Epicatechin Protects Against Post-Cardiac Arrest Brain Injury in Aged Rats via NRG1-Mediated Suppression of Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
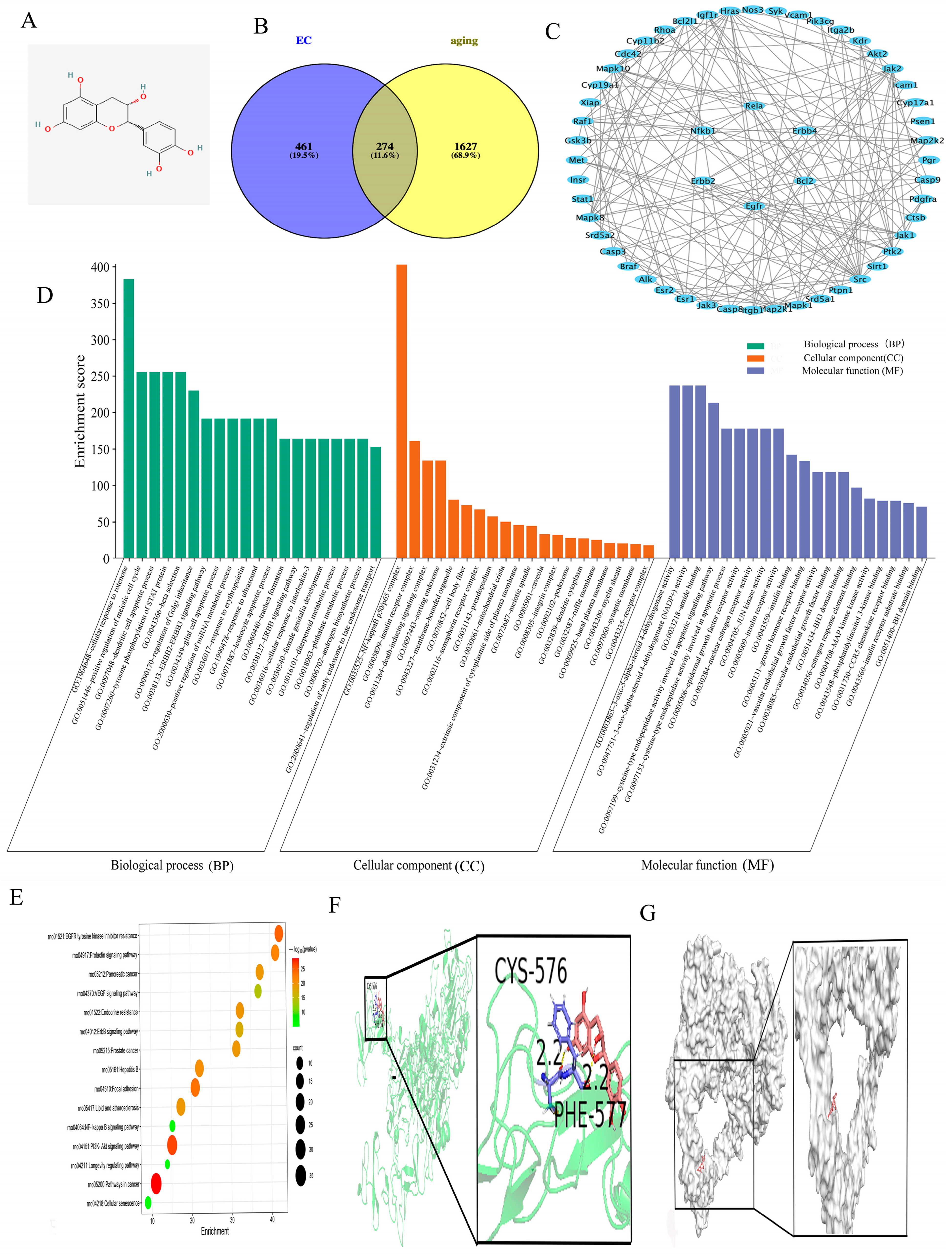
2.1. Experimental Methods of Network Pharmacology
2.2. Grouping and Administration of EC in Naturally Aged Rats
2.3. Grouping of CA/CPR in Aged Rats
2.4. CA/CPR Model
2.5. Survival Observation and Neurological Evaluation
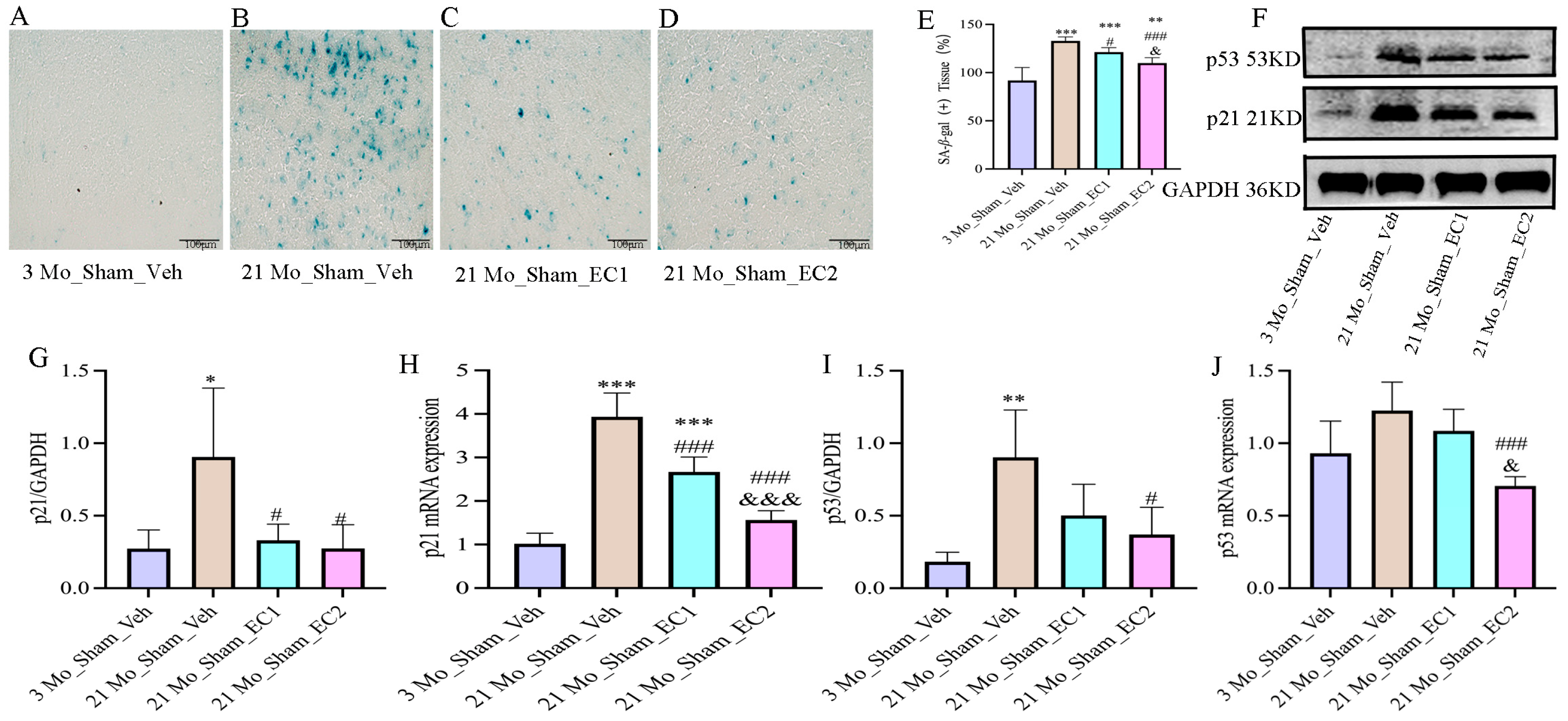
2.6. β-Galactosidase Staining
2.7. Hematoxylin and Eosin Staining
2.8. Transmission Electron Microscopy (TEM)
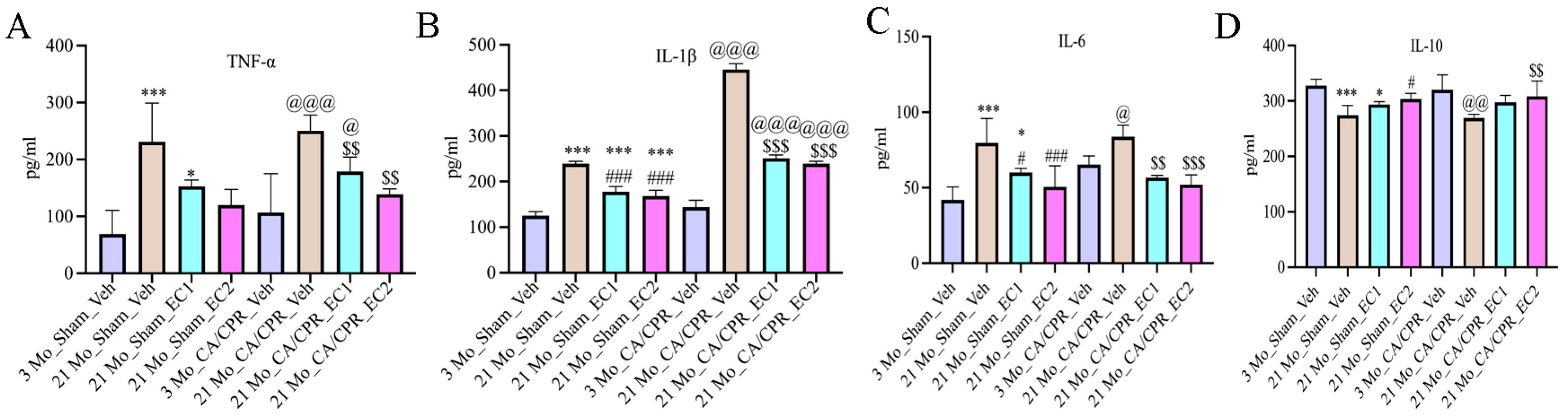
2.9. Serum Levels of TNF-α, IL-1β, IL-6 and IL-10 Were Quantified by Adopting Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. RNA Extraction and RT-qPCR
2.11. Western Blot Analysis
2.12. Statistical Analyses
3. Results
3.1. Network Pharmacology Provides a Theoretical Basis for Understanding the Interaction Between ECs and Aging-Related Signaling Pathways
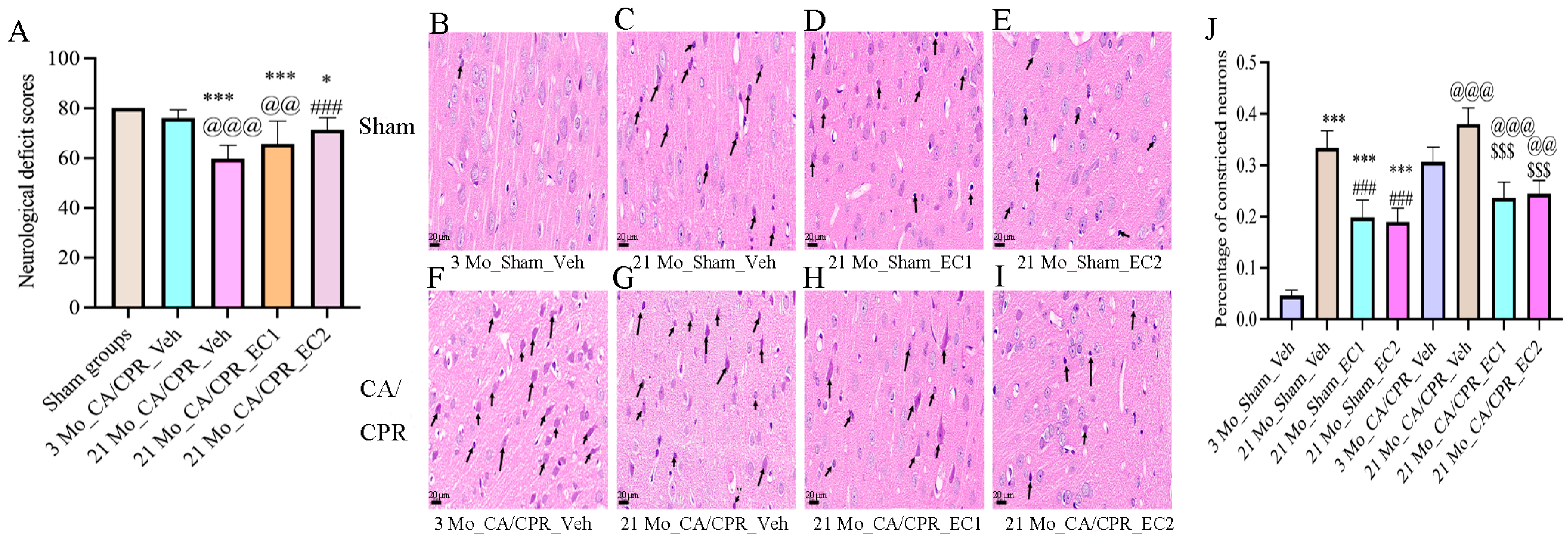
3.2. EC Increased Neurological Deficit Scores and Survival Rates After CA/CPR
3.3. EC Improved the Structure of Neurons After CA/CPR
3.4. EC Down-Regulated Aging Indicators
3.5. EC Attenuated Inflammatory Aging
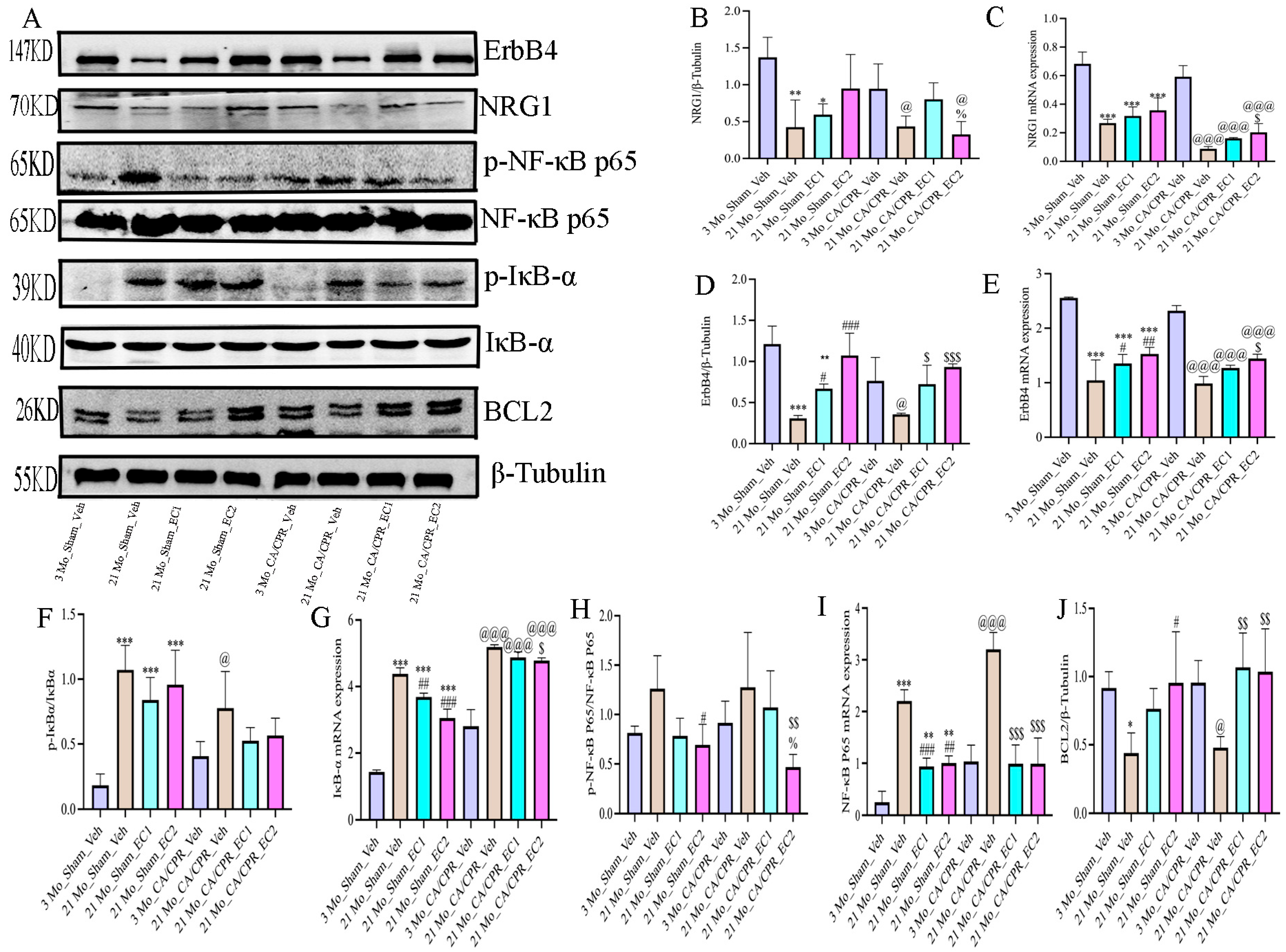
3.6. EC Modulates the NRG1-NF-κB Pathway in the Context of Aging-Related Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Uyar, B.; Palmer, D.; Kowald, A.; Escobar, H.M.; Barrantes, I.; Möller, S.; Akalin, A.; Fuellen, G. Single-cell analyses of aging, inflammation and senescence. Ageing Res. Rev. 2020, 64, 101156. [Google Scholar] [CrossRef]
- Menardo, J.; Tang, Y.; Ladrech, S.; Lenoir, M.; Casas, F.; Michel, C.; Bourien, J.; Ruel, J.; Rebillard, G.; Maurice, T.; et al. Oxidative Stress, Inflammation, and Autophagic Stress as the Key Mechanisms of Premature Age-Related Hearing Loss in SAMP8 Mouse Cochlea. Antioxid. Redox Signal. 2012, 16, 263–274. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated neuroinflammation and sickness behavior in aged mice after activation of the peripheral innate immune system. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Zhang, S.; Li, C.; Zhang, L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 2020, 87, 1–10. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Field, J.M.; Hazinski, M.F.; Sayre, M.R.; Chameides, L.; Schexnayder, S.M.; Hemphill, R.; Samson, R.A.; Kattwinkel, J.; Berg, R.A.; Bhanji, F.; et al. Part 1: Executive Summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S640–S656. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Wen, S.; Huang, K.; Huang, J.; Chu, X.; Wang, F.; Pang, L. Novel Controlled and Targeted Releasing Hydrogen Sulfide System Exerts Combinational Cerebral and Myocardial Protection after Cardiac Arrest. J. Nanobiotechnol. 2021, 19, 40. [Google Scholar] [CrossRef]
- Roine, R.O.; Kajaste, S.; Kaste, M. Neuropsychological Sequelae of Cardiac Arrest. JAMA 1993, 269, 237–242. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Jiang, X.; Xu, J.; Zhang, M.; Liu, F.; Lin, Y.; Tao, J.; He, J.; Zhou, X.; et al. A Nanotherapy of Octanoic Acid Ameliorates Cardiac Arrest/Cardiopulmonary Resuscitation-Induced Brain Injury via RVG29-and Neutrophil Membrane-Mediated Injury Relay Targeting. ACS Nano 2023, 17, 3528–3548. [Google Scholar] [CrossRef]
- Hayashida, K.; Ichinose, F. Response by Hayashida and Ichinose to Letter Regarding Article, ‘Improvement in Outcomes After Cardiac Arrest and Resuscitation by Inhibition of S-Nitrosoglutathione Reductase’. Circulation 2019, 140, E192–E193. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xie, L.; Wang, W.; Zhao, G.; Tian, X.; Chen, M. FK866 Alleviates Cerebral Pyroptosis and Inflammation Mediated by Drp1 in a Rat Cardiopulmonary Resuscitation Model. Int. Immunopharmacol. 2020, 89, 107032. [Google Scholar] [CrossRef]
- Wang, W.Y.; Xie, L.; Zou, X.S.; Li, N.; Yang, Y.G.; Wu, Z.J.; Tian, X.Y.; Zhao, G.Y.; Chen, M.H. Inhibition of Extracellular Signal-Regulated Kinase/Calpain-2 Pathway Reduces Neuroinflammation and Necroptosis after Cerebral Ischemia-Reperfusion Injury in a Rat Model of Cardiac Arrest. Int. Immunopharmacol. 2021, 93, 107377. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Carter, J.; Traystman, R.J.; Wagner, D.H.; Herson, P.S. Pro-inflammatory T-lymphocytes rapidly infiltrate into the brain and contribute to neuronal injury following cardiac arrest and cardiopulmonary resuscitation. J. Neuroimmunol. 2014, 274, 132–140. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Johansson, P.I.; Kjaergaard, J.; Wanscher, M.; Ostrowski, S.R.; Bjerre, M.; Hassager, C. Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation 2017, 121, 179–186. [Google Scholar] [CrossRef]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 2. Tea, Wine, Fruit Juices, and Chocolate Milk. J. Agric. Food Chem. 2000, 48, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin Attenuates Atherosclerosis and Exerts Anti-Inflammatory Effects on Diet-Induced Human-CRP and NFκB in Vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin Mitigates High-Fructose-Associated Insulin Resistance by Modulating Redox Signaling and Endoplasmic Reticulum Stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef]
- Shariati, S.; Kalantar, H.; Pashmforoosh, M.; Mansouri, E.; Khodayar, M.J. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed. Pharmacother. 2019, 114, 108776. [Google Scholar] [CrossRef]
- Jiang, C.; Zhuge, X.; Li, D.; Chen, M.; Hu, W.; Xie, L. Epicatechin-Mediated Modulation of the Nrf2/HO-1 Pathway Alleviates Senile Cerebral Ischemic/Reperfusion Injury. Food Sci. Nutr. 2024, 12, 6521–6533. [Google Scholar] [CrossRef]
- Chen, M.-H.; Liu, T.-W.; Xie, L.; Song, F.-Q.; He, T.; Zeng, Z.-Y.; Mo, S.-R. Ventricular Fibrillation Induced by Transoesophageal Cardiac Pacing: A New Model of Cardiac Arrest in Rats. Resuscitation 2007, 74, 546–551. [Google Scholar] [CrossRef]
- Jia, X.; Koenig, M.A.; Shin, H.-C.; Zhen, G.; Pardo, C.A.; Hanley, D.F.; Thakor, N.V.; Geocadin, R.G. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008, 76, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Geocadin, R.; Ghodadra, R.; Kimura, T.; Lei, H.; Sherman, D.; Hanley, D.; Thakor, N. A novel quantitative EEG injury measure of global cerebral ischemia. Clin. Neurophysiol. 2000, 111, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Fishbein, M.C.; Braunwald, E.; Maroko, P.R. Effect of propranolol on mitochondrial morphology during acute myocardial ischemia. Am. J. Cardiol. 1978, 41, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Julian, M.W.; Blaho, D.V.; Pfeiffer, D.R. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit. Care Med. 2002, 30, 276–284. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Zhang, Q.; Liu, F.; Wang, J.; Wei, C.; Liu, F.; Li, J. NFKB2 Inhibits NRG1 Transcription to Affect Nucleus Pulposus Cell Degeneration and Inflammation in Intervertebral Disc Degeneration. Mech. Ageing Dev. 2021, 197, 111511. [Google Scholar] [CrossRef]
- Brown, J.P.; Wei, W.; Sedivy, J.M. Bypass of Senescence After Disruption of p21CIP1/WAF1 Gene in Normal Diploid Human Fibroblasts. Science 1997, 277, 831–834. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Lee, S.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. A Senescence Program Controlled by p53 and p16INK4a Contributes to the Outcome of Cancer Therapy. Cell 2002, 109, 335–346. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Artimovič, P.; Špaková, I.; Macejková, E.; Pribulová, T.; Rabajdová, M.; Mareková, M.; Zavacká, M. The ability of microRNAs to regulate the immune response in ischemia/reperfusion inflammatory pathways. Genes. Immun. 2024, 25, 277–296. [Google Scholar] [CrossRef]
- Wang, H.; Xi, Z.; Deng, L.; Pan, Y.; He, K.; Xia, Q. Macrophage Polarization and Liver Ischemia-Reperfusion Injury. Int. J. Med. Sci. 2021, 18, 1104–1113. [Google Scholar] [CrossRef]
- Vazquez-Prieto, M.A.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2012, 527, 113–118, Correction in Arch. Biochem. Biophys. 2024, 756, 109992. [Google Scholar] [CrossRef]
- Tabata, S.; Matsuda, K.; Soeda, S.; Nagai, K.; Izumi, Y.; Takahashi, M.; Motomura, Y.; Nagasato, A.I.; Moro, K.; Bamba, T.; et al. NFκB dynamics-dependent epigenetic changes modulate inflammatory gene expression and induce cellular senescence. FEBS J. 2024, 291, 4951–4968. [Google Scholar] [CrossRef]
- Harada, M.; Su-Harada, K.; Kimura, T.; Ono, K.; Ashida, N. Sustained activation of NF-κB through constitutively active IKKβ leads to senescence bypass in murine dermal fibroblasts. Cell Cycle 2024, 23, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lu, X.; Zhang, X. Noncanonical NF-κB Signaling Pathway in Liver Diseases. J. Clin. Transl. Hepatol. 2020, 9, 81–89. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, P.; Zhao, R.; Zhang, Y.; Wang, X.; Cui, W. Neuregulin-1 Regulates the Conversion of M1/M2 Microglia Phenotype via ErbB4-Dependent Inhibition of the NF-κB Pathway. Mol. Biol. Rep. 2022, 49, 3975–3986. [Google Scholar] [CrossRef]
- Zhang, W.; Dao, J.-J.; Li, Q.; Liu, C.; Qiao, C.-M.; Cui, C.; Shen, Y.; Zhao, W. Neuregulin 1 Mitigated Prolactin Deficiency through Enhancing TRPM8 Signaling under the Influence of Melatonin in Senescent Pituitary Lactotrophs. Int. J. Biol. Macromol. 2024, 275, 133659. [Google Scholar] [CrossRef]
- Baik, T.-K.; Kim, Y.-J.; Kang, S.-M.; Song, D.-Y.; Min, S.S.; Woo, R.-S. Blocking the Phosphatidylinositol 3-Kinase Pathway Inhibits Neuregulin-1-Mediated Rescue of Neurotoxicity Induced by Aβ1–42. J. Pharm. Pharmacol. 2016, 68, 1021–1029. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M.; Fan, Y.; Zhang, J.; Liu, L.; Li, Y.; Zhang, Q.; Xie, H.; Jiang, C.; Wu, J.; et al. Intermittent Theta-Burst Stimulation Improves Motor Function by Inhibiting Neuronal Pyroptosis and Regulating Microglial Polarization via TLR4/NFκB/NLRP3 Signaling Pathway in Cerebral Ischemic Mice. J. Neuroinflamm. 2022, 19, 141. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-kappaB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Shakeri, H.; Gevaert, A.B.; Schrijvers, D.M.; De Meyer, G.R.Y.; De Keulenaer, G.W.; Guns, P.-J.D.F.; Lemmens, K.; Segers, V.F. Neuregulin-1 attenuates stress-induced vascular senescence. Cardiovasc. Res. 2018, 114, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.-C.; Lin, S.-Z.; Chiang, M.-F.; Yang, H.-I.; Thajeb, P.; Li, H. Neuregulin-1 Reduces Ischemia-Induced Brain Damage in Rats. Neurobiol. Aging 2004, 25, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Zhu, N.; Tsantoulas, C.; Ma, Z.; Grist, J.; Loeb, J.A.; Bennett, D.L.H. Neuregulin-ErbB Signaling Promotes Microglial Proliferation and Chemotaxis Contributing to Microgliosis and Pain after Peripheral Nerve Injury. J. Neurosci. 2010, 30, 5437–5450. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Wilson, N.O.; Anderson, L.; Pitts, S.; Patrickson, J.; Liu, M.; Ford, B.D.; Stiles, J.K. Neuregulin-1 Attenuates Mortality Associated with Experimental Cerebral Malaria. J. Neuroinflamm. 2014, 11, 9. [Google Scholar] [CrossRef]

| Group | Survival Rat | n (Final Survival Quantity) | CPR Duration |
|---|---|---|---|
| 3 Mo_Sham_Veh | 6/6 (100%) | 6 | - |
| 21 Mo_Sham_Veh | 6/6 (100%) | 6 | - |
| 21 Mo_Sham_EC1 | 9/9 (100%) | 9 | - |
| 21 Mo_Sham_EC2 | 10/10 (100%) | 10 | - |
| 3 Mo_CA/CPR_Veh | 6/8 (75%) | 6 | 99.83 ± 14.69 |
| 21 Mo_CA/CPR_Veh | 6/14 (42.9%) * | 6 | 96.00 ± 14.79 |
| 21 Mo_CA/CPR_EC1 | 6/11 (54.5%) | 6 | 106.5 ± 19.90 |
| 21 Mo_CA/CPR_EC2 | 6/10 (60%) | 6 | 106.83 ± 14.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-H.; Huang, F.; Du, Z.-L.; Xie, L. Epicatechin Protects Against Post-Cardiac Arrest Brain Injury in Aged Rats via NRG1-Mediated Suppression of Neuroinflammation. Curr. Issues Mol. Biol. 2025, 47, 793. https://doi.org/10.3390/cimb47100793
Wang H-H, Huang F, Du Z-L, Xie L. Epicatechin Protects Against Post-Cardiac Arrest Brain Injury in Aged Rats via NRG1-Mediated Suppression of Neuroinflammation. Current Issues in Molecular Biology. 2025; 47(10):793. https://doi.org/10.3390/cimb47100793
Chicago/Turabian StyleWang, Hui-Hui, Fan Huang, Zi-Long Du, and Lu Xie. 2025. "Epicatechin Protects Against Post-Cardiac Arrest Brain Injury in Aged Rats via NRG1-Mediated Suppression of Neuroinflammation" Current Issues in Molecular Biology 47, no. 10: 793. https://doi.org/10.3390/cimb47100793
APA StyleWang, H.-H., Huang, F., Du, Z.-L., & Xie, L. (2025). Epicatechin Protects Against Post-Cardiac Arrest Brain Injury in Aged Rats via NRG1-Mediated Suppression of Neuroinflammation. Current Issues in Molecular Biology, 47(10), 793. https://doi.org/10.3390/cimb47100793





