Beyond the Cup: Coffee Extracts as Modulators of Periodontal Inflammation and Bone Remodeling
Abstract
1. Introduction
2. Materials and Methods
Search Strategy
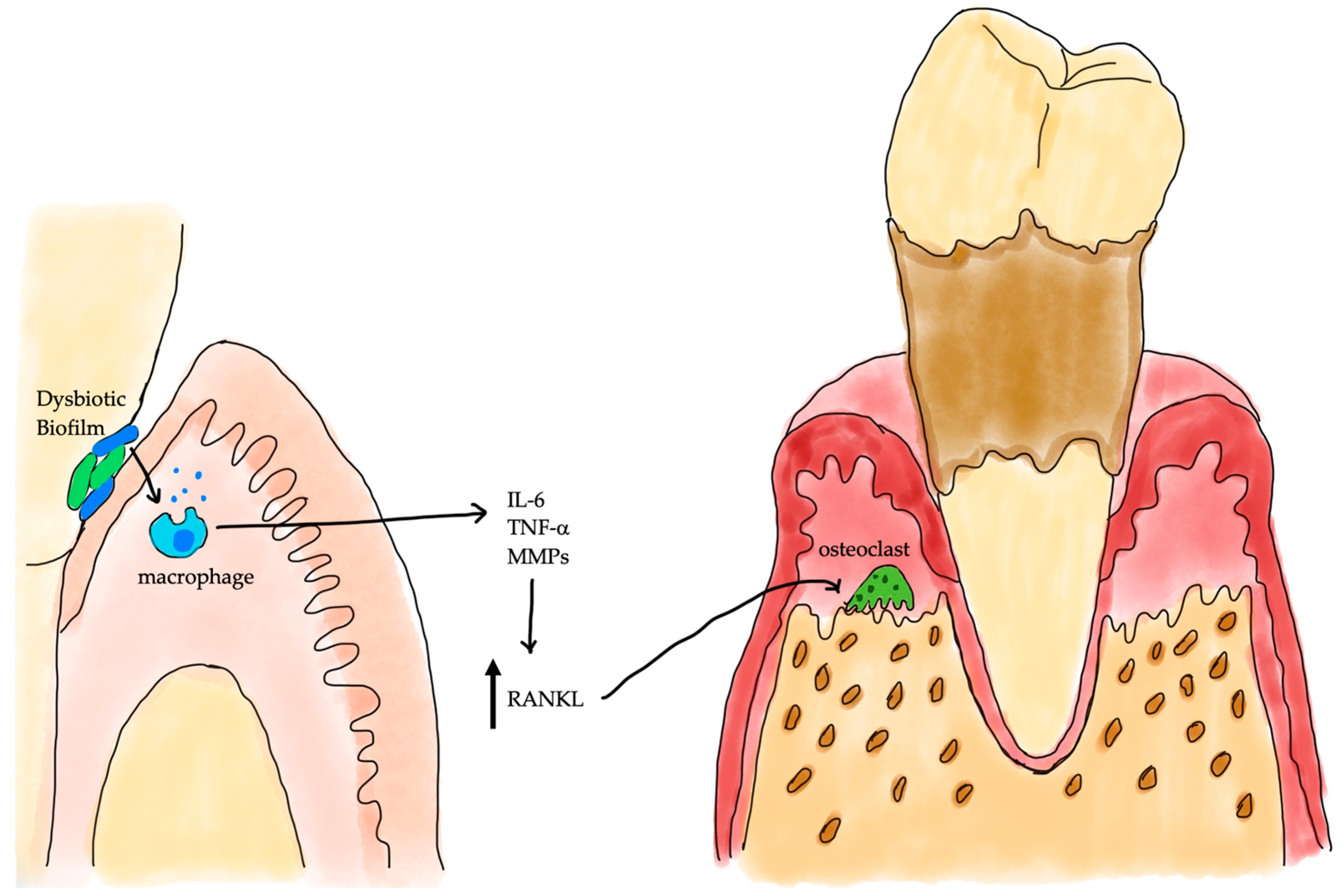
3. Periodontal Inflammation and Alveolar Bone Loss
4. Bioactive Compounds in Coffee Extracts
| Bioactive Compound | Primary Effects on Periodontal Health | Proposed Mechanism of Action | Key Takeaway for Periodontal Therapy |
|---|---|---|---|
| Caffeine [10,32,34,35] | Biphasic effects. High doses may exacerbate bone loss and impair healing. Low doses may have mild anti-inflammatory effects. | Adenosine receptor antagonism; suppression of cytokines like TNF-α. | A primary source of risk. Its removal or strict dose control in therapeutic formulations is critical. |
| Chlorogenic Acids (CGA) * [11,36,37] | Potent anti-inflammatory, antioxidant, and antimicrobial effects. Promotes osteoblast function and suppresses osteoclast activity. | Inhibits NF-κB and MAPK signaling; activates Nrf2 antioxidant pathway; reduces P. gingivalis protease activity. | mitigates periodontitis by reducing P. gingivalis virulence, suppressing oxidative stress and inflammatory signaling (NF-κB, MAPK), and restoring bone balance through enhanced osteoblast activity and inhibition of osteoclastogenesis |
| Diterpenes (Cafestol & Kahweol) [38] | Anti-inflammatory and antioxidant properties. | Modulate detoxification enzymes; enhance the Nrf2 antioxidant response. | Beneficial, but levels are heavily dependent on coffee species (higher in Arabica) and preparation (removed by paper filters). |
| Melanoidins [39,40] | Strong antioxidant and antimicrobial activity. | Formed during roasting via the Maillard reaction. Exhibit metal chelation and free radical scavenging properties. | Contribute to the overall antioxidant capacity of roasted coffee, but their complex structure makes specific roles hard to study. |
5. Variability in Composition: Source, Processing and Composition
6. Properties of Coffee
6.1. Anti-Inflammatory Properties
6.2. Antioxidant Effects
7. Identifying Optimal Coffee Components for Bone Health
7.1. Analysis of Which Coffee Components Most Effectively Support Periodontal Bone Health
| Coffee Type | Caffeine Content | Chlorogenic Acids | Polyphenols | Diterpenes | Antioxidant/Bone Implication |
|---|---|---|---|---|---|
| Espresso | High per mL | High | Moderate to High | High (unfiltered) | Potent antioxidants may support bone health if intake is moderate [46] |
| Drip Coffee | Moderate | Moderate to High | High | Low (filtered) | Suppresses osteoclastogenesis; favorable for bone [47] |
| Instant Coffee | High (varies by brand) | Moderate (preserved by freeze-drying) | Moderate | Low | Offers antioxidant support; lower diterpenes; caffeine impact depends on dose [48] |
7.2. Impact of Roasting Levels and Brewing Methods on Coffee’s Bioactive Components
| Study Type | Reference | Model & Sample Size | Primary Outcomes & Findings |
|---|---|---|---|
| In vitro | Tsou, 2019 [11] | P. gingivalis cultures | Coffee extract & chlorogenic acid inhibited bacterial growth and gingipain activity |
| Sari, 2023 [54] | Robusta coffee extract vs. periopathogens | Antibacterial activity linked to caffeine, trigonelline, flavonoids, chlorogenic acid | |
| Song, 2022 [10] | Human oral keratinocytes (LPS-stimulated) | Coffee extract reduced pro-inflammatory cytokines (IL-6, IL-8) | |
| Lonati, 2022 [55] | Cell stress models (endothelial cells) | Coffee phenolics activated Nrf2/HO-1 antioxidant pathway and reduced ROS | |
| Yi, 2016 [56] | Human PDL cells under compression | Caffeine enhanced RANKL-mediated osteoclastogenesis |
| Study Type | Reference | Model & Sample Size | Primary Outcomes & Findings |
|---|---|---|---|
| In vivo | Macedo, 2015 [57] | Rat extraction socket bone healing | Coffee & caffeine delayed alveolar bone repair; caffeine stronger effect |
| Bezerra, 2008 [58] | Ligature periodontitis in rats | High caffeine intake worsened alveolar bone loss | |
| Yi, 2016 [56] | Rat orthodontic tooth movement | Caffeine accelerated tooth movement via increased osteoclast activity | |
| Moreno, 2024 [59] | Rat orthodontic model | Caffeine induced alveolar bone loss via RANK/RANKL/OPG signaling during tooth movement | |
| Sakamoto, 2001 [60] | Rat diet/bone metabolism | Coffee intake did not induce bone loss under that experimental regime | |
| Folwarczna, 2017 [61] | Rat skeletal bone model | Moderate caffeine dosing had mixed/largely neutral effects on bone parameters | |
| Kobayashi, 2020 [12] | Aged rats, periodontal tissues | Coffee reduced oxidative stress and alveolar bone loss |
8. Clinical Evidence and Human Studies in Periodontitis
| Study/Analysis | Design & Population | Findings |
|---|---|---|
| Hamburg City Health Study (n = 6209) [13] | Cross-sectional | High intake (≥7 cups/day) causes increased periodontitis prevalence; moderate intake shows no association |
| VA Dental Longitudinal Study [62] | Older men, prospective | Higher coffee consumption decreases alveolar bone loss over time |
| NHANES 2024 (biomarker-based) [63] | Urinary metabolites (1-methyluric acid, 1,7-dimethylxanthine) | Positive correlation with periodontitis severity |
| Mendelian randomization analysis [34] | Genetic epidemiology | Weak but significant causal link between genetically predicted coffee intake & increased periodontitis risk (minimal effect size) |
9. Safety and Considerations for Periodontal Applications
10. Future Directions and Research Gaps
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGA | Chlorogenic acid |
| IL-1β | Interleukin—1 beta |
| TNF-α | Tumor Necrosis Factor—alpha |
| PGE | Prostaglandin E2 |
| AMP | Adenosine Mono Phosphate |
| Nrf2/HO-1 | Nuclear factor erythroid 2—related factor 2/Heme Oxygenase-1 |
| NF-κB | Nuclear factor kappa-B |
| RANKL | Receptor Activator of Nuclear Factor κB Ligand |
| TLR2 | Toll-like Receptor 2 |
| OPG | Osteoprotegerin |
| RANK | Receptor Activator of Nuclear Factor κB |
| ROS | Reactive Oxygen Species |
| IL-6 | Interleukin-6 |
| 5-CQA | 5-Caffeoylquinic acid |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| MAPK | Mitogen-Activated Protein Kinase |
| NMDA | N-Methyl-D-Aspartate |
| DNA | Deoxyribonucleic Acid |
| NHANES | National Health and Nutrition Examination Survey |
| RCT | Randomized Controlled Trial |
| BMP-2 | Bone Morphogenetic Protein-2 |
| LPS | Lipopolysaccharide |
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontol. 2000 2001, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A. Periodontal Disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Uzun Saylan, B.C.; Yılmaz, B.; Öztürk, V.Ö.; Atmaca, H.; Emingil, G. Evaluation of annexin A1, carbonic anhydrase 1, and elongation factor 1-gamma levels in periodontal diseases. BMC Oral Health 2025, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef]
- Periodontal Disease: A Systemic Condition. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/prd.12616 (accessed on 4 June 2025).
- Hajishengallis, G. Immune evasion strategies of Porphyromonas gingivalis. J. Oral Biosci. JAOB Jpn. Assoc. Oral Biol. 2011, 53, 233–240. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Mattei, A.; Cristiano, L.; Mancini, L.; Torge, D.; Varvara, G.; Macchiarelli, G.; Marchetti, E. Morphological and Biological Evaluations of Human Periodontal Ligament Fibroblasts in Contact with Different Bovine Bone Grafts Treated with Low-Temperature Deproteinisation Protocol. Int. J. Mol. Sci. 2022, 23, 5273. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Tang, C.-H.; Ho, T.-L.; Wang, W.-L.; Yao, C.-H. Chlorogenic acid prevents ovariectomized-induced bone loss by facilitating osteoblast functions and suppressing osteoclast formation. Aging 2024, 16, 4832–4840. [Google Scholar] [CrossRef]
- Song, J.; Kim, B.; Kim, O.; Yang, Y.; Liu, D.; Fu, W.; Ma, G.; Kim, Y.; Kim, O. Effect of Coffee on Lipopolysaccharide-Induced Immortalized Human Oral Keratinocytes. Foods 2022, 11, 2199. [Google Scholar] [CrossRef]
- Tsou, S.-H.; Hu, S.-W.; Yang, J.-J.; Yan, M.; Lin, Y.-Y. Potential Oral Health Care Agent from Coffee against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef]
- Kobayashi, T.; Maruyama, T.; Yoneda, T.; Miyai, H.; Azuma, T.; Tomofuji, T.; Ekuni, D.; Morita, M. Effects of Coffee Intake on Oxidative Stress During Aging-related Alterations in Periodontal Tissue. In Vivo 2020, 34, 615–622. [Google Scholar] [CrossRef]
- Struppek, J.; Walther, C.; Kaymaz, K.; Zyriax, B.-C.; Wenzel, J.-P.; Senftinger, J.; Nikorowitsch, J.; Heydecke, G.; Seedorf, U.; Beikler, T.; et al. The association between coffee consumption and periodontitis: A cross-sectional study of a northern German population. Clin. Oral Investig. 2022, 26, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Hwang, E.; Park, J.-B. Association between Consumption of Coffee and the Prevalence of Periodontitis: The 2008–2010 Korea National Health and Nutrition Examination Survey. PLoS ONE 2016, 11, e0158845. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Choi, Y.; Park, J.; Park, H.R.; Kim, K.; Kim, Y.H. Association between coffee consumption and periodontal diseases: A systematic review and meta-analysis. BMC Oral Health 2022, 22, 272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Parfitt, A.M. Targeted and nontargeted bone remodeling: Relationship to basic multicellular unit origination and progression. Bone 2002, 30, 5–7. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, L.; Chen, Y.; Han, X.; Sun, L.; Chen, H.; Chen, Q. Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front. Endocrinol. 2023, 14, 1109296. [Google Scholar] [CrossRef]
- Tamura, H.; Maekawa, T.; Domon, H.; Sirisereephap, K.; Isono, T.; Hirayama, S.; Hiyoshi, T.; Sasagawa, K.; Takizawa, F.; Maeda, T.; et al. Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas gingivalis Lipopolysaccharide. Pharmaceuticals 2023, 16, 303. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Yasuhara, R.; Miyamoto, Y.; Takami, M.; Imamura, T.; Potempa, J.; Yoshimura, K.; Kamijo, R. Lysine-specific gingipain promotes lipopolysaccharide- and active-vitamin D3-induced osteoclast differentiation by degrading osteoprotegerin. Biochem. J. 2009, 419, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Treatment of Aggressive Periodontitis—Teughels—2014—Periodontology 2000—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/prd.12020 (accessed on 10 August 2025).
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Caffeine (1, 3, 7-trimethylxanthine) in Foods: A Comprehensive Review on Consumption, Functionality, Safety, and Regulatory Matters—Heckman—2010—Journal of Food Science—Wiley Online Library. Available online: https://ift.onlinelibrary.wiley.com/doi/full/10.1111/j.1750-3841.2010.01561.x (accessed on 14 July 2025).
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int. J. Food Sci. Nutr. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Raeis-Abdollahi, E.; Raise-Abdullahi, P.; Rashidy-Pour, A.; Meamar, M.; Askari, H. Chapter Eight—Coffee’s protective mechanisms against neurodegeneration. In Progress in Brain Research; Moradikor, N., Chatterjee, I., Eds.; Neuroscience of Coffee Part A.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 288, pp. 167–200. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Bramantoro, T.; Krismariono, A.; Amir, M.S.; Nugraha, A.P.; Irmalia, W.R.; Zulfiana, A.A. Chapter 38—Coffee and periodontal health: To protect or to harm. In Coffee in Health and Disease Preventio, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 423–430. ISBN 978-0-443-13868-3. [Google Scholar]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef]
- Liao, W.-Z.; Zhou, Z.-Y.; Lin, Z.-K.; Xie, S.-J.; Zheng, Y.-F.; Wang, J.-T.; Zheng, J.-H.; Chen, H.-K.; Chen, W.-S.; Guo, X.-G. Coffee consumption and periodontitis: A Mendelian Randomization study. Genes Nutr. 2023, 18, 13. [Google Scholar] [CrossRef]
- Bramantoro, T.; Zulfiana, A.A.; Amir, M.S.; Irmalia, W.R.; Mohd Nor, N.A.; Nugraha, A.P.; Krismariono, A. The contradictory effects of coffee intake on periodontal health: A systematic review of experimental and observational studies. F1000Research 2022, 11, 924. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Park, C.M.; Yoon, H.-S. Chlorogenic Acid as a Positive Regulator in LPS-PG-Induced Inflammation via TLR4/MyD88-Mediated NF-κB and PI3K/MAPK Signaling Cascades in Human Gingival Fibroblasts. Mediators Inflamm. 2022, 2022, 2127642. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Rufián Henares, J.A.; Morales, F.J. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Wu, X.; Wei, Y.; Li, X.; Ji, J. Antibacterial properties of Maillard reaction products: Molecular mechanisms and influencing factors (Review). Biomed. Rep. 2025, 23, 141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Jiang, L. Coffee and tea consumption on the risk of osteoporosis: A meta-analysis. Front. Nutr. 2025, 12, 1559835. [Google Scholar] [CrossRef]
- Berman, N.K.; Honig, S.; Cronstein, B.N.; Pillinger, M.H. The effects of caffeine on bone mineral density and fracture risk. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2022, 33, 1235–1241. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, S.; Zhang, J.; Wei, X.; Wang, Z.; Han, B. Osteogenic mechanism of chlorogenic acid and its application in clinical practice. Front. Pharmacol. 2024, 15, 1396354. [Google Scholar] [CrossRef]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef]
- Doss, H.M.; Samarpita, S.; Ganesan, R.; Rasool, M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κB signalling pathway. Life Sci. 2018, 207, 284–295. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, S.; Ma, W.; Wang, Q.; Li, Y.; Xia, C.; Xu, Y.; Zhang, T.; Yang, L.; Zhou, M. The Impact of Instant Coffee and Decaffeinated Coffee on the Gut Microbiota and Depression-Like Behaviors of Sleep-Deprived Rats. Front. Microbiol. 2022, 13, 778512. [Google Scholar] [CrossRef] [PubMed]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Nosal, B.M.; Sakaki, J.R.; Kim, D.-O.; Chun, O.K. Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body’s antioxidant status. Food Sci. Biotechnol. 2022, 31, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Alongi, M.; Cervantes-Flores, M.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Valenzuela-Ramírez, A.A.; Rojas-Contreras, J.A.; Guerrero-Romero, F.; Gamboa-Gómez, C.I. Influence of coffee roasting degree on inflammatory and oxidative stress markers in high-fructose and saturated fat-fed rats. Food Res. Int. Ott. Ont 2023, 165, 112530. [Google Scholar] [CrossRef]
- Nishida, Y.; Shimada, K.; Horibe, K.; Seki, K.; Murai, Y.; Sogawa, C.; Murakami, S.; Nakamura, H.; Masuda, Y.; Sogawa, N. Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice. Appl. Sci. 2023, 13, 4129. [Google Scholar] [CrossRef]
- Mestanza, M.; Mori-Culqui, P.L.; Chavez, S.G. Changes of polyphenols and antioxidants of arabica coffee varieties during roasting. Front. Nutr. 2023, 10, 1078701. [Google Scholar] [CrossRef]
- Sari, D.S.; Pujiastuti, P.; Fatmawati, D.W.A.; Mardiyana, M.A.; Wulandari, A.T.; Arina, Y.M.D. Inhibiting the growth of periopathogenic bacteria and accelerating bone repair processes by using robusta coffee bean extract. Saudi Dent. J. 2023, 35, 322–329. [Google Scholar] [CrossRef]
- Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; Del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules 2022, 27, 1049. [Google Scholar] [CrossRef]
- Yi, J.; Yan, B.; Li, M.; Wang, Y.; Zheng, W.; Li, Y.; Zhao, Z. Caffeine may enhance orthodontic tooth movement through increasing osteoclastogenesis induced by periodontal ligament cells under compression. Arch. Oral Biol. 2016, 64, 51–60. [Google Scholar] [CrossRef]
- Macedo, R.M.; Brentegani, L.G.; Lacerda, S.A. de Effects of Coffee Intake and Intraperitoneal Caffeine on Bone Repair Process—A Histologic and Histometric Study. Braz. Dent. J. 2015, 26, 175–180. [Google Scholar] [CrossRef]
- Bezerra, J.P.; da Silva, L.R.F.; de Alvarenga Lemos, V.A.; Duarte, P.M.; Bastos, M.F. Administration of High Doses of Caffeine Increases Alveolar Bone Loss in Ligature-Induced Periodontitis in Rats. J. Periodontol. 2008, 79, 2356–2360. [Google Scholar] [CrossRef]
- Moreno, M.C.; Cavalcante, G.R.G.; Pirih, F.Q.; de Paula Soares, V.; Klein, K.P.; da Silveira, É.J.D.; da Silva, J.S.P.; Lins, R.D.A.U.; de Araujo, A.A.; de Sousa Lopes, M.L.D.; et al. Caffeine induces alveolar bone loss in rats submitted to orthodontic movement via activation of receptor activator of nuclear factor κB, receptor activator of nuclear factor κB ligand, and osteoprotegerin pathway. Am. J. Orthod. Dentofac. Orthop. 2024, 166, 148–159. [Google Scholar] [CrossRef]
- Sakamoto, W.; Nishihira, J.; Fujie, K.; Iizuka, T.; Handa, H.; Ozaki, M.; Yukawa, S. Effect of coffee consumption on bone metabolism. Bone 2001, 28, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Janas, A.; Cegieła, U.; Pytlik, M.; Śliwiński, L.; Matejczyk, M.; Nowacka, A.; Rudy, K.; Krivošíková, Z.; Štefíková, K.; et al. Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes. Nutrients 2017, 9, 1196. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Kaye, E.K.; Garcia, R.I. Coffee Consumption and Periodontal Disease in Males. J. Periodontol. 2014, 85, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ge, R.; Wu, Y.; Wu, Y.; Yang, H.; Yu, Y.; Deng, Q.; Qiu, Y.; He, B.; Yan, F.; et al. The associations of coffee consumption, coffee types, and caffeine metabolites with periodontitis: Results from NHANES 2009-2014. J. Periodontol. 2024, 95, 778–788. [Google Scholar] [CrossRef]
- Song, I.-S.; Han, K.; Ryu, J.-J.; Choi, Y.-J.; Park, J.-B. Coffee Intake as a Risk Indicator for Tooth Loss in Korean Adults. Sci. Rep. 2018, 8, 2392. [Google Scholar] [CrossRef]
- Antonio, J.; Newmire, D.E.; Stout, J.R.; Antonio, B.; Gibbons, M.; Lowery, L.M.; Harper, J.; Willoughby, D.; Evans, C.; Anderson, D.; et al. Common questions and misconceptions about caffeine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2024, 21, 2323919. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mody, J.; Aleisa, D.; Modh, H.; Sainani, P.; Dibart, S.; Ma, W. Beyond the Cup: Coffee Extracts as Modulators of Periodontal Inflammation and Bone Remodeling. Curr. Issues Mol. Biol. 2025, 47, 827. https://doi.org/10.3390/cimb47100827
Mody J, Aleisa D, Modh H, Sainani P, Dibart S, Ma W. Beyond the Cup: Coffee Extracts as Modulators of Periodontal Inflammation and Bone Remodeling. Current Issues in Molecular Biology. 2025; 47(10):827. https://doi.org/10.3390/cimb47100827
Chicago/Turabian StyleMody, Janvi, Deamah Aleisa, Harshal Modh, Purnima Sainani, Serge Dibart, and Weiyuan Ma. 2025. "Beyond the Cup: Coffee Extracts as Modulators of Periodontal Inflammation and Bone Remodeling" Current Issues in Molecular Biology 47, no. 10: 827. https://doi.org/10.3390/cimb47100827
APA StyleMody, J., Aleisa, D., Modh, H., Sainani, P., Dibart, S., & Ma, W. (2025). Beyond the Cup: Coffee Extracts as Modulators of Periodontal Inflammation and Bone Remodeling. Current Issues in Molecular Biology, 47(10), 827. https://doi.org/10.3390/cimb47100827






