involving mPNGase, AMFR (GP78), and other ER-associated degradation components, which provide a mechanistic framework in which deglycosylation precedes ubiquitination [
23,
24]. By screening a two-hybrid yeast library using mPNGase as bait, a previous study identified AMFR as one of the interacting proteins that forms a complex with mPNGase [
23]. The cytoplasmic tail of AMFR was found to be involved in this interaction [
23]. The formation of a ternary complex composed of the E3 enzyme AMFR, p97, mPNGase, and mHR23B was proposed to bridge the ER and the proteasome [
24]. According to this model, the first step involves the deglycosylation of the substrate by mPNGase, followed by ubiquitination. In the next step, the misfolded, deglycosylated, and ubiquitinated protein is channelled to the proteasome by mHR23B [
24]. Thus, further investigation will be necessary to determine whether the removal of N-glycosylated residues from PD-L1 is directly or indirectly promoted by GP78 in cancer cells, providing a deeper understanding of the events that occur before ubiquitination.
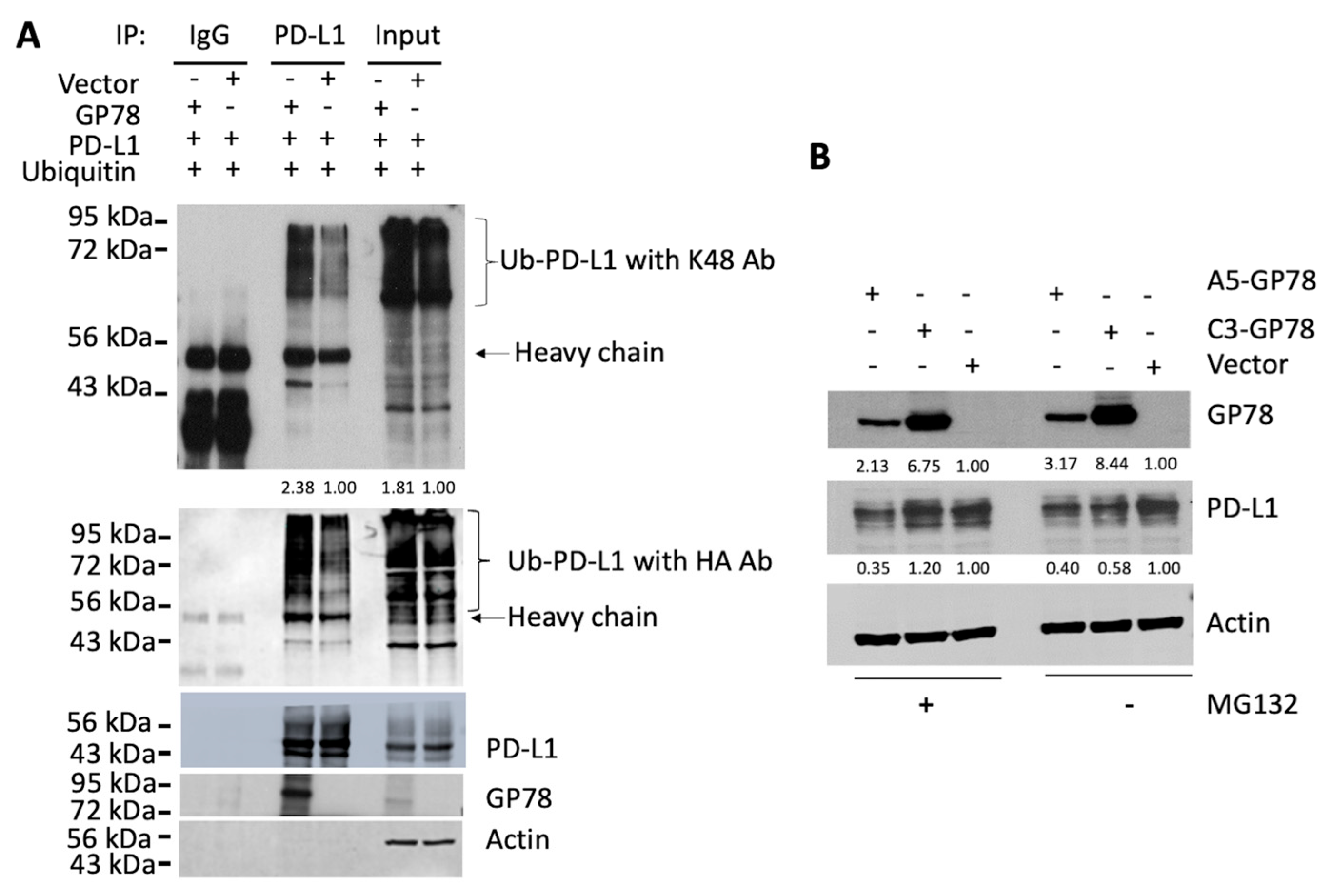
PD-L1 homeostasis is regulated by its proteasomal degradation. Our study not only demonstrated the physical interaction of PD-L1 with GP78 but also showed that PD-L1 polyubiquitination by GP78 occurred through a K48-linked ubiquitin chain (
Figure 2). GP78 is an ER-associated protein, while PD-L1 is primarily expressed on the cell surface. It is unclear how these two proteins, located in different compartments, interact. We speculate that PD-L1, after synthesis, is transported to the plasma membrane through the secretory pathway. It is known that cells can utilize various signaling mechanisms to retrotranslocate proteins from their native cell organelles, and one of the ways is by exposing the degradation signal (degron) of the substrate and subsequent recognition by E3 ubiquitin ligase complexes during a misfolding event [
25,
26]. E3 ligase is typically anchored to the endoplasmic reticulum and mediates substrate recognition via degron, followed by ubiquitination and subsequent degradation. The substrate and E3 ligase, located in different organelles, can utilize molecular glues that bind to both the E3 ligase and the substrate, bringing them into close proximity for a series of degradation events to occur. Studies have shown that adaptor proteins are recruited to bring the cognate substrate into close proximity to Cullin-RING ligases (CRLs) for the formation of the E3 ligase complex [
27].
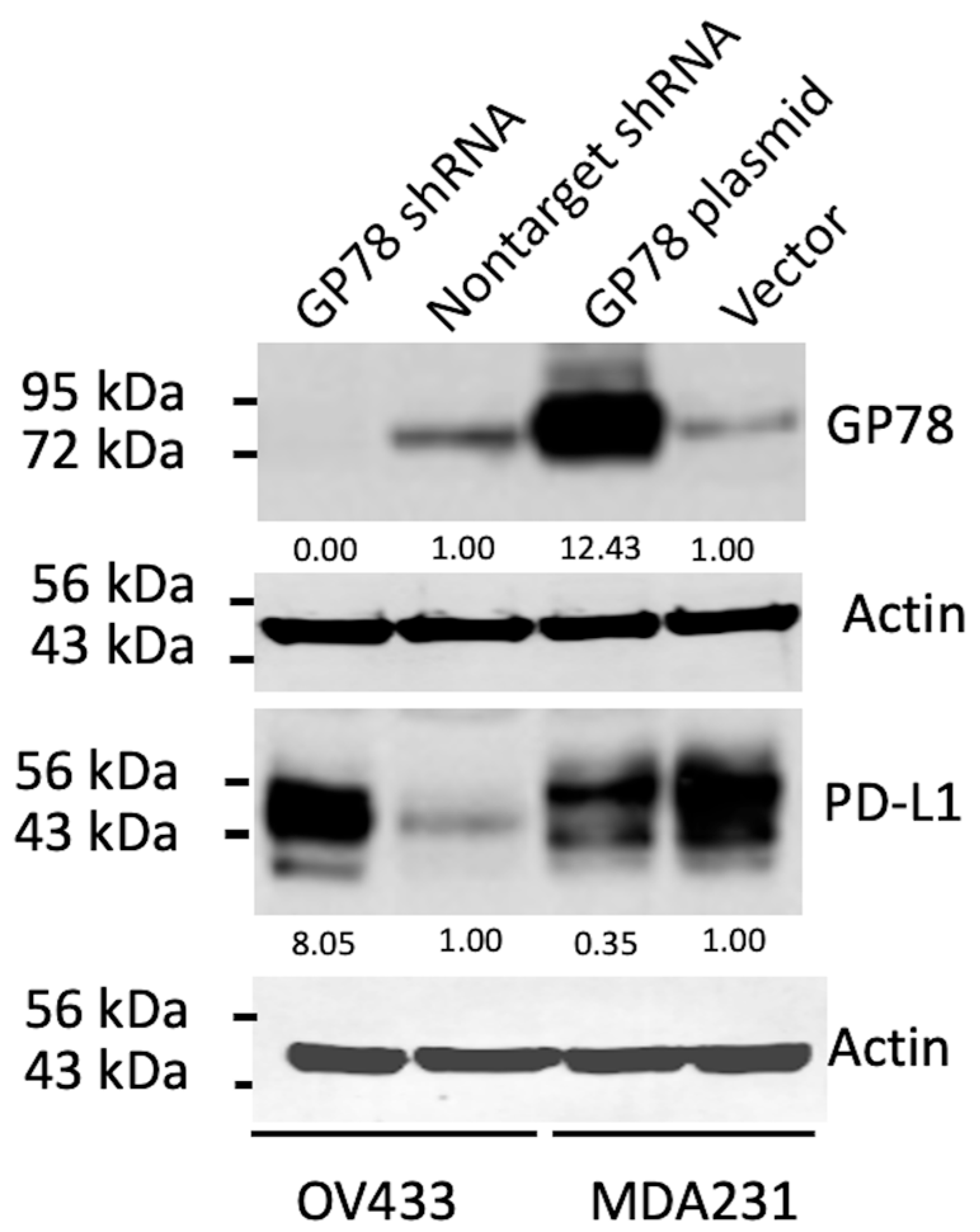
While further study is needed to confirm their interaction and subsequent impact on PD-L1 protein levels, our results demonstrate that GP78 alters the steady-state levels of endogenous PD-L1 compared with control vector–transfected cells, which maintain consistent basal PD-L1 expression. Specifically, GP78 overexpression decreased PD-L1 levels in MDA-MB-231 cells, whereas GP78 knockdown in OV433 cells increased PD-L1 protein levels (
Figure 1). These findings provide indirect evidence that GP78 regulates the stability of PD-L1. Furthermore, ubiquitination assays (
Figure 2) demonstrate that GP78 promotes K48-linked ubiquitination of PD-L1, a modification well established for targeting proteins for proteasomal degradation. It is established that proteins bearing K48-linked polyubiquitin chains are primarily targeted for degradation by the ubiquitin-proteasome system (UPS) [
28], whereas K63-linked polyubiquitin chains are involved in endocytosis [
29]. In addition to regulating PD-L1 protein expression, it is possible that GP78 may also impact PD-L1 mRNA, which will be addressed in future studies.
5. Conclusions
Taken together, our study has identified PD-L1 as a substrate of GP78, which promotes the polyubiquitination of PD-L1 and its subsequent degradation. It remains to be determined whether GP78 is involved in the deglycosylation of PD-L1. It has been reported that after entering the ER-associated degradation (ERAD) pathway, some glycoproteins undergo deglycosylation before being ubiquitinated by an E3 ligase, resulting in proteasomal degradation. A study revealed that the E3 ligase Murine double minute 2 (MDM2) facilitates the deglycosylation of PD-1 by the glycosidase NGLY1 before the ubiquitination event [
30]. However, as discussed earlier, the E3 ligase can participate in the ubiquitination event through the recognition of the degron on the substrate, and it cannot be ruled out that deglycosylation must occur before ubiquitination.
Furthermore, GP78 binding to AMF has been demonstrated to enhance MMP3 expression, resulting in increased cell motility and invasiveness [
31]. Therefore, an in vivo approach to the development of immunotherapeutic strategies can involve targeting AMF binding to its monoclonal antibody, as previously reported [
32], thus inhibiting GP78-AMF signaling and allowing GP78 to interact more readily with PD-L1 for degradation. We speculate that the degradation of PD-L1 by GP78 E3 ligase reduces the abundance of PD-L1 protein on the surface of tumor cells. As a result, the binding of PD-L1 to PD-1 on T cells is reduced, reducing the inhibition of T cells, thus enhancing tumor immune surveillance and ultimately improving the overall anti-tumor immune response. However, if tumors express a low level of GP78, those tumors can express a high level of PD-L1 due to GP78-mediated downregulation. Tumors with high levels of PD-L1 are believed to be more responsive to PD-1/PD-L1-based immunotherapies. Thus, the negative regulation of PD-L1 protein levels by the E3 ubiquitin ligase GP78 can enhance cancer immune surveillance and also serve as an alternative biomarker to predict whether cancer patients can benefit from PD-1/PD-L1-based cancer immunotherapies. These two possibilities require further investigation.