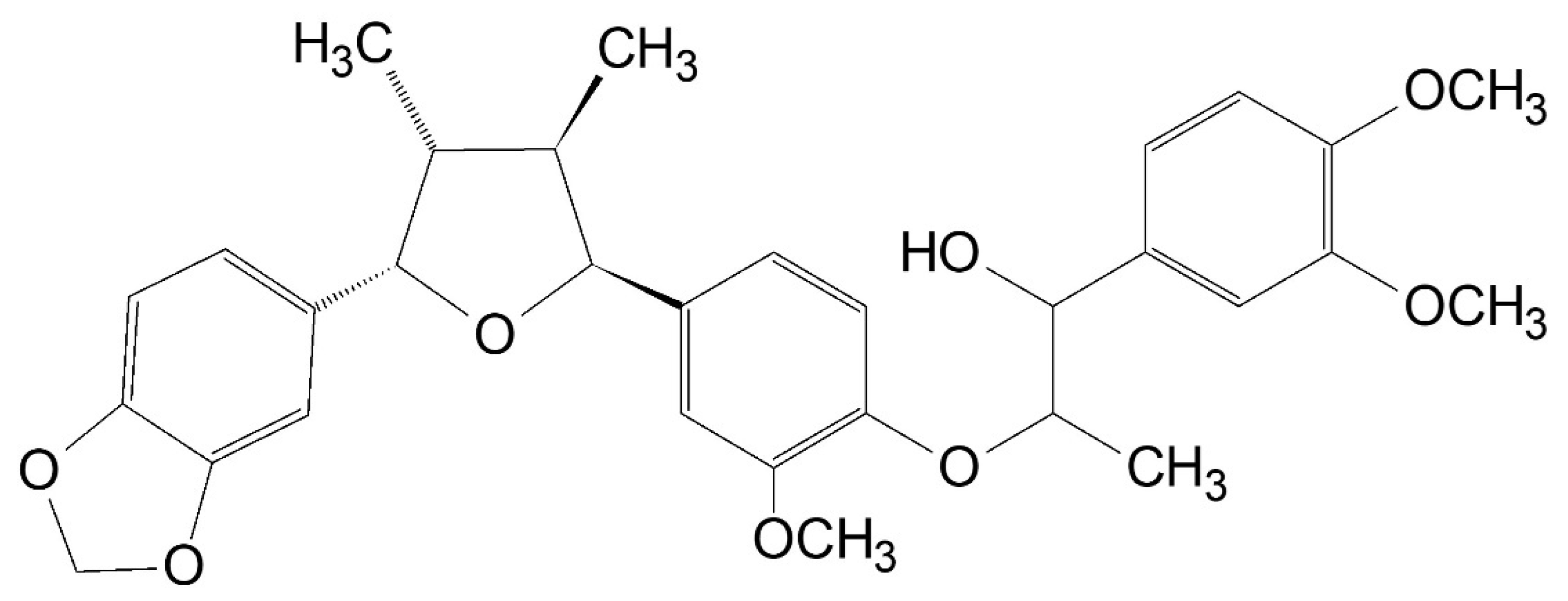
Saucerneol D Suppresses the Growth of Helicobacter pylori and Their Virulence Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.3. RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.4. Anti-H. pylori SecA or Anti-H. pylori Polyclonal Antibody Production
2.5. Protein Extraction and Western Blot
2.6. Urease Activity Test
2.7. Statistical Analysis
3. Results
3.1. Determination of the MIC
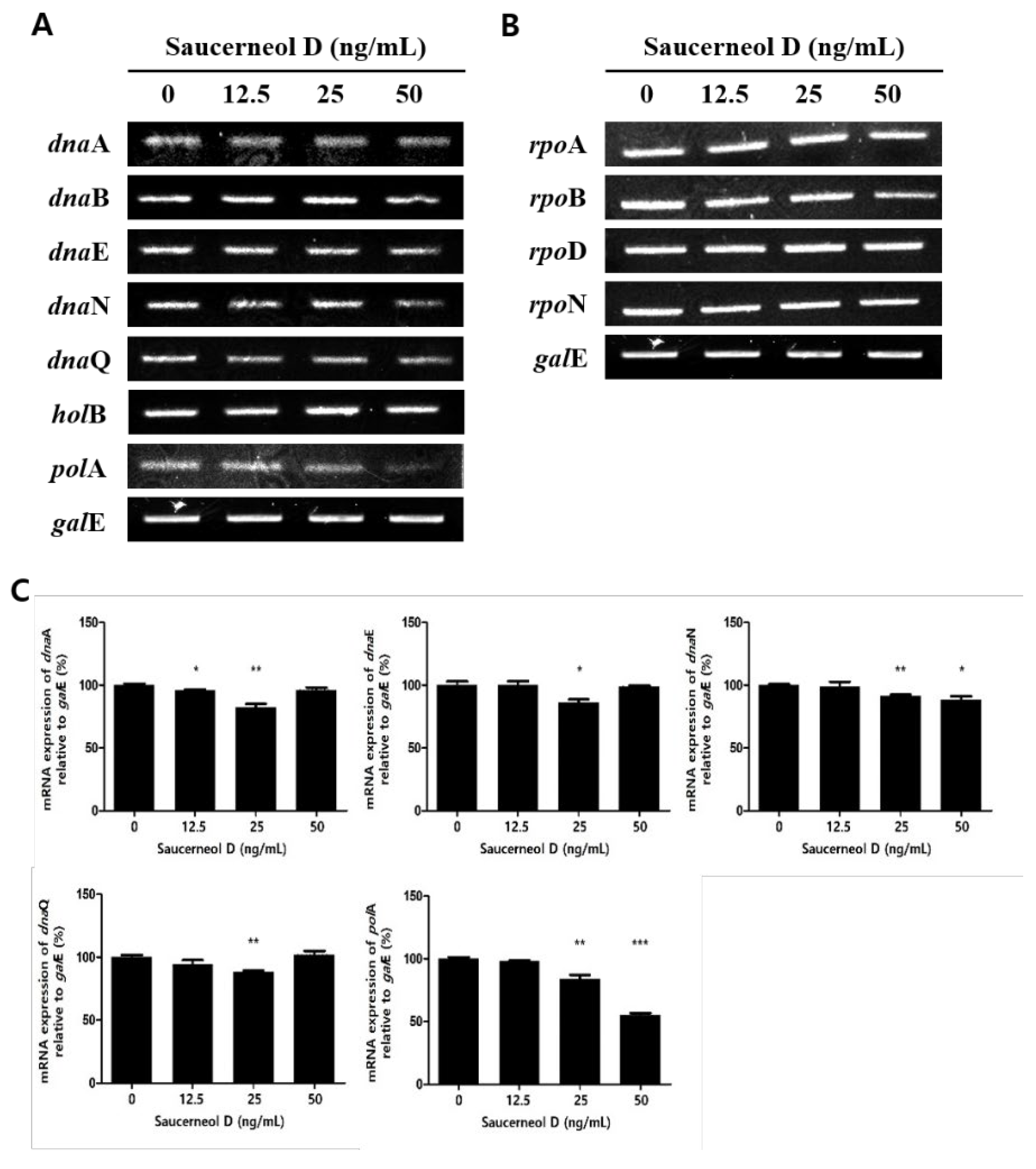
3.2. Effect on Replication and Transcription Genes in Saucerneol D-Treated H. pylori
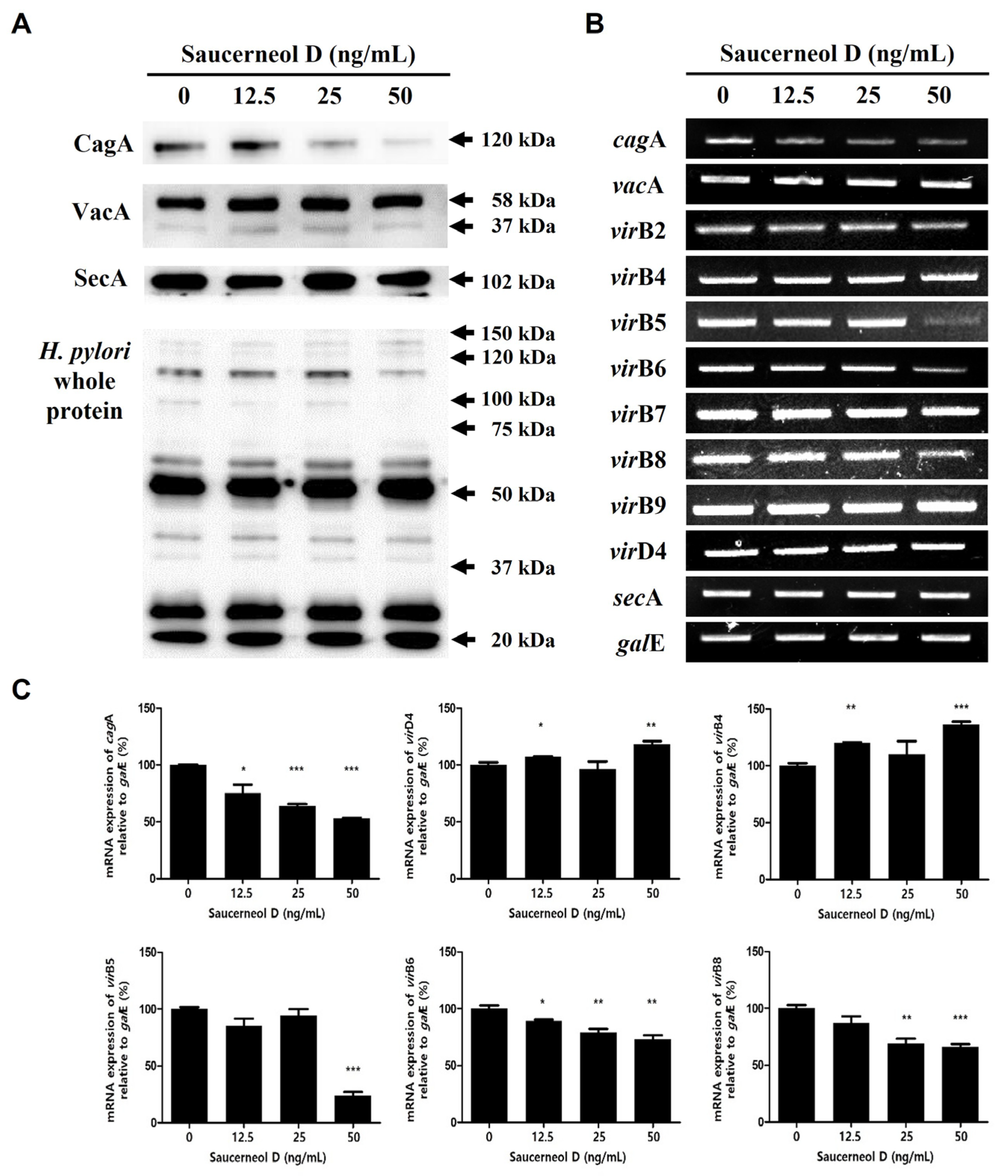
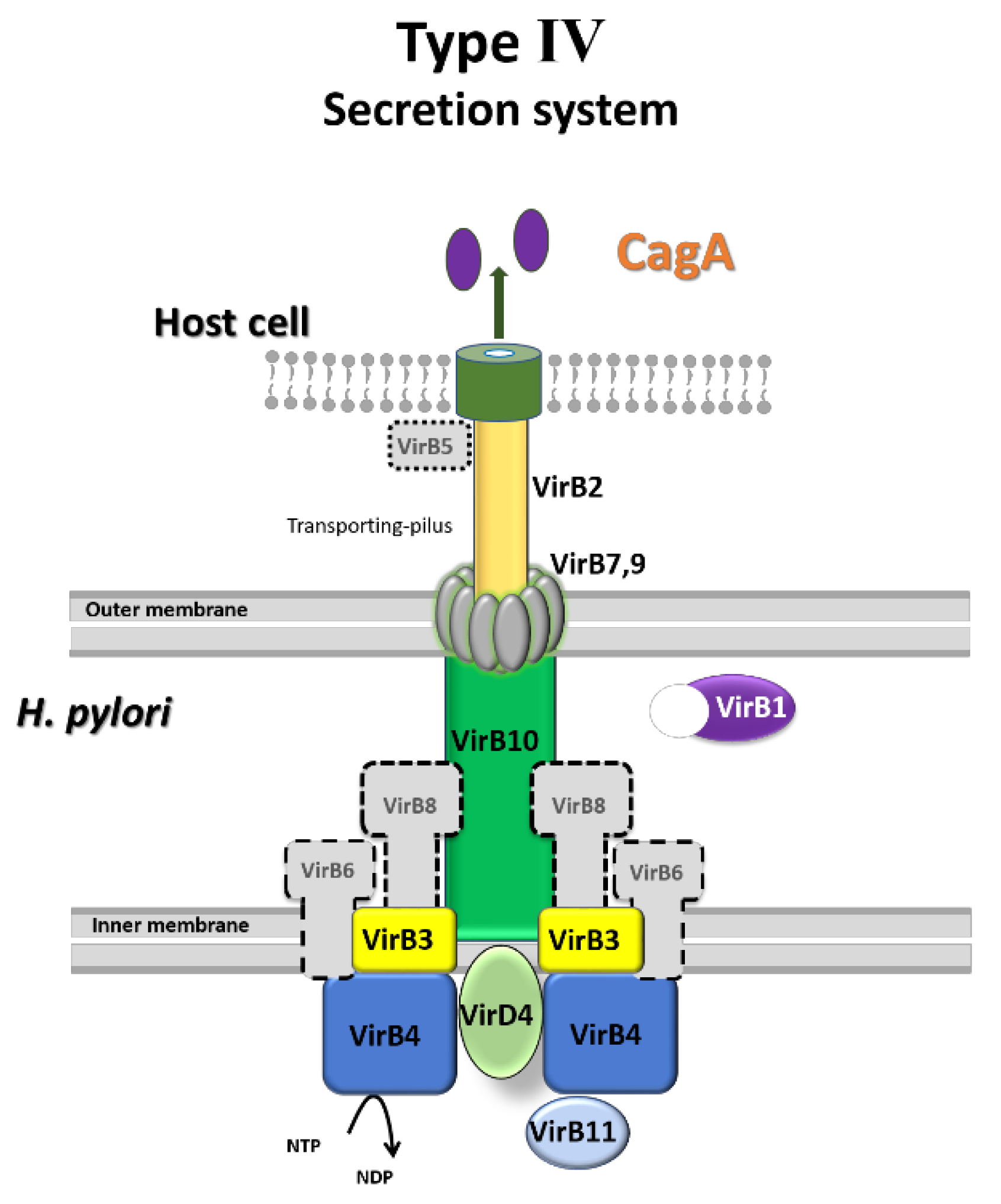
3.3. Effect on CagA, VacA, and Their Secretion Systems in Saucerneol D-Treated H. pylori
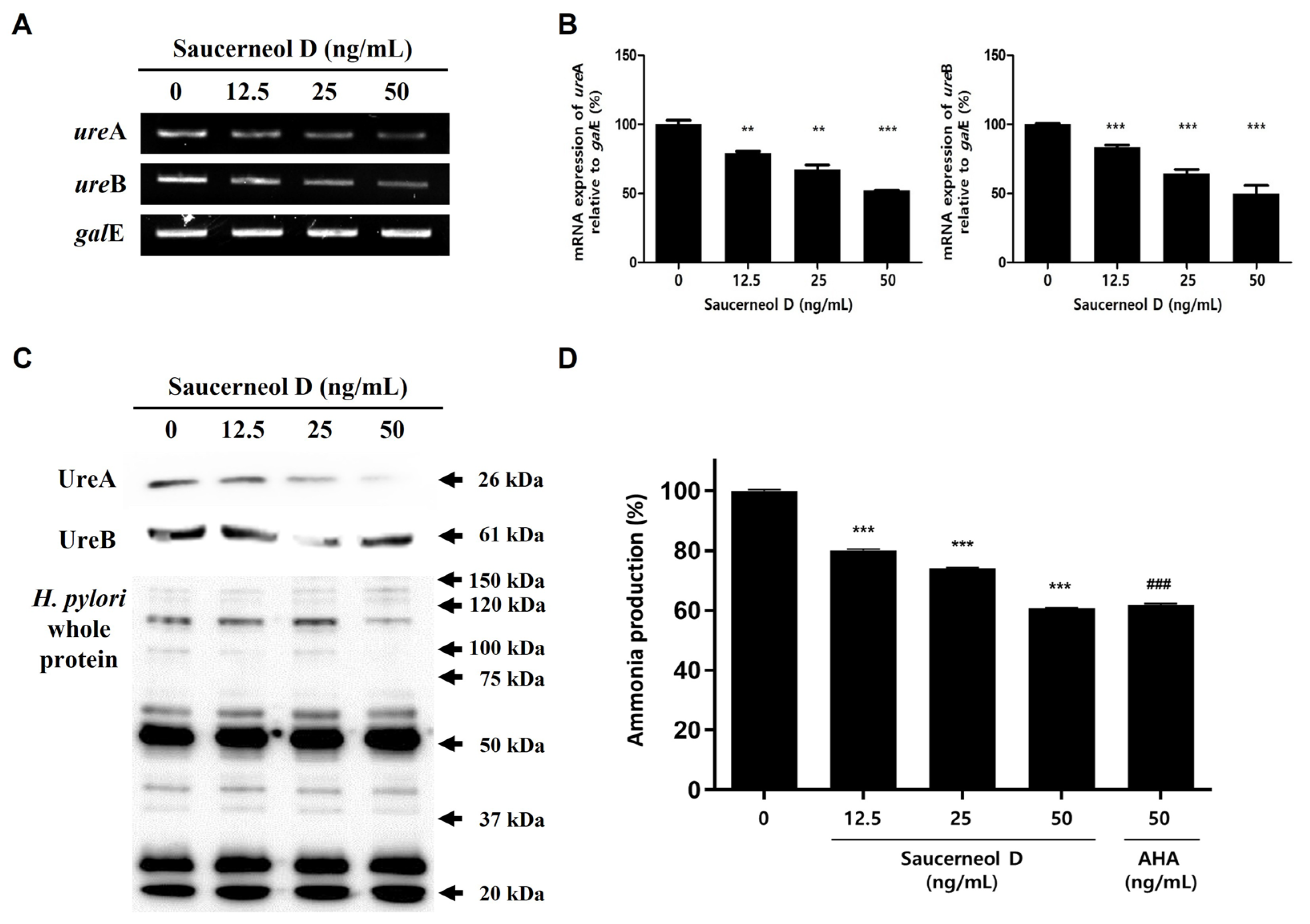
3.4. Downregulation of Urease in Saucerneol D-Treated H. pylori
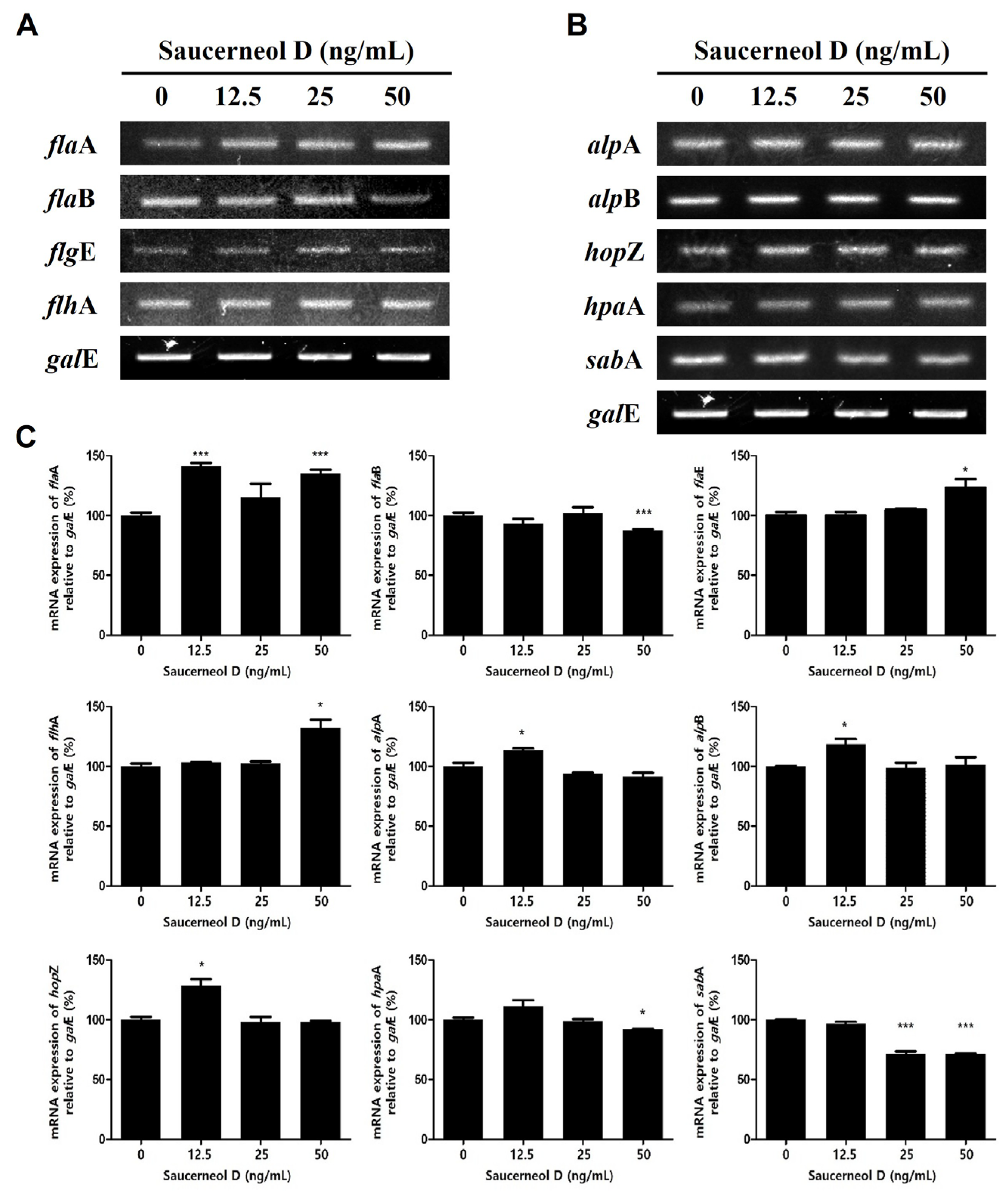
3.5. Effect on Flagella and Adhesion in Saucerneol D-Treated H. pylori
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santacroce, L.; Topi, S.; Bottalico, L.; Charitos, I.A.; Jirillo, E. Current Knowledge about Gastric Microbiota with Special Emphasis on Helicobacter pylori-Related Gastric Conditions. Curr. Issues Mol. Biol. 2024, 46, 4991–5009. [Google Scholar] [CrossRef]
- Møller, H.; Heseltine, E.; Vainio, H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int. J. Cancer 1995, 60, 587–589. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, S.J.; Lee, Y.C.; Yang, H.J.; Park, S.Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.H.; Nam, S.Y.; et al. Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J. Intern. Med. 2021, 36, 807–838. [Google Scholar] [CrossRef]
- Valada, P.; Mata, A.; Brito, R.M.M.; Gonçalves, T.; Medeiros, J.A.; Nogueira, C. Detection of Helicobacter pylori and the Genotypes of Resistance to Clarithromycin, Fluoroquinolones, and Metronidazole in Gastric Biopsies: An In Silico Analysis to Help Understand Antibiotic Resistance. Curr. Issues Mol. Biol. 2025, 47, 187. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Infection, Its Laboratory Diagnosis, and Antimicrobial Resistance: A Perspective of Clinical Relevance. Clin. Microbiol. Rev. 2022, 35, e0025821. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef]
- Merino, E.; Flores-Encarnacion, M.; Aguilar-Gutierrez, G.R. Functional interaction and structural characteristics of unique components of Helicobacter pylori T4SS. FEBS J. 2017, 284, 3540–3549. [Google Scholar] [CrossRef]
- Higuchi, M.; Tsutsumi, R.; Higashi, H.; Hatakeyama, M. Conditional gene silencing utilizing the lac repressor reveals a role of SHP-2 in cagA-positive Helicobacter pylori pathogenicity. Cancer Sci. 2004, 95, 442–447. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Fahimi, F.; Tohidkia, M.R.; Fouladi, M.; Aghabeygi, R.; Samadi, N.; Omidi, Y. Pleiotropic cytotoxicity of VacA toxin in host cells and its impact on immunotherapy. Bioimpacts 2017, 7, 59–71. [Google Scholar] [CrossRef]
- Kim, I.J.; Blanke, S.R. Remodeling the host environment: Modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA). Front. Cell Infect. Microbiol. 2012, 2, 37. [Google Scholar] [CrossRef]
- Boquet, P.; Ricci, V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012, 20, 165–174. [Google Scholar] [CrossRef]
- Ricci, V.; Galmiche, A.; Doye, A.; Necchi, V.; Solcia, E.; Boquet, P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 2000, 11, 3897–3909. [Google Scholar] [CrossRef]
- Ricci, V.; Sommi, P.; Fiocca, R.; Romano, M.; Solcia, E.; Ventura, U. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J. Pathol. 1997, 183, 453–459. [Google Scholar] [CrossRef]
- Stingl, K.; Altendorf, K.; Bakker, E.P. Acid survival of Helicobacter pylori: How does urease activity trigger cytoplasmic pH homeostasis? Trends Microbiol. 2002, 10, 70–74. [Google Scholar] [CrossRef]
- Koh, M.S. Antimicrobial Activity of Saururus chinensis Baill Extract. J. Korean Soc. Food Sci. Nutr. 2004, 33, 1098–1105. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Pyo, M.; Yun, J.; Hwang, B.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Saucerneol D inhibits dendritic cell activation by inducing heme oxygenase-1, but not by directly inhibiting toll-like receptor 4 signaling. J. Ethnopharmacol. 2015, 166, 92–101. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Park, S.Y.; Hwang, J.W.; Park, P.J.; Lim, J.H.; Choi, D.K. Attenuation of inflammatory-mediated neurotoxicity by Saururus chinensis extract in LPS-induced BV-2 microglia cells via regulation of NF-κB signaling and anti-oxidant properties. BMC Complement. Altern. Med. 2014, 14, 502. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, J.B.; Lee, P.; Kim, S.H. Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 3385. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, M.H.; Kim, H.W.; Yang, J.Y.; Woo, H.J.; Park, M.; Moon, C.; Kim, S.-H.; Kim, J.-B. Riboflavin Inhibits Growth of Helicobacter pylori by Down-regulation of polA and dnaB Genes. Korean Soc. Biomed. Lab. Sci. 2020, 26, 288–295. [Google Scholar] [CrossRef]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.-H.; Kim, J.-B. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, N.; Kim, S.H.; Park, M.; Woo, H.J.; Kim, H.W.; Yang, J.Y.; Rhee, K.-J.; Kim, J.B. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agent Cancer 2014, 9, 43. [Google Scholar] [CrossRef]
- Kim, S.H.; Woo, H.; Park, M.; Rhee, K.J.; Moon, C.; Lee, D.; Seo, W.D.; Kim, J.B. Cyanidin 3-O-glucoside reduces Helicobacter pylori VacA-induced cell death of gastric KATO III cells through inhibition of the SecA pathway. Int. J. Med. Sci. 2014, 11, 742–747. [Google Scholar] [CrossRef]
- Dunn, B.E.; Phadnis, S.H. Structure, function and localization of Helicobacter pylori urease. Yale J. Biol. Med. 1998, 71, 63–73. [Google Scholar] [PubMed]
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869. [Google Scholar] [CrossRef]
- Kersulyte, D.; Mukhopadhyay, A.K.; Velapatiño, B.; Su, W.; Pan, Z.; Garcia, C.; Hernandez, V.; Valdez, Y.; Mistry, R.S.; Gilman, R.H.; et al. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 2000, 182, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Testino, G.; Cornaggia, M.; Valentini, M. Helicobacter pylori, pre-neoplastic changes, gastric cancer: A point of view. Eur. J. Gastroenterol. Hepatol. 1999, 11, 357–359. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhou, L.Y.; Song, Z.Q.; Zhang, J.Z.; He, L.H.; Ding, Y. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: A prospective serial study. World J. Gastroenterol. 2015, 21, 2786–2792. [Google Scholar] [CrossRef]
- Evariste, T.K.; Yoshio, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Nitharwal, R.G.; Verma, V.; Dasgupta, S.; Dhar, S.K. Helicobacter pylori chromosomal DNA replication: Current status and future perspectives. FEBS Lett. 2011, 585, 7–17. [Google Scholar] [CrossRef][Green Version]
- Joyce, C.M.; Kelley, W.S.; Grindley, N.D. Nucleotide sequence of the Escherichia coli polA gene and primary structure of DNA polymerase I. J. Biol. Chem. 1982, 257, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Hapfelmeier, S.; Domke, N.; Zambryski, P.C.; Baron, C. VirB6 is required for stabilization of VirB5 and VirB3 and formation of VirB7 homodimers in Agrobacterium tumefaciens. J. Bacteriol. 2000, 182, 4505–4511. [Google Scholar] [CrossRef] [PubMed]
- Baron, C. VirB8: A conserved type IV secretion system assembly factor and drug target. Biochem. Cell Biol. 2006, 84, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.C.; Monzo, P.; Kaddai, V.; Doye, A.; Ricci, V.; Boquet, P. Helicobacter pylori VacA cytotoxin: A probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol. Biol. Cell 2005, 16, 4852–4866. [Google Scholar] [CrossRef]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Leyton, D.L.; Rossiter, A.E.; Henderson, I.R. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nat. Rev. Microbiol. 2012, 10, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Karita, M.; Morshed, M.G.; Okita, K.; Nakazawa, T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect. Immun. 1994, 62, 3586–3589. [Google Scholar] [CrossRef]
- Karita, M.; Tsuda, M.; Nakazawa, T. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J. Clin. Gastroenterol. 1995, 21, S160–S163. [Google Scholar]
- Smoot, D.T.; Mobley, H.; Chippendale, G.R.; Lewison, J.; Resau, J. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect. Immun. 1990, 58, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Souza, M.; de Moraes, J.A.; Da Silva, V.N.; Helal-Neto, E.; Uberti, A.F.; Scopel-Guerra, A.; Scopel-Guerra, A.; Olivera-Severo, D.; Carlini, C.R.; Christina Barja-Fidalgo, C. Helicobacter pylori urease induces pro-inflammatory effects and differentiation of human endothelial cells: Cellular and molecular mechanism. Helicobacter 2019, 24, e12573. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Benoit, S.L.; Misra, S.K.; Sharp, J.S.; Maier, R.J. Noncatalytic antioxidant role for Helicobacter pylori urease. J. Bacteriol. 2018, 200, e00124. [Google Scholar] [CrossRef]
- Bryk, R.; Griffin, P.; Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 2000, 407, 211–215. [Google Scholar] [CrossRef]
- Schirm, M.; Soo, E.C.; Aubry, A.J.; Austin, J.; Thibault, P.; Logan, S.M. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 2003, 48, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Loconte, V.; Kekez, I.; Matkovic-Calogovic, D.; Zanotti, G. Structural characterization of FlgE2 protein from Helicobacter pylori hook. FEBS J. 2017, 284, 4328–4342. [Google Scholar] [CrossRef]
- Tsang, J.; Hoover, T.R. Basal Body Structures Differentially Affect Transcription of RpoN- and FliA-Dependent Flagellar Genes in Helicobacter pylori. J. Bacteriol. 2015, 197, 1921–1930. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.L.; Cheng, D.D.; Xu, W.T.; Lu, N.H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Aspholm, M.; Olfat, F.O.; Nordén, J.; Sondén, B.; Lundberg, C.; Sjöström, R.; Altraja, S.; Odenbreit, S.; Haas, R.; Wadström, T.; et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2006, 2, e110. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J. Infect. Dev. Ctries. 2008, 2, 174–181. [Google Scholar] [CrossRef]
- Magalhães, A.; Reis, C. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz. J. Med. Biol. Res. 2010, 43, 611–618. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequences (5′-3′) | Product Length (bp) | Annealing Temperature (°C) | Cycles | Reference | |
|---|---|---|---|---|---|---|
| Forward | Reverse | |||||
| dnaA | GGGCATGACTTTAGCGGTTA | TTAACGAATTGCACGCCAAC | 128 | 55 | 24 | [21] |
| dnaB | AATGGGCCGTTTATCGTCTC | CAAATCCGCTTGCAACTACG | 231 | 55 | 30 | |
| dnaE | AATCCACCGGCTCCAAATAC | GCCAAACAAGTGTGGGAGTA | 184 | 55 | 30 | |
| dnaN | GTTAGCGGTGGTTGAAAACG | CGGTTTCGCTATGCTCAGAA | 233 | 55 | 30 | |
| dnaQ | CGCATGAAGCTTTGCAAGAA | GCATAGGCTCTATGGCTGAC | 244 | 55 | 30 | |
| holB | TGCAAGCCTTTTTGAACACC | CGCGTTTTGGGCTTCTATAC | 196 | 55 | 30 | |
| polA | TTTCCAAGCTCCCTAATCGC | ATACGGGATTTTGCCGAGTC | 134 | 55 | 30 | [22] |
| rpoA | AGCGACACGTCTTCAGTAAC | ACAGCACCTTTGATCCCATC | 224 | 55 | 30 | [21] |
| rpoB | TTTAGGTAAGCGCGTGGATT | AATCAGCTTTGGATGGAACG | 301 | 59 | 24 | |
| rpoD | TCATCATCATTGCCGACTGG | GTCATGCGCAAACACATTCA | 152 | 55 | 27 | |
| rpoN | GCCCTTGAAATCGTGCTTAC | ATGATGAGAGCTACCCGACA | 250 | 55 | 27 | |
| cagA | GTCATAATGGCATAGAACCTGAA | ATTCCCTAGGGCGTCTAAATAA | 407 | 59 | 27 | [23] |
| vacA | AAACGACAAGAAAGAGATCAGT | CCAGCAAAAGGCCCATCAA | 291 | 57 | 22 | |
| secA | AAAAATTTGACGCTGTGATCC | CCCCCAAGCTCCTTAATTTC | 274 | 47 | 27 | |
| ureA | GCCAATGGTAAATTAGTT | CTCCTTAATTGTTTTTAC | 411 | 40 | 20 | [24] |
| ureB | CCATCCACGAACACATGGTA | TCTATCCCTACCCCACAACC | 252 | 50 | 20 | |
| virB2 | CAGTCGCCTGACCTCTTTTGA | CGGTCACCAGTCCTGCAAC | 156 | 62 | 30 | |
| virB4 | GTTATAGGGGCAACCGGAAG | TTGAACGCGTCATTCAAAGC | 449 | 62 | 30 | |
| virB5 | TACAAGCGTCTGTGAAGCAG | GACCAACCAACAAGTGCTCA | 436 | 62 | 36 | |
| virB6 | CCTCAACACCGCCTTTGGTA | TAGCCGCTAGCAATCTGGTG | 225 | 62 | 30 | |
| virB7 | GATTACGCTCATAGGCGATGC | TGGCTGACTTCCTTGCAACA | 202 | 62 | 30 | |
| virB8 | GTTGATCCTTGCGATCCCTCA | CGCCGCTGTAACGAGTATTG | 218 | 62 | 25 | |
| virB9 | GCATGTCCTCTAGTCGTTCCA | TATCGTAGATGCGCCTGACC | 269 | 62 | 34 | |
| virD4 | CCGCAAGTTTCCATAGTGTC | GCGAGTTGGGAAACTGAAGA | 263 | 62 | 30 | |
| flhA | TCATTGGAGGGTTTTTAGTGG | GGTGCGAGTGGCGACAAT | 155 | 60 | 35 | |
| flaA | TAGACACCACCAACGCTAAA | TGCATTCTAGGGGGTTGTAT | 239 | 62 | 32 | |
| flaB | GTCAATGGCGTGAATGATTA | ATTCACGGTCCCAATTTCTA | 213 | 52 | 32 | |
| flgE | CCGATCAAATCCTTAACACC | AGGCTTAAAAACATGCGAAC | 381 | 52 | 30 | |
| sabA | AAAGCATTCAAAACGCCAAC | CCCGCATAAAGACTCCAAAA | 163 | 60 | 26 | |
| hopZ | GCGCCGTTACTAGCATGATCA | GAAATCTTTCGGCGCGTTT | 101 | 60 | 26 | |
| hpaA | GAGCGTGGTGGCTTTGTTAGT | TCGCTAGCTGGATGGTAATTCA | 90 | 60 | 26 | |
| alpA | GCACGATCGGTAGCCAGACT | ACACATTCCCCGCATTCAAG | 90 | 60 | 27 | |
| alpB | ACGCTAAGAAACAGCCCTCAAC | TCATGCGTAACCCCACATCA | 82 | 60 | 28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Woo, H.J.; Yang, Z.; Zhao, T.; Yang, J.Y.; Kim, S.-H. Saucerneol D Suppresses the Growth of Helicobacter pylori and Their Virulence Factors. Curr. Issues Mol. Biol. 2025, 47, 828. https://doi.org/10.3390/cimb47100828
Kim SM, Woo HJ, Yang Z, Zhao T, Yang JY, Kim S-H. Saucerneol D Suppresses the Growth of Helicobacter pylori and Their Virulence Factors. Current Issues in Molecular Biology. 2025; 47(10):828. https://doi.org/10.3390/cimb47100828
Chicago/Turabian StyleKim, Su Man, Hyun Jun Woo, Zhongduo Yang, Tiankun Zhao, Ji Yeong Yang, and Sa-Hyun Kim. 2025. "Saucerneol D Suppresses the Growth of Helicobacter pylori and Their Virulence Factors" Current Issues in Molecular Biology 47, no. 10: 828. https://doi.org/10.3390/cimb47100828
APA StyleKim, S. M., Woo, H. J., Yang, Z., Zhao, T., Yang, J. Y., & Kim, S.-H. (2025). Saucerneol D Suppresses the Growth of Helicobacter pylori and Their Virulence Factors. Current Issues in Molecular Biology, 47(10), 828. https://doi.org/10.3390/cimb47100828







