The Combination of Oolonghomobisflavan B and Diallyl Disulfide Induces Apoptotic Cell Death via 67-kDa Laminin Receptor/Cyclic Guanosine Monophosphate in Acute Myeloid Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Quantitative Reverse Transcription PCR (qRT-PCR)
2.5. cGMP Assays
2.6. Acid Sphingomyelinase (ASM) Activity Measurement
2.7. Statistical Analysis
3. Results
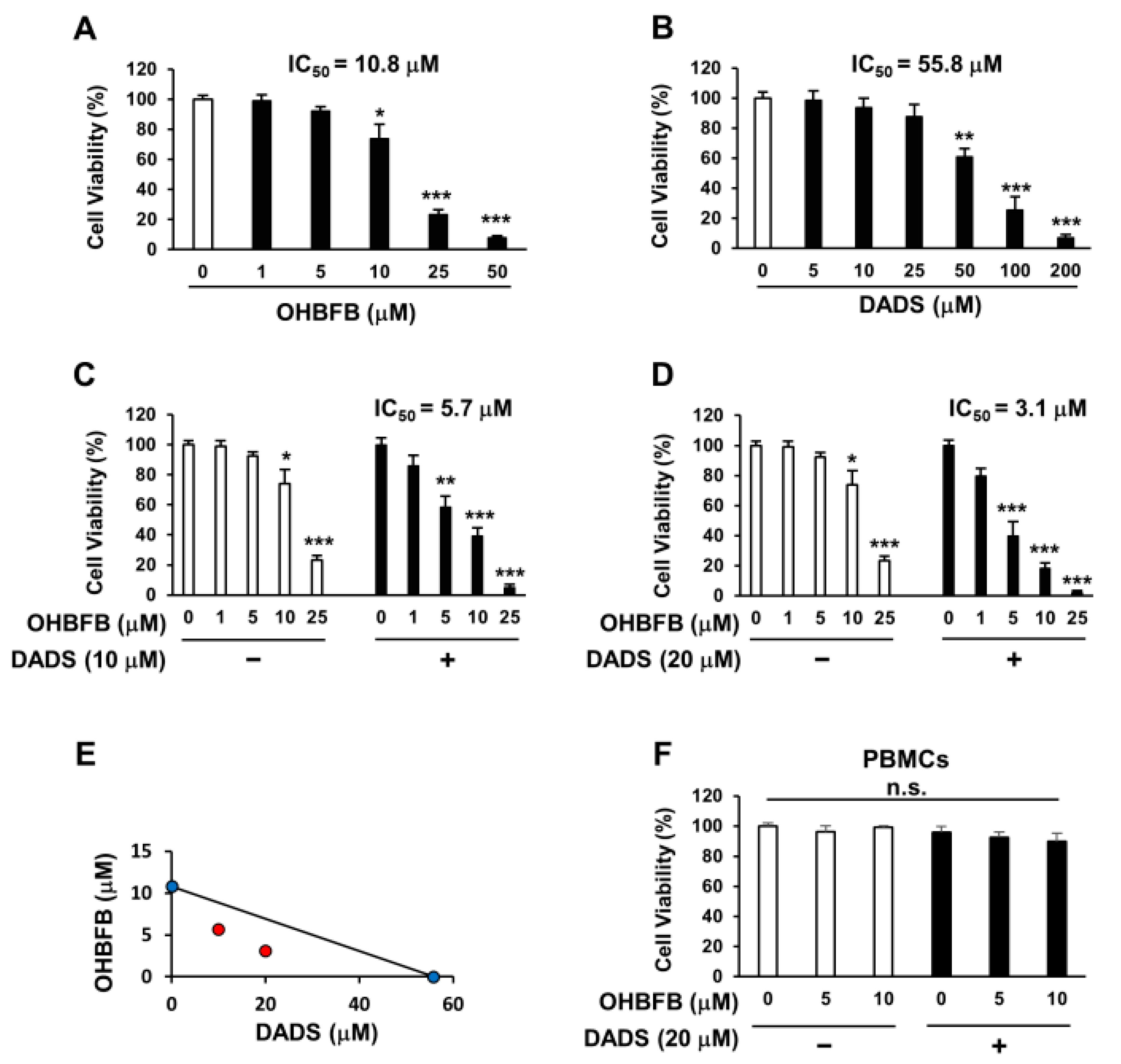
3.1. Synergistic Induction of Cell Death in HL60 Cells by the Combination of OHBFB and DADS
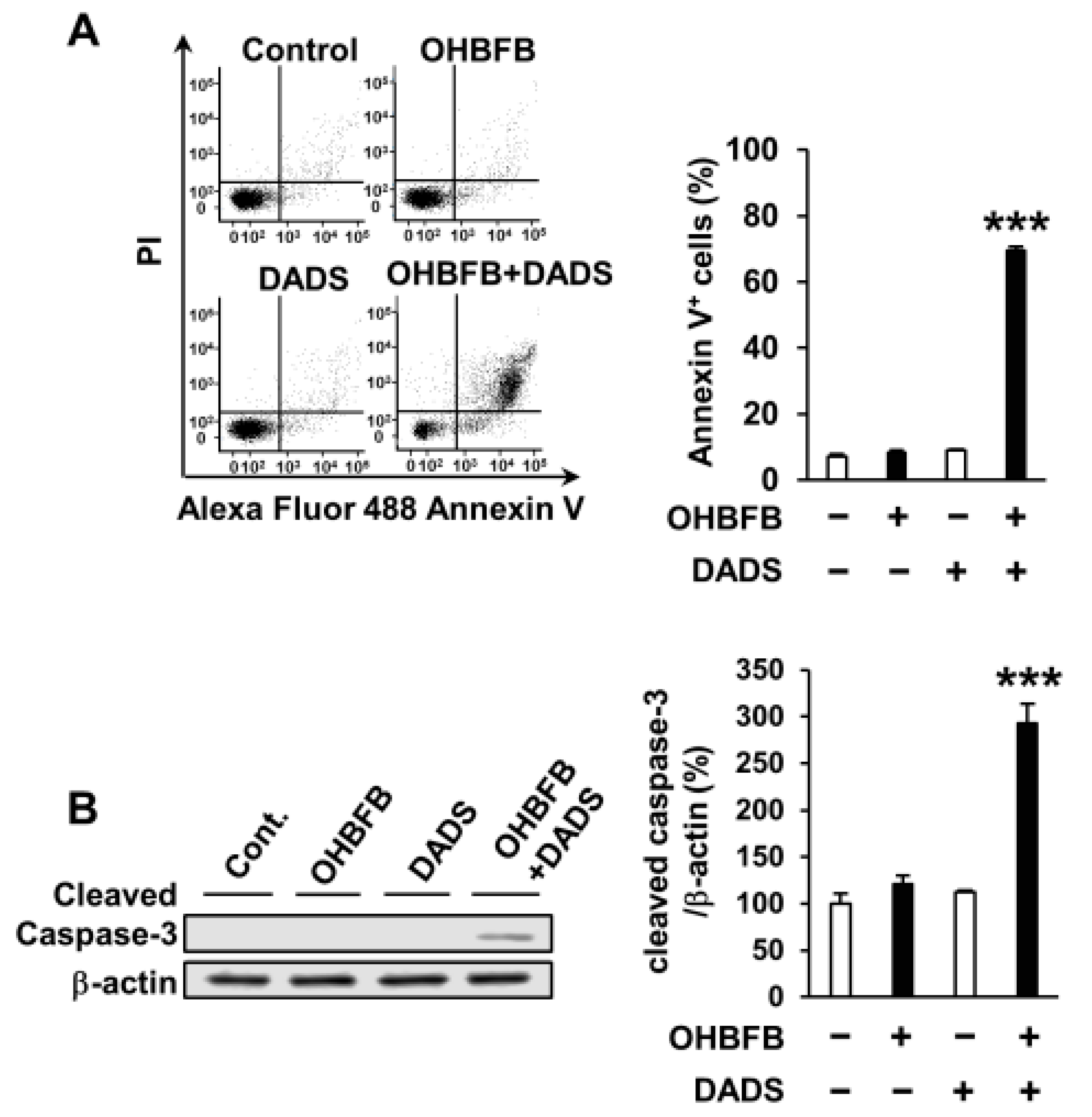
3.2. Combination of OHBFB and DADS Induces Apoptotic Cell Death
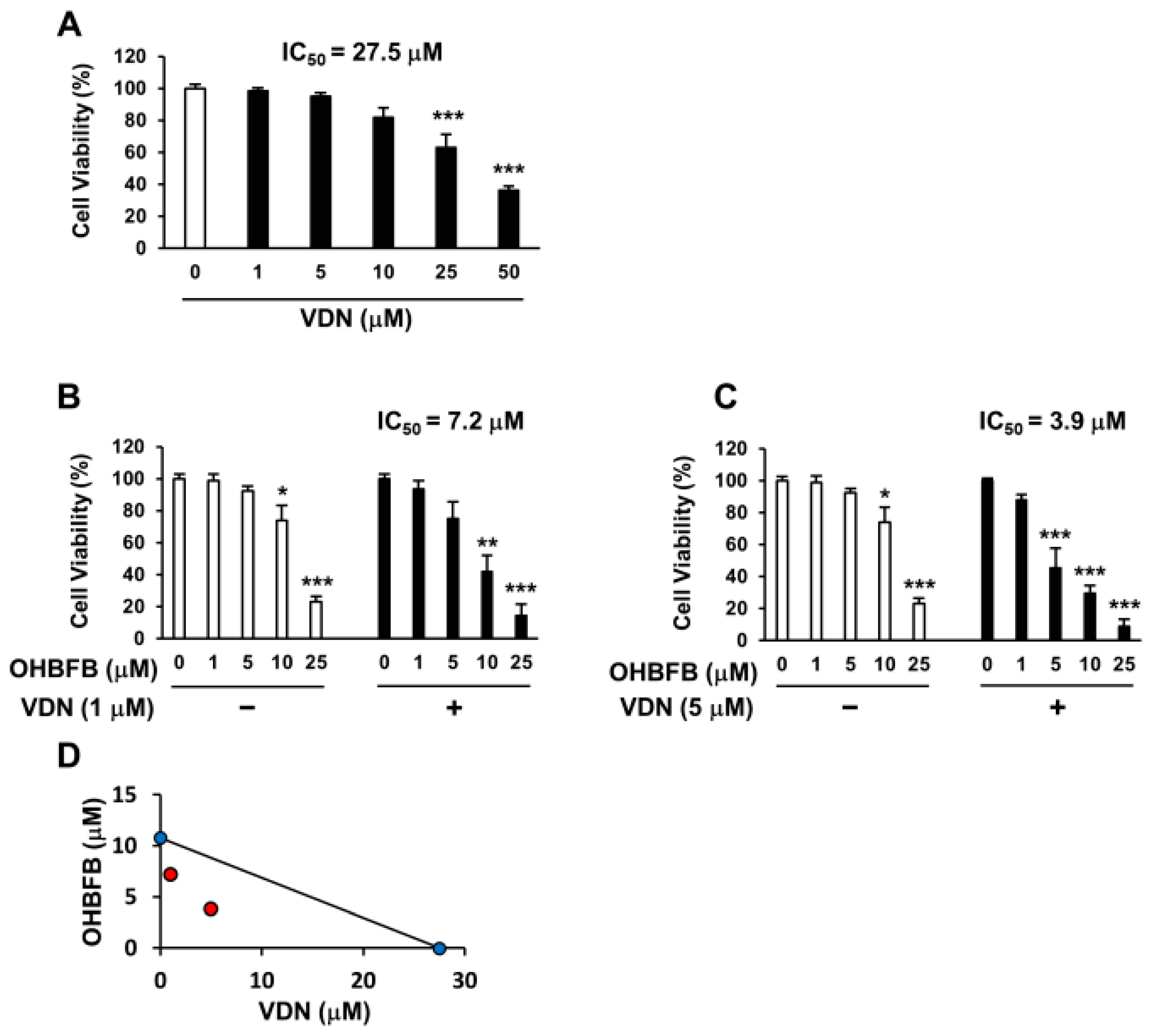
3.3. Vardenafil Synergistically Potentiates Cell Death Induced by OHBFB
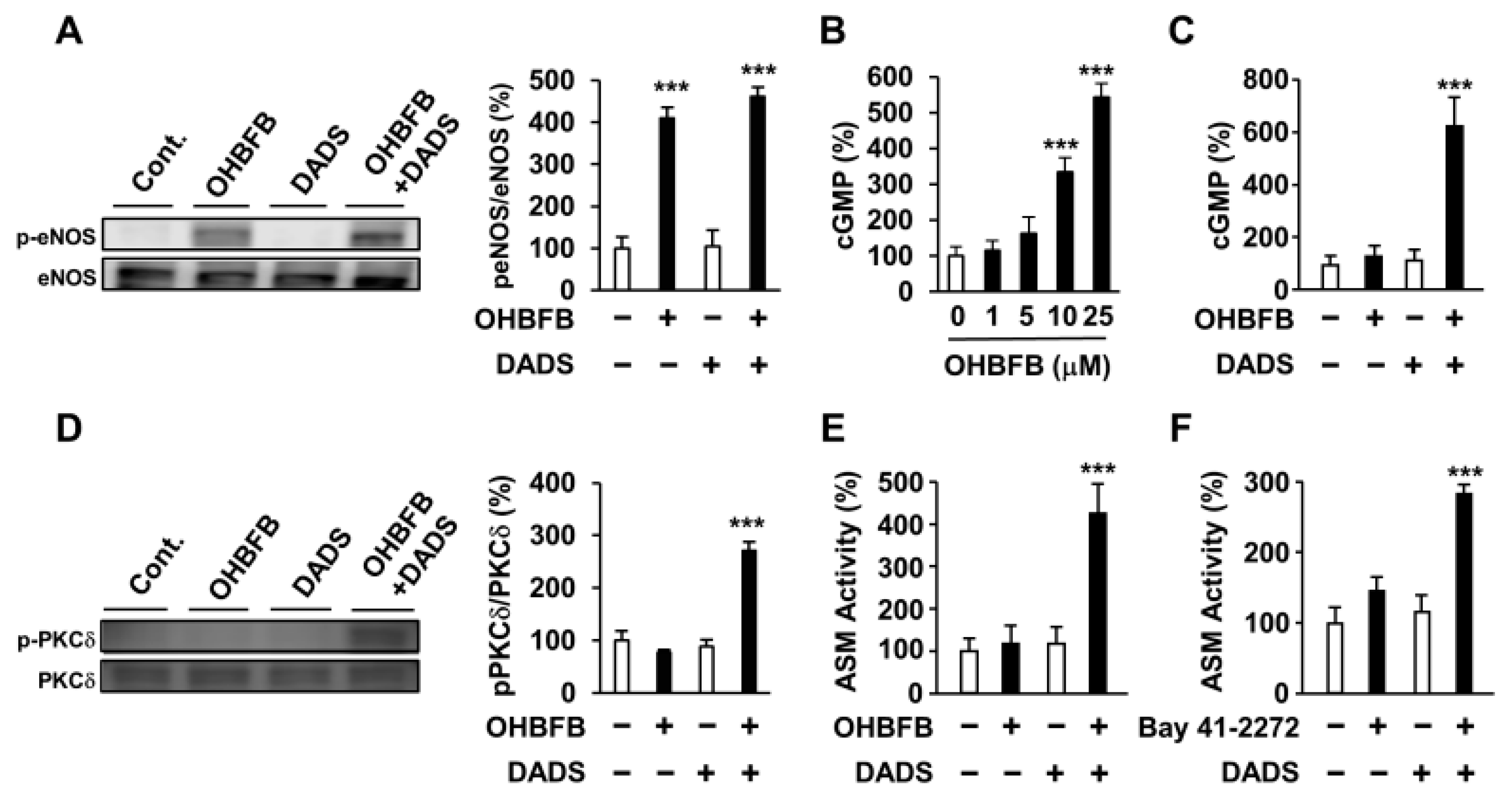
3.4. DADS Potentiates OHBFB-Induced Activation of cGMP Pathway
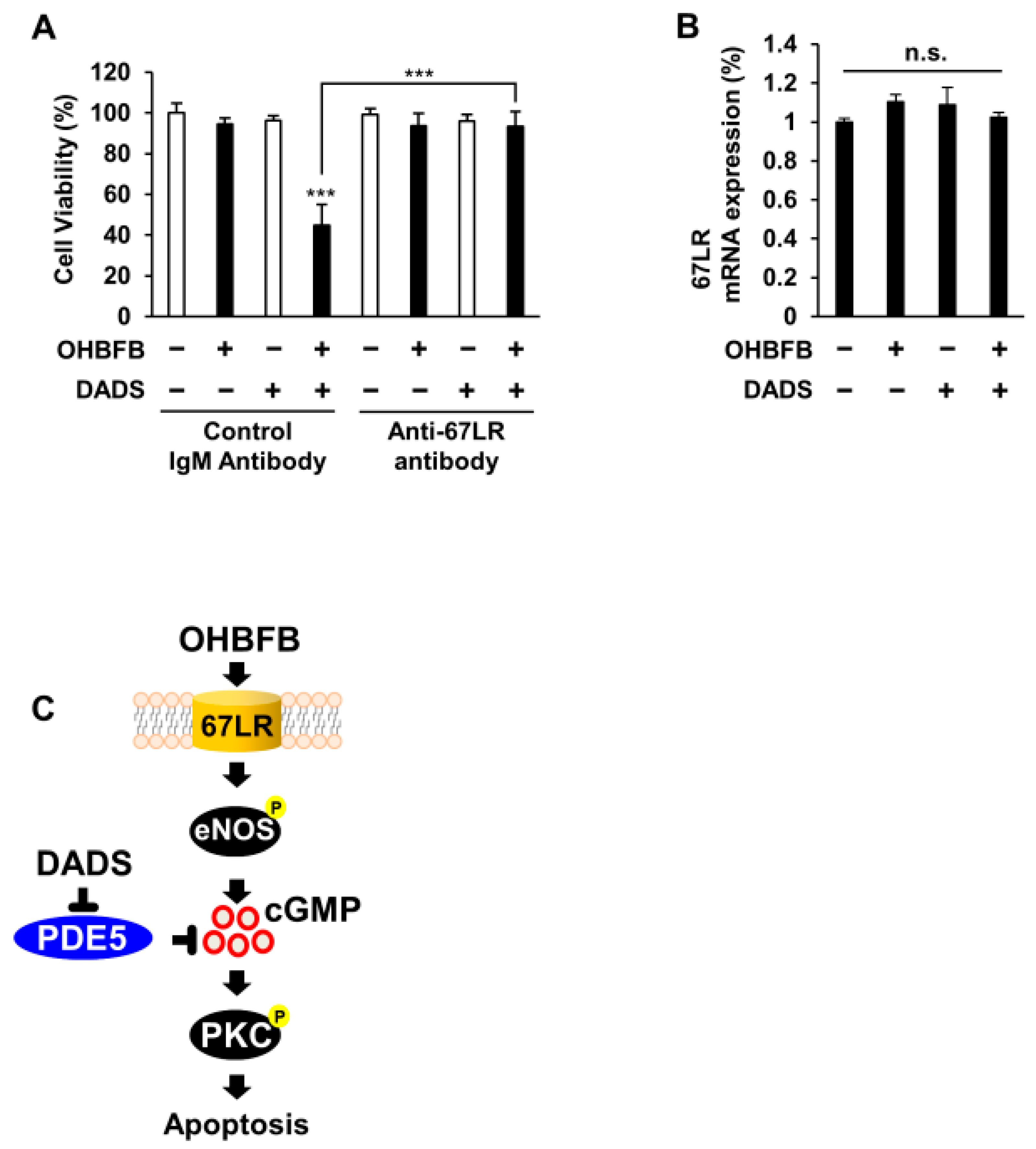
3.5. Combined OHBFB and DADS Induce Cell Death via 67LR in HL60 Cells without Affecting Normal Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kumazoe, M.; Lee, K.W.; Fujimura, Y.; Tachibana, H. 67-kDa laminin receptor mediates oolonghomobisflavan B-induced cell growth inhibition in melanoma. Phytomedicine 2023, 118, 154970. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; LaPlant, B.; Bowen, D.A.; Roos, M.; Secreto, C.R.; Ghosh, A.K.; Kabat, B.F.; Lee, M.J.; et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol. 2009, 27, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Achaintre, D.; Zamora-Ros, R.; Jenab, M.; Boutron-Ruault, M.C.; Carbonnel, F.; Savoye, I.; Kaaks, R.; Kühn, T.; Boeing, H.; et al. A prospective evaluation of plasma polyphenol levels and colon cancer risk. Int. J. Cancer 2018, 143, 1620–1631. [Google Scholar] [CrossRef]
- Zabel, U.; Kleinschnitz, C.; Oh, P.; Nedvetsky, P.; Smolenski, A.; Müller, H.; Kronich, P.; Kugler, P.; Walter, U.; Schnitzer, J.E.; et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat. Cell Biol. 2002, 4, 307–311. [Google Scholar] [CrossRef]
- Warner, T.D.; Mitchell, J.A.; Sheng, H.; Murad, F. Effects of cyclic GMP on smooth muscle relaxation. Adv. Pharmacol. 1994, 26, 171–194. [Google Scholar]
- Buechler, W.A.; Ivanova, K.; Wolfram, G.; Drummer, C.; Heim, J.M.; Gerzer, R. Soluble guanylyl cyclase and platelet function. Ann. N. Y. Acad. Sci. 1994, 714, 151–157. [Google Scholar]
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Porst, H.; Rosen, R.; Padma-Nathan, H.; Goldstein, I.; Giuliano, F.; Ulbrich, E.; on behalf of T Bandel and the Vardenafil Study Group. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: The first at-home clinical trial. Int. J. Impot. Res. 2001, 13, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors in the Management of Erectile Dysfunction. Pharm. Ther. 2013, 38, 407–419. [Google Scholar]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef]
- Arunkumar, A.; Vijayababu, M.R.; Venkataraman, P.; Senthilkumar, K.; Arunakaran, J. Chemoprevention of rat prostate carcinogenesis by diallyl disulfide, an organosulfur compound of garlic. Biol. Pharm. Bull. 2006, 29, 375–379. [Google Scholar] [CrossRef][Green Version]
- Lemar, K.M.; Aon, M.A.; Cortassa, S.; O’Rourke, B.; Müller, C.T.; Lloyd, D. Diallyl disulphide depletes glutathione in Candida albicans: Oxidative stress-mediated cell death studied by two-photon microscopy. Yeast 2007, 24, 695–706. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017, 7, 6665. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Fujimura, Y.; Tachibana, H. Diallyl disulfide potentiates anti-obesity effect of green tea in high-fat/high-sucrose diet-induced obesity. J. Nutr. Biochem. 2019, 64, 152–161. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hirotsu, K.; Kumazoe, M.; Goto, Y.; Sugihara, K.; Suda, T.; Tsurudome, Y.; Suzuki, T.; Yamashita, S.; Kim, Y.; et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012, 443, 525–534. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Song, Z.J.; Ng, M.Y.; Lee, Z.W.; Dai, W.; Hagen, T.; Moore, P.K.; Huang, D.; Deng, L.W.; Tan, C.H. Hydrogen sulfide donors in research and drug development. MedChemComm 2014, 5, 557–570. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, H.; Guth, A.; König, J.; Forestier, N.; Cremers, B.; Hennen, B.; Böhm, M.; Sybrecht, G.W. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation 2001, 104, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Vicenzi, M.; Arena, R.; Guazzi, M.D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 2011, 4, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Morris, G.Z.; Corbin, J.D. Molecular mechanisms that could contribute to prolonged effectiveness of PDE5 inhibitors to improve erectile function. Int. J. Impot. Res. 2008, 20, 333–342. [Google Scholar] [CrossRef]
- Linder, A.E.; McCluskey, L.P.; Cole, K.R., 3rd; Lanning, K.M.; Webb, R.C. Dynamic association of nitric oxide downstream signaling molecules with endothelial caveolin-1 in rat aorta. J. Pharmacol. Exp. Ther. 2005, 314, 9–15. [Google Scholar] [CrossRef]
- Russwurm, M.; Wittau, N.; Koesling, D. Guanylyl cyclase/PSD-95 interaction: Targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J. Biol. Chem. 2001, 276, 44647–44652. [Google Scholar] [CrossRef]
- Schoser, B.G.; Behrends, S. Soluble guanylyl cyclase is localized at the neuromuscular junction in human skeletal muscle. Neuroreport 2001, 12, 979–981. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Di Giacomo, V.; Rapino, M.; Zara, S.; Rana, R.A. Ionizing radiation induces apoptotic signal through protein kinase Cdelta (delta) and survival signal through Akt and cyclic-nucleotide response element-binding protein (CREB) in Jurkat T cells. Biol. Bull. 2009, 217, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Yamashita, S.; Kim, Y.H.; Kumazoe, M.; Huang, Y.; Yamada, K.; Tachibana, H. Oxygen partial pressure modulates 67-kDa laminin receptor expression, leading to altered activity of the green tea polyphenol, EGCG. FEBS Lett. 2012, 586, 3441–3447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.; Park, S.-J. The Combination of Oolonghomobisflavan B and Diallyl Disulfide Induces Apoptotic Cell Death via 67-kDa Laminin Receptor/Cyclic Guanosine Monophosphate in Acute Myeloid Leukemia Cells. Curr. Issues Mol. Biol. 2024, 46, 2444-2455. https://doi.org/10.3390/cimb46030154
Bae J, Park S-J. The Combination of Oolonghomobisflavan B and Diallyl Disulfide Induces Apoptotic Cell Death via 67-kDa Laminin Receptor/Cyclic Guanosine Monophosphate in Acute Myeloid Leukemia Cells. Current Issues in Molecular Biology. 2024; 46(3):2444-2455. https://doi.org/10.3390/cimb46030154
Chicago/Turabian StyleBae, Jaehoon, and Su-Jin Park. 2024. "The Combination of Oolonghomobisflavan B and Diallyl Disulfide Induces Apoptotic Cell Death via 67-kDa Laminin Receptor/Cyclic Guanosine Monophosphate in Acute Myeloid Leukemia Cells" Current Issues in Molecular Biology 46, no. 3: 2444-2455. https://doi.org/10.3390/cimb46030154
APA StyleBae, J., & Park, S.-J. (2024). The Combination of Oolonghomobisflavan B and Diallyl Disulfide Induces Apoptotic Cell Death via 67-kDa Laminin Receptor/Cyclic Guanosine Monophosphate in Acute Myeloid Leukemia Cells. Current Issues in Molecular Biology, 46(3), 2444-2455. https://doi.org/10.3390/cimb46030154








