Prognostic Value of P63 Expression in Muscle-Invasive Bladder Cancer and Association with Molecular Subtypes—Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
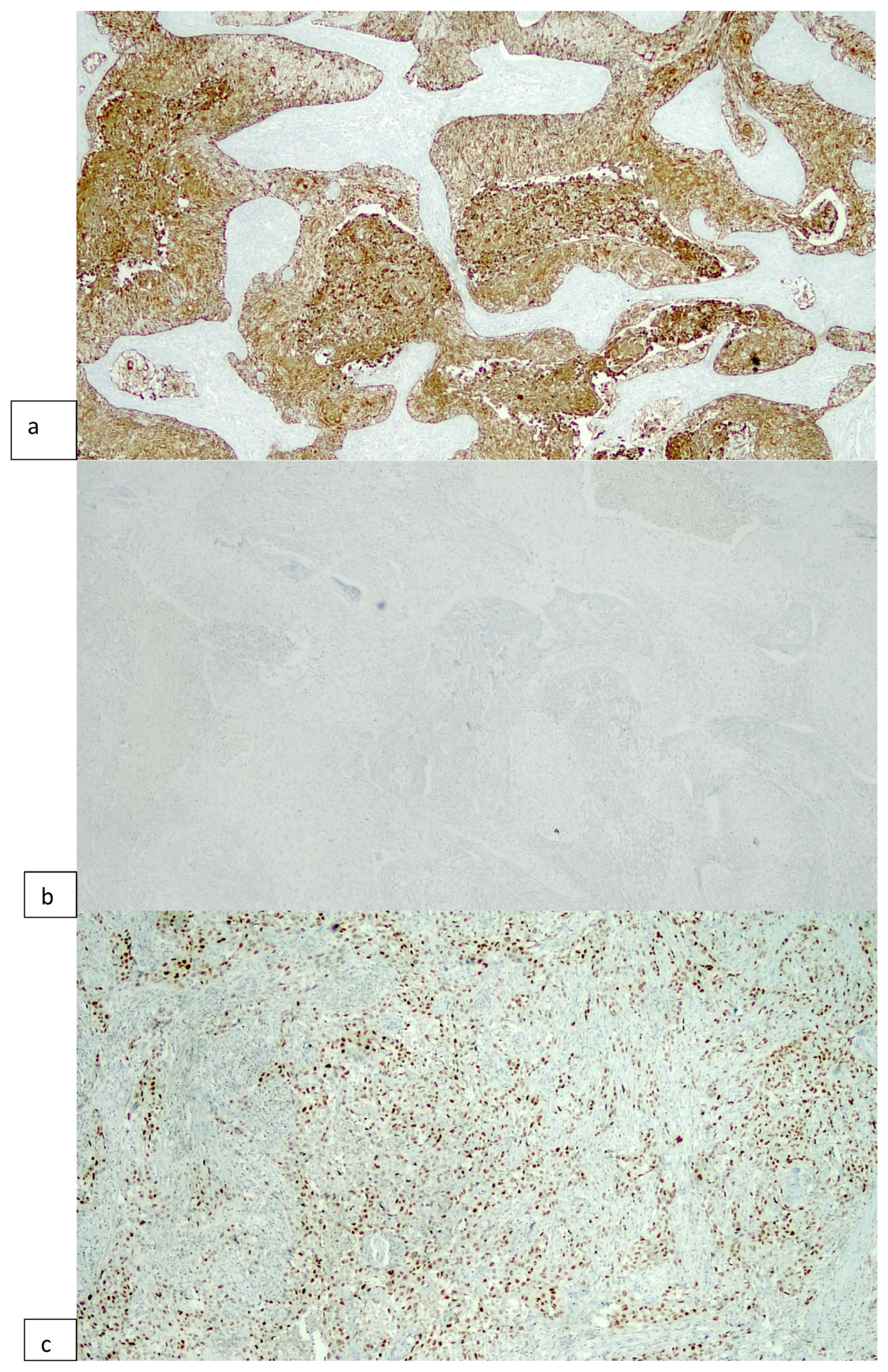
2.2. Immunohistochemical Procedures
2.3. Statistical Analysis
3. Results
3.1. Patients Clinicopathological Characteristics
3.2. P63 Expression and Molecular Subtyping
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://gco.iarc.fr/today/ (accessed on 31 January 2023).
- Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; Palou, J.; Van Rhijn, B.W.G.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS); EAU: Arnhem, The Netherlands, 2022. [Google Scholar]
- Witjes, J.A.; Bruins, H.M.; Carrión, A.; Cathomas, R.; Compérat, E.M.; Efstathiou, J.A.; Kietkau, R.; Gakis, G.; Van der Heijden, A.G.; Lorch, A.; et al. (Eds.) EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer; EAU: Arnhem, The Netherlands, 2022. [Google Scholar]
- Matulewicz, R.S.; Steinberg, G.D. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar] [PubMed]
- Sanguedolce, F.; Cormio, A.; Bufo, P.; Carrieri, G.; Cormio, L. Molecular markers in bladder cancer: Novel research frontiers. Crit. Rev. Clin. Lab. Sci. 2015, 52, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; De Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Landriscina, M.; Conteduca, V.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 2: Subtypes and Divergent Differentiation. Int. J. Mol. Sci. 2022, 23, 7844. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Landriscina, M.; Conteduca, V.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 1: General Issues and Marker Expression. Int. J. Mol. Sci. 2022, 23, 7819. [Google Scholar] [CrossRef]
- Woodstock, D.L.; Sammons, M.A.; Fischer, M. p63 and p53: Collaborative Partners or Dueling Rivals? Front. Cell Dev. Biol. 2021, 9, 701986. [Google Scholar] [CrossRef]
- Pokorná, Z.; Vysloužil, J.; Hrabal, V.; Vojtěšek, B.; Coates, P.J. The foggy world(s) of p63 isoform regulation in normal cells and cancer. J. Pathol. 2021, 254, 454–473. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Haghayeghi, K.; Lu, S.; Matoso, A.; Schiff, S.F.; Mueller-Leonhard, C.; Amin, A. Association of current molecular subtypes in urothelial carcinoma with patterns of muscularis propria invasion. Virchows Arch. 2021, 479, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cormio, L.; Sanguedolce, F.; Cormio, A.; Massenio, P.; Pedicillo, M.C.; Cagiano, S.; Calò, G.; Pagliarulo, V.; Carrieri, G.; Bufo, P. Human epidermal growth factor receptor 2 expression is more important than bacillus calmette guerin treatment in predicting the outcome of T1G3 bladder cancer. Oncotarget 2017, 8, 25433–25441. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jang, I.; Kim, K.; Jung, M.; Lee, C.; Park, J.H.; Kim, Y.A.; Moon, K.C. Comprehensive Gene Expression Analyses of Immunohistochemically Defined Subgroups of Muscle-Invasive Urinary Bladder Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 628. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Bondaruk, J.; Yao, H.; Wang, Z.; Zhang, L.; Lee, S.; Lee, J.G.; Cogdell, D.; Zhang, M.; Yang, G.; et al. Assessment of Luminal and Basal Phenotypes in Bladder Cancer. Sci. Rep. 2020, 10, 9743. [Google Scholar] [CrossRef]
- Zhai, Q.; Deng, F.M. Diagnostic Values of Immunohistochemistry in Bladder Cancer. In Urinary Bladder Pathology; Zhou, H., Guo, C.C., Ro, J.Y., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Sanguedolce, F.; Russo, D.; Mancini, V.; Selvaggio, O.; Calò, B.; Carrieri, G.; Cormio, L. Morphological and Immunohistochemical Biomarkers in Distinguishing Prostate Carcinoma and Urothelial Carcinoma: A Comprehensive Review. Int. J. Surg. Pathol. 2019, 27, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Bontoux, C.; Rialland, T.; Cussenot, O.; Compérat, E. A four-antibody immunohistochemical panel can distinguish clinico-pathological clusters of urothelial carcinoma and reveals high concordance between primary tumor and lymph node metastases. Virchows Arch. 2021, 478, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, G.; Eriksson, P.; Lövgren, K.; Marzouka, N.A.; Bernardo, C.; Nordentoft, I.; Dyrskjøt, L.; Liedberg, F.; Höglund, M. Discordant molecular subtype classification in the basal-squamous subtype of bladder tumors and matched lymph-node metastases. Mod. Pathol. 2018, 31, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, L.; Chebil, G.; Marzouka, N.A.; Liedberg, F.; Sjödahl, G. Low Frequency of Intratumor Heterogeneity in Bladder Cancer Tissue Microarrays. Bladder Cancer 2018, 4, 327–337. [Google Scholar] [CrossRef]
- Compérat, E.; Camparo, P.; Haus, R.; Chartier-Kastler, E.; Bart, S.; Delcourt, A.; Houlgatte, A.; François, R.; Capron, F.; Vieillefond, A. Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma. A tissue microarray study of 158 cases. Virchows Arch. 2006, 448, 319–324. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Wahbbah, M.A.; Selem, M.; Abdou, A.G.; Sultan, S.M. Correlation between immunohistochemical expression of Ki-67and P63 and aggressiveness of urinary bladder urothelial carcinoma. J. Immunoass. Immunochem. 2021, 42, 188–201. [Google Scholar] [CrossRef]
- Compérat, E.; Bièche, I.; Dargère, D.; Ferlicot, S.; Laurendeau, I.; Benoît, G.; Vieillefond, A.; Verret, C.; Vidaud, M.; Capron, F.; et al. p63 gene expression study and early bladder carcinogenesis. Urology 2007, 70, 459–462. [Google Scholar] [CrossRef]
- Moussa, R.A.; Khalil, E.Z.I.; Ali, A.I. Prognostic Role of Epithelial-Mesenchymal Transition Markers “E-Cadherin, β-Catenin, ZEB1, ZEB2 and p63” in Bladder Carcinoma. World J. Oncol. 2019, 10, 199–217. [Google Scholar] [CrossRef]
- Stacy, A.J.; Craig, M.P.; Sakaram, S.; Kadakia, M. ΔNp63α and microRNAs: Leveraging the epithelial-mesenchymal transition. Oncotarget 2017, 8, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; He, Y.F.; Tan, J.S.; Jiang, Y.; Xu, Q.; Cheng, H.L. The DeltaN p63 Promotes EMT and Metastasis in Bladder Cancer by the PTEN/AKT Signalling Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 9566055. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Kawakami, S.; Fujii, Y.; Saito, K.; Ohtsuka, Y.; Iwai, A.; Ando, N.; Takizawa, T.; Kageyama, Y.; Kihara, K. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res. 2003, 9, 5501–5507. [Google Scholar] [PubMed]
- Paner, G.P.; Annaiah, C.; Gulmann, C.; Rao, P.; Ro, J.Y.; Hansel, D.E.; Shen, S.S.; Lopez-Beltran, A.; Aron, M.; Luthringer, D.J.; et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum. Pathol. 2014, 45, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Babjuk, M.; Bellmunt, J.; Bruins, H.M.; De Reijke, T.M.; De Santis, M.; Gillessen, S.; James, N.; Mclennan, S.; Palou, J.; et al. EAU-ESMO Consensus Statements on the Management of Advanced and Variant Bladder Cancer—An international collaborative multistakeholder effort: Under the auspices of the EAU-ESMO Guidelines Committees. Eur. Urol. 2020, 77, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Seisen, T.; Compérat, E.; Larré, S.; Mazerolles, C.; Gobet, F.; Fetissof, F.; Fromont, G.; Safsaf, A.; d’Arcier, B.F.; et al. Prognostic interest in discriminating muscularis mucosa invasion (T1a vs. T1b) in nonmuscle invasive bladder carcinoma: French national multicenter study with central pathology review. J. Urol. 2013, 189, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Shah, J.B.; Tran, M.; Svatek, R.; Marquis, L.; Lee, I.L.; Yu, D.; Adam, L.; Wen, S.; Shen, Y.; et al. p63 expression defines a lethal subset of muscle-invasive bladder cancers. PLoS ONE 2012, 7, e30206. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Feng, C.; Gao, P.; Ding, G.; Zhou, Z.; Jiang, H.; Wu, Z.; Ding, Q. Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy. Int. Urol. Nephrol. 2016, 48, 495–501. [Google Scholar] [CrossRef]
- Burgess, E.F.; Livasy, C.; Trufan, S.; Zhu, J.; O’Connor, H.F.; Hartman, A.; Clark, P.E.; Grigg, C.; Raghavan, D. Clinical outcomes associated with expression of aurora kinase and p53 family members in muscle-invasive bladder cancer. Mol. Clin. Oncol. 2022, 16, 102. [Google Scholar] [CrossRef]
- Steurer, S.; Riemann, C.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; Fraune, C.; et al. p63 expression in human tumors and normal tissues: A tissue microarray study on 10,200 tumors. Biomark. Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Sjödahl, G.; Lövgren, K.; Lauss, M.; Patschan, O.; Gudjonsson, S.; Chebil, G.; Aine, M.; Eriksson, P.; Månsson, W.; Lindgren, D.; et al. Toward a molecular pathologic classification of urothelial carcinoma. Am. J. Pathol. 2013, 183, 681–691. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Weyerer, V.; Weisser, R.; Moskalev, E.A.; Haller, F.; Stoehr, R.; Eckstein, M.; Zinnall, U.; Gaisa, N.T.; Compérat, E.; Perren, A.; et al. Distinct Genetic Alterations and Luminal Molecular Subtype in Nested Variant of Urothelial Carcinoma. Histopathology 2019, 75, 865–875. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Russo, D.; Calò, B.; Cindolo, L.; Carrieri, G.; Cormio, L. Diagnostic and prognostic roles of CK20 in the pathology of urothelial lesions. A systematic review. Pathol. Res. Pract. 2019, 215, 152413. [Google Scholar] [CrossRef] [PubMed]
- Marquis, L.; Tran, M.; Choi, W.; Lee, I.L.; Huszar, D.; Siefker-Radtke, A.; Dinney, C.; McConkey, D.J. p63 expression correlates with sensitivity to the Eg5 inhibitor ZD4877 in bladder cancer cells. Cancer Biol. Ther. 2012, 13, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zangen, R.; Ratovitski, E.; Sidransky, D. ΔNp63α levels correlate with clinical tumor response to cisplatin. Cell Cycle 2005, 4, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Viale, G.; Gelber, R.D.; Bottiglieri, L.; Gelber, S.; Pruneri, G.; Ghisini, R.; Balduzzi, A.; Pietri, E.; D’Alessandro, C.; et al. Pathologic complete remission rate after cisplatin-based primary chemotherapy in breast cancer: Correlation with p63 expression. Cancer Chemother. Pharmacol. 2008, 61, 965–971. [Google Scholar] [CrossRef] [PubMed]

| P63-Negative (n = 22) | P63-Positive (n = 43) | p Value | |
|---|---|---|---|
| Age, mean (range) | 71 (68–78) | 76 (70–82) | 0.077 |
| Gender, n (%) | |||
| Female | 3 (14%) | 5 (12%) | 0.8 |
| Male | 19 (86%) | 38 (88%) | |
| Papillary architecture, n (%) | |||
| Absent | 20 (91%) | 36 (84%) | 0.4 |
| Present | 2 (9%) | 7 (16%) | |
| DD/HS, n (%) | |||
| Absent | 12 (55%) | 35 (81%) | 0.022 |
| Present | 10 (45%) | 8 (19%) | |
| Anaplasia, n (%) | |||
| Absent | 20 (91%) | 35 (81%) | 0.3 |
| Present | 2 (9%) | 8 (19%) | |
| CIS, n (%) | |||
| Absent | 14 (64%) | 36 (84%) | 0.069 |
| Present | 8 (36%) | 7 (16%) | |
| pT, n (%) | |||
| 2 | 4 (18%) | 7 (16%) | 0.8 |
| 3 | 11 (50%) | 25 (58%) | |
| 4 | 7 (32%) | 11 (26%) | |
| Pelvic nodal involvement, n (%) | |||
| Absent | 12 (55%) | 21 (49%) | 0.7 |
| Present | 10 (45%) | 22 (51%) | |
| LVI, n (%) | |||
| Absent | 9 (41%) | 23 (53%) | 0.3 |
| Present | 13 (59%) | 20 (47%) | |
| PNI, n (%) | |||
| Absent | 16 (73%) | 24 (56%) | 0.2 |
| Present | 6 (27%) | 19 (44%) | |
| Pattern of MP invasion, n (%) | |||
| 1 | 11 (50%) | 37 (86%) | 0.002 |
| 2 | 11 (50%) | 6 (14%) | |
| Necrosis, n (%) | |||
| Absent | 9 (41%) | 16 (37%) | 0.8 |
| Present | 13 (59%) | 27 (63%) |
| P63-Negative (n = 22) | P63-Positive (n = 43) | p Value | |
|---|---|---|---|
| Algorithm #1, n (%) | |||
| Bas | 0 (0%) | 17 (40%) | 0.004 |
| Lum | 8 (38%) | 15 (35%) | |
| DP | 1 (5%) | 1 (2%) | |
| DN | 12 (57%) | 10 (23%) | |
| Algorithm #2, n (%) | |||
| Bas | 0 (0%) | 5 (12%) | 0.003 |
| Lum | 18 (82%) | 25 (58%) | |
| DP | 1 (5%) | 13 (30%) | |
| DN | 3 (14%) | 0 (0%) |
| Grade | Stage | Treatment | Clone | Target | Scoring Method | Findings | Reference |
|---|---|---|---|---|---|---|---|
| HG | MIBCs | NAC + RC | 4A4 | ∆Np63 isoform | Overexpression > 50% stained cells | Baseline p63 (HR 2.02; 95% CI = 0.51–8.1; p = 0.313) protein expression did not predict for OS. | [34] |
| LG + HG | NMIBCs + MIBCs | NA | DAK-p63 | TAp63 and ΔNp63 isoforms | Low (≤median), high (>median) | Higher P63 expression showed a significant association with low-grade tumors (p < 0.05). | [23] |
| HG | NMIBCs + MIBCs | RC | 4A4 | ∆Np63 isoform | Combined intensity and percentage of stained cells | Lower P63 expression showed a significant association with higher-stage (p = 0.0004), nodal metastases (p = 0.0013), and poor prognosis (p = 0.0005). | [28] |
| LG + HG | NMIBCs + MIBCs | RC | 4A4 | ∆Np63 isoform | Negative (<10%), weak (10–80%), high (80–100%) stained cells | Higher P63 expression showed a significant association with poor OS (p < 0.001) in patients with MIBC. | [25] |
| LG + HG | NMIBCs + MIBCs | RC | NA | NA | 0 (≤10%), + (>10%) stained cells | Positive P63 expression was an independent factor for worse survival (p = 0.033) in all patients. | [33] |
| LG + HG | NMIBCs + MIBCs | TUR, RC | 4A4 | ∆Np63 isoform | 0 (<10%), 1 (10–80%), 2 (80–100%) stained cells | P63 expression distinguished between PUNLMP/NILGC and NIHGC/pT1 (p = 4.105). | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanguedolce, F.; Falagario, U.G.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Ascani, S.; Tortorella, S.; Busetto, G.M.; Cormio, A.; Carrieri, G.; et al. Prognostic Value of P63 Expression in Muscle-Invasive Bladder Cancer and Association with Molecular Subtypes—Preliminary Report. Curr. Issues Mol. Biol. 2024, 46, 2456-2467. https://doi.org/10.3390/cimb46030155
Sanguedolce F, Falagario UG, Zanelli M, Palicelli A, Zizzo M, Ascani S, Tortorella S, Busetto GM, Cormio A, Carrieri G, et al. Prognostic Value of P63 Expression in Muscle-Invasive Bladder Cancer and Association with Molecular Subtypes—Preliminary Report. Current Issues in Molecular Biology. 2024; 46(3):2456-2467. https://doi.org/10.3390/cimb46030155
Chicago/Turabian StyleSanguedolce, Francesca, Ugo Giovanni Falagario, Magda Zanelli, Andrea Palicelli, Maurizio Zizzo, Stefano Ascani, Simona Tortorella, Gian Maria Busetto, Angelo Cormio, Giuseppe Carrieri, and et al. 2024. "Prognostic Value of P63 Expression in Muscle-Invasive Bladder Cancer and Association with Molecular Subtypes—Preliminary Report" Current Issues in Molecular Biology 46, no. 3: 2456-2467. https://doi.org/10.3390/cimb46030155
APA StyleSanguedolce, F., Falagario, U. G., Zanelli, M., Palicelli, A., Zizzo, M., Ascani, S., Tortorella, S., Busetto, G. M., Cormio, A., Carrieri, G., & Cormio, L. (2024). Prognostic Value of P63 Expression in Muscle-Invasive Bladder Cancer and Association with Molecular Subtypes—Preliminary Report. Current Issues in Molecular Biology, 46(3), 2456-2467. https://doi.org/10.3390/cimb46030155








