Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Product
2.2. Ex Vivo Assessment of the Anti-Pollution Activity of ZP on Human Living Skin Explants
2.2.1. Skin Explants
2.2.2. Pollutants and Treatment
2.2.3. Sampling and Histological Processing
2.2.4. Immuno-Histochemistry
2.2.5. Biochemical Assays—MDA and IL-1α
2.2.6. Image Analysis and Statistics
2.3. In Vitro Analysis of the Anti-Pollution Capacity of ZP in Human Keratinocytes
2.3.1. Cell Culture Conditions
2.3.2. Antioxidant Assessment against UD-Induced Oxidative Stress
2.3.3. Antioxidant Assessment against UD-Plus-UVA-Induced Oxidative Stress (Photopollution Model)
2.3.4. Anti-Inflammatory Assessment against UD-Induced IL-6 and IL-1α Production
2.3.5. Evaluation of AhR, Nrf2, and CYP1A1 Protein Levels
2.4. In Vitro Analysis of the Anti-Pollution Capacity of ZP in Human Pulmonary Fibroblasts
2.4.1. Cell Culture Conditions
2.4.2. Antioxidant Assessment against UD Induced Oxidative Stress
2.4.3. Study of mRNA Expression of bcl-2 and Bax Genes by RT-qPCR
2.5. In Vitro Antioxidant Assessment against UD-Induced Oxidative Stress in Human Endothelial Cells (HUVECs)
2.6. Heartbeat Quantification in Medaka Embryos Exposed to Urban Dust
3. Results
3.1. Ex Vivo Assessment of the Anti-Pollution Activity of ZP on Human Living Skin Explants
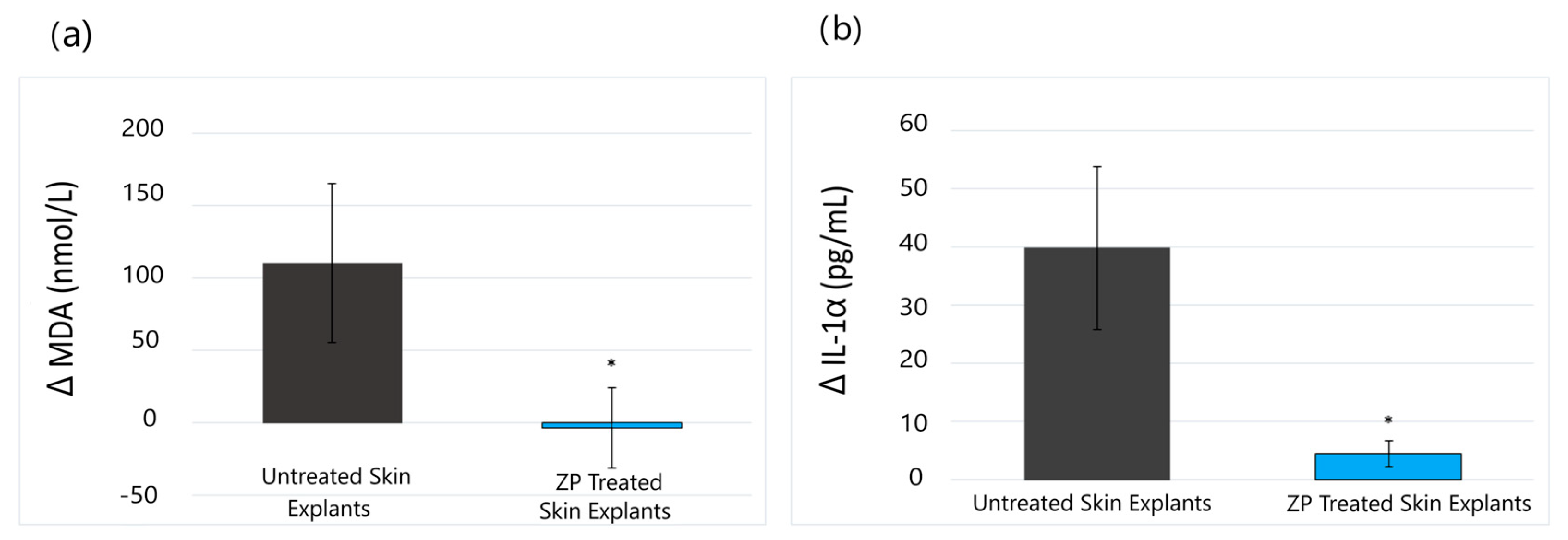
3.1.1. Lipid Peroxidation (MDA) and Anti-Inflammatory (IL-1α) Assay Results
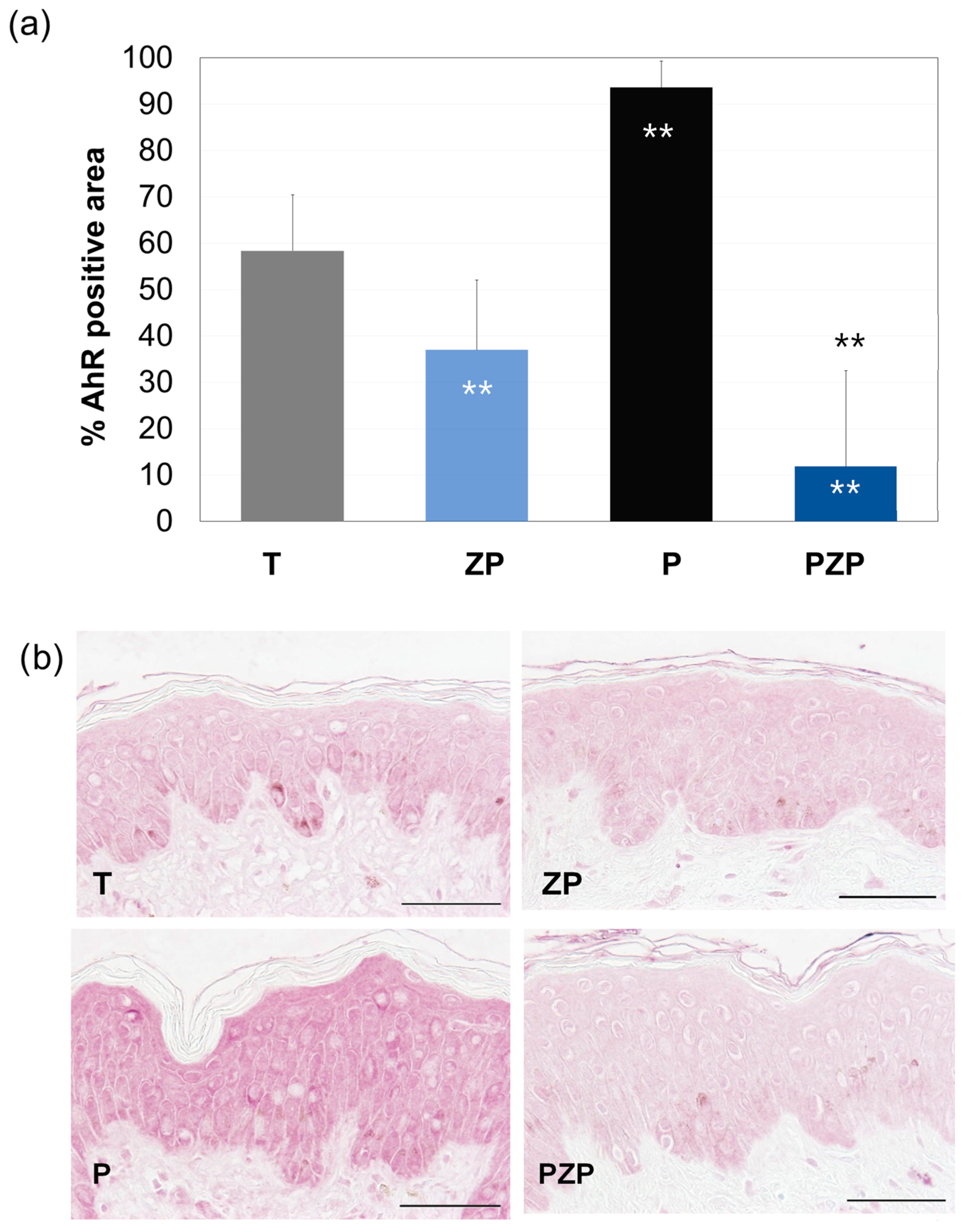
3.1.2. AhR Expression
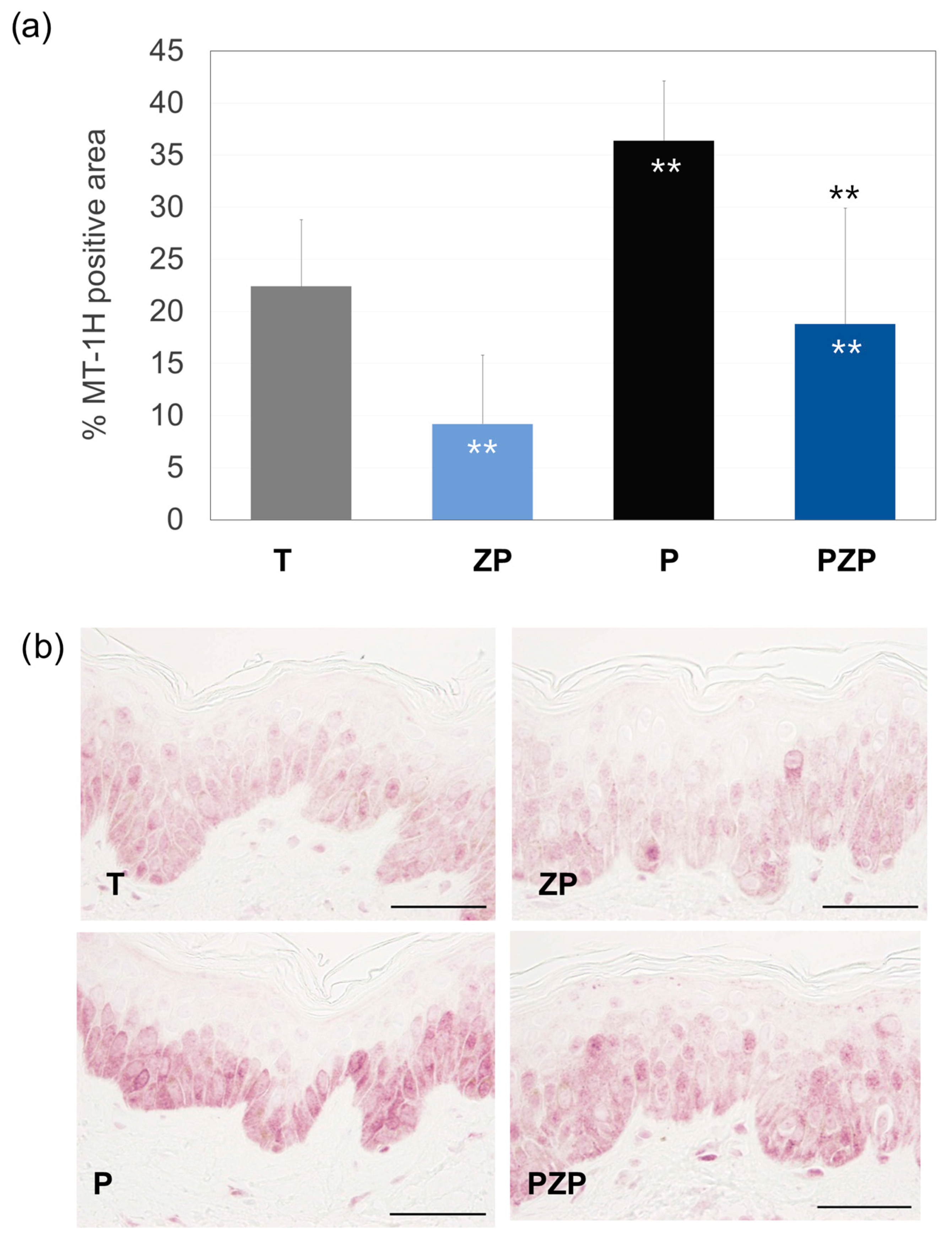
3.1.3. Metallothionein (MT-1H) Expression
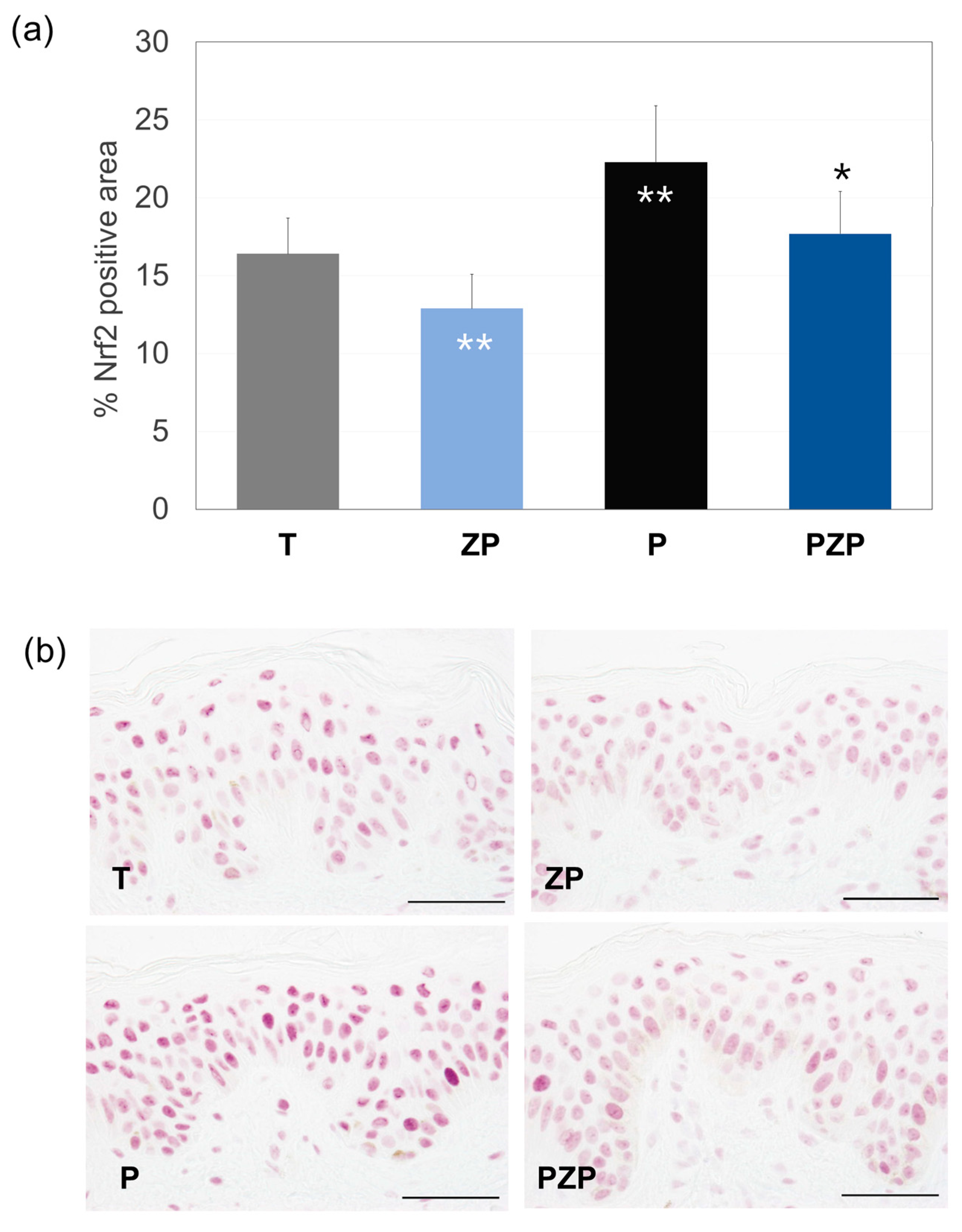
3.1.4. Nrf2 Expression
3.2. In Vitro Analysis of the Anti-Pollution Capacity of ZP in Human Keratinocytes
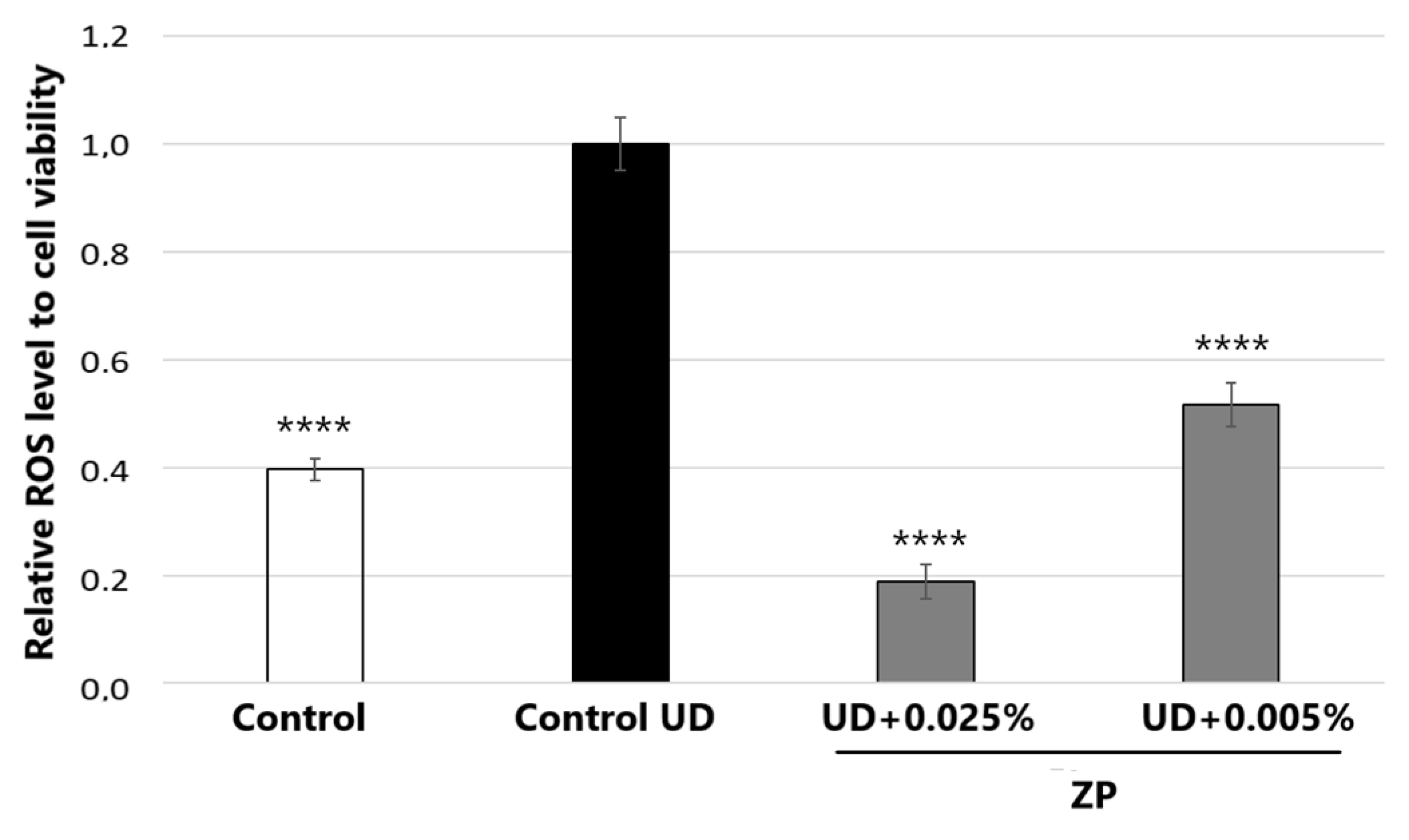
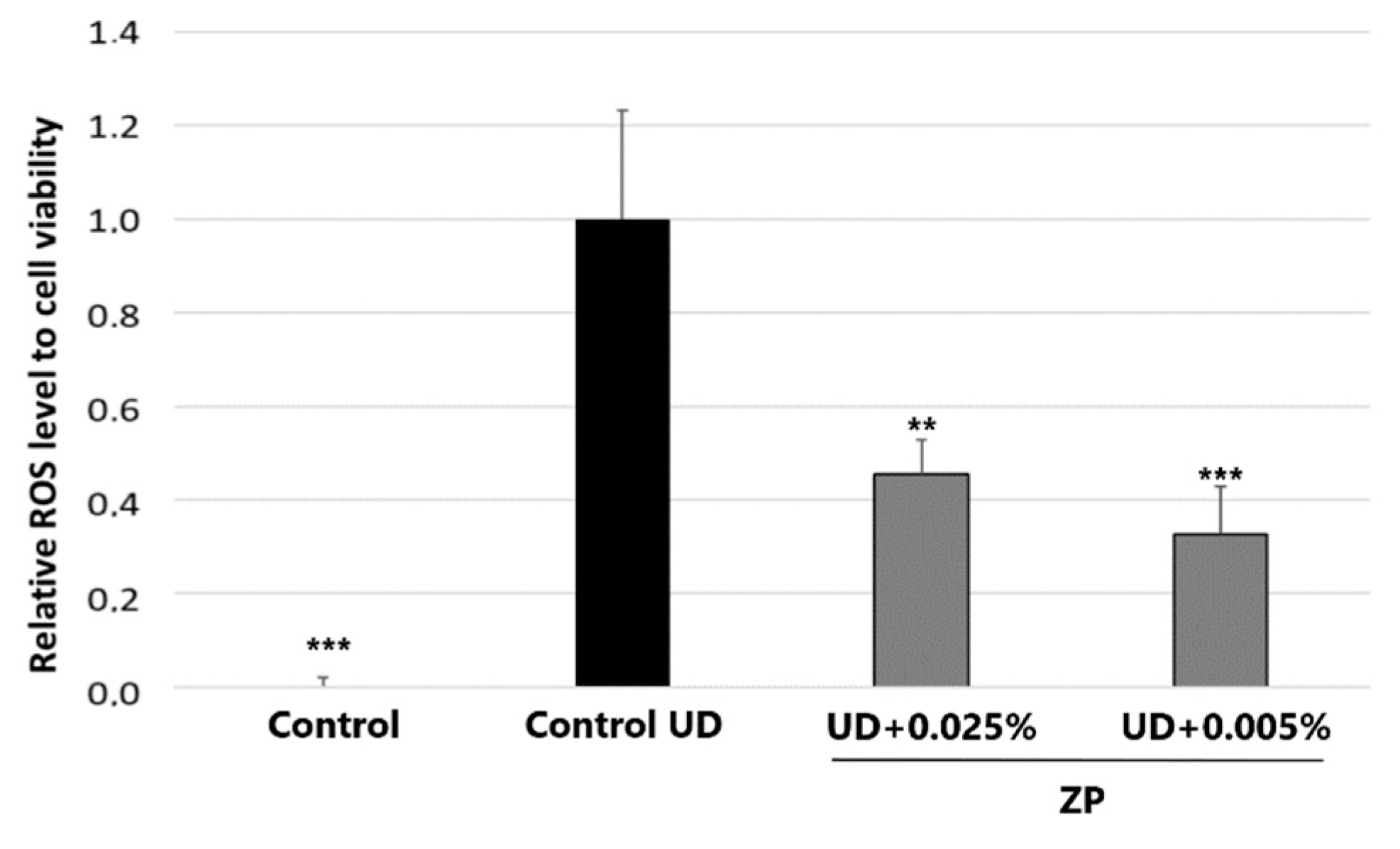
3.2.1. Antioxidant Assessment against UD-Induced Oxidative Stress
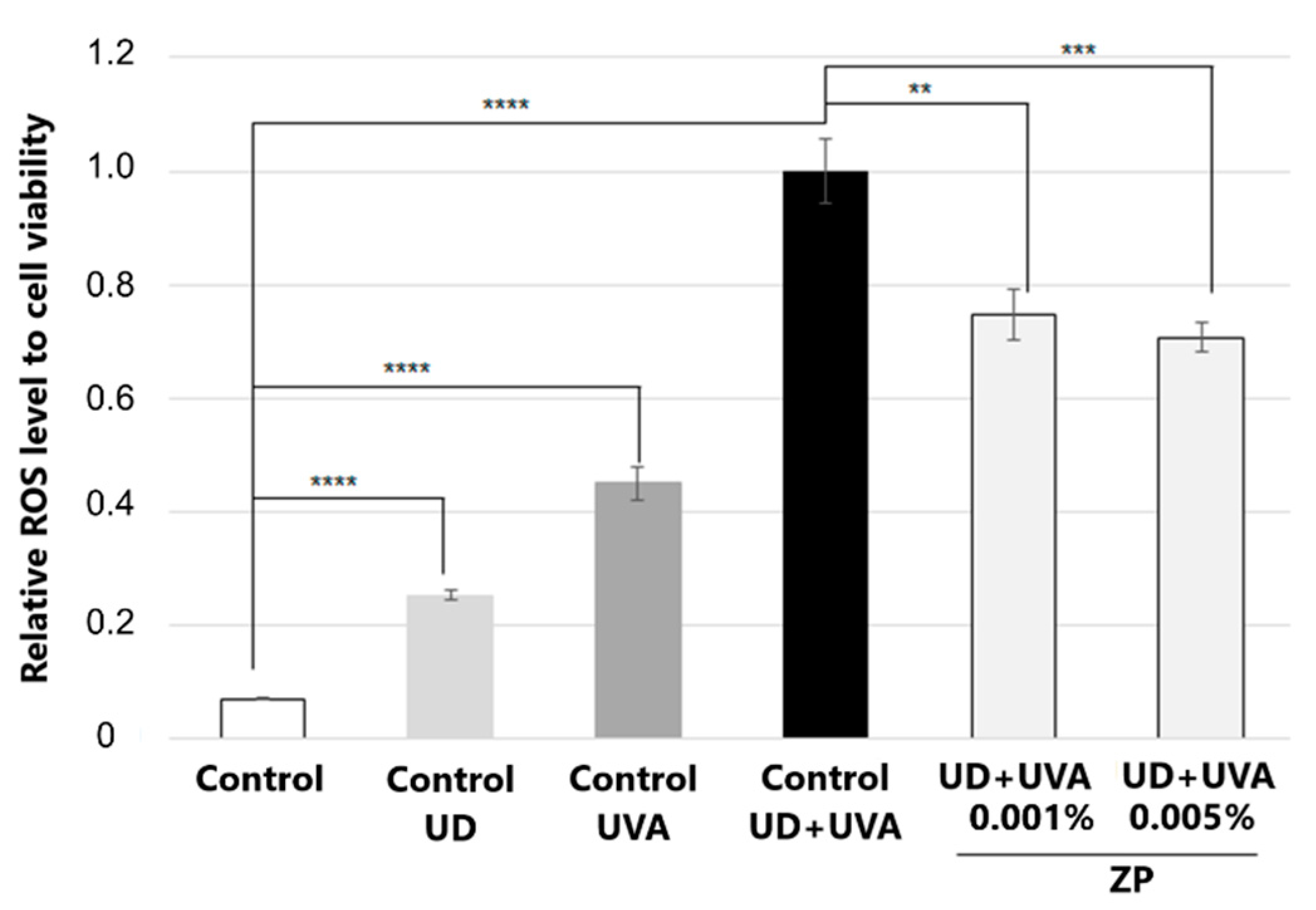
3.2.2. Antioxidant Assessment against UD-Plus-UVA-Induced Oxidative Stress
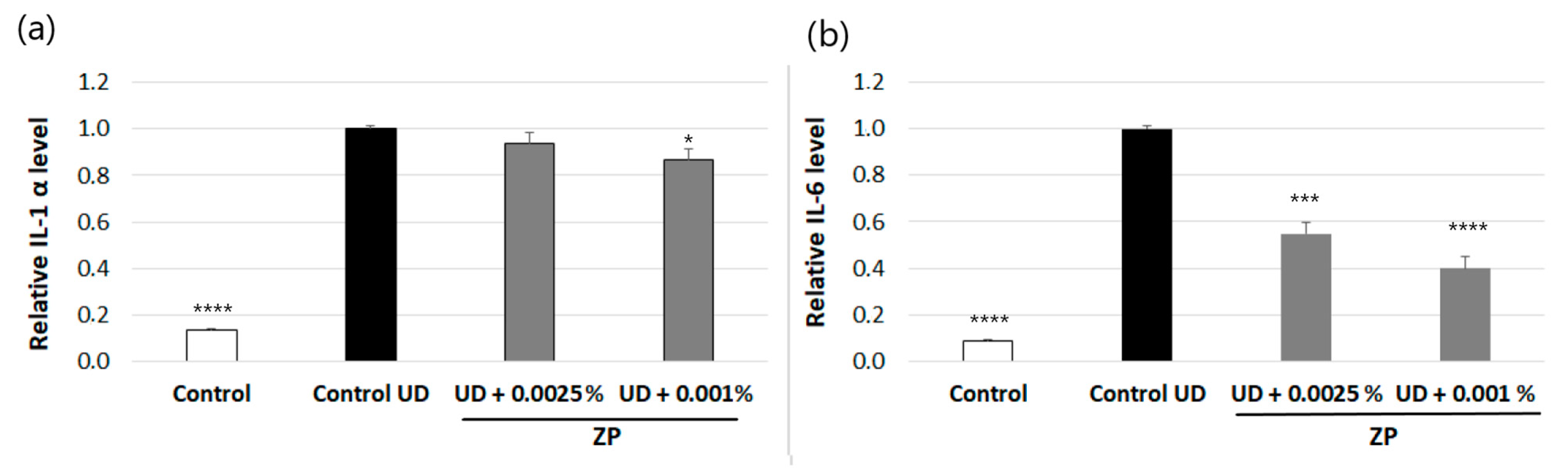
3.2.3. Anti-Inflammatory Assessment against UD-Induced IL-6 and IL-1α Production
3.2.4. Evaluation of AhR, Nrf2, and CYP1A1 Protein Levels in Human Keratinocytes
3.3. In Vitro Analysis of the Anti-Pollution Capacity of ZP in Human Pulmonary Fibroblasts
3.3.1. Antioxidant Assessment against UD-Induced Oxidative Stress
3.3.2. Study of mRNA Expression of BAX-2 and FAS Genes by RT-qPCR in HPF
3.4. In Vitro Antioxidant Assessment against UD-Induced Oxidative Stress in Human Endothelial Cells (HUVECs)
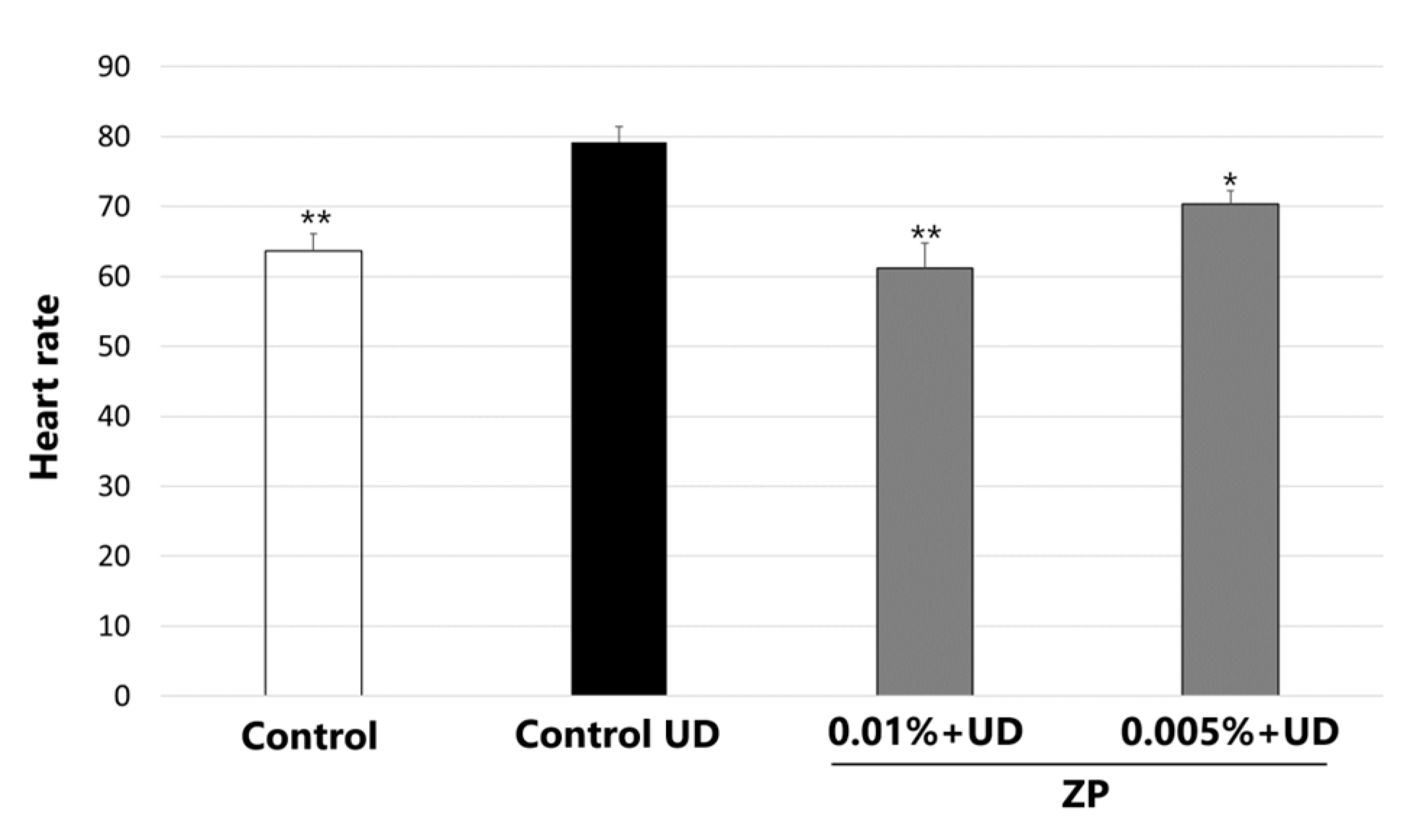
3.5. Heartbeat Quantification in Medaka Embryos Exposed to Urban Dust
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boogaard, H.; Patton, A.P.; Atkinson, R.W.; Brook, J.R.; Chang, H.H.; Crouse, D.L.; Fussell, J.C.; Hoek, G.; Hoffmann, B.; Kappeler, R.; et al. Long-Term Exposure to Traffic-Related Air Pollution and Selected Health Outcomes: A Systematic Review and Meta-Analysis. Environ. Int. 2022, 164, 107262. [Google Scholar] [CrossRef]
- Air Pollution. Available online: https://www.who.int/health-topics/air-pollution (accessed on 18 August 2023).
- Kim, K.E.; Cho, D.; Park, H.J. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Schikowski, T.; Hüls, A. Air Pollution and Skin Aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef]
- Fadadu, R.P.; Abuabara, K.; Balmes, J.R.; Hanifin, J.M.; Wei, M.L. Air Pollution and Atopic Dermatitis, from Molecular Mechanisms to Population-Level Evidence: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2526. [Google Scholar] [CrossRef]
- Lowe, M.E.; Akhtari, F.S.; Potter, T.A.; Fargo, D.C.; Schmitt, C.P.; Schurman, S.H.; Eccles, K.M.; Motsinger-Reif, A.; Hall, J.E.; Messier, K.P. The Skin Is No Barrier to Mixtures: Air Pollutant Mixtures and Reported Psoriasis or Eczema in the Personalized Environment and Genes Study (PEGS). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Yang, R.; Yu, H.; Shang, S.; Hu, Y. Short-Term Exposure to Ambient Fine Particulate Matter and Psoriasis: A Time-Series Analysis in Beijing, China. Front. Public Health 2022, 10, 1015197. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Pollution as a Risk Factor for the Development of Melasma and Other Skin Disorders of Facial Hyperpigmentation—Is There a Case to Be Made? J. Drugs Dermatol. 2015, 14, 337–341. [Google Scholar] [PubMed]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air Pollution-induced Tanning of Human Skin. Br. J. Dermatol. 2021, 185, 1026–1034. [Google Scholar] [CrossRef]
- El Haddad, C.; Gerbaka, N.-E.; Hallit, S.; Tabet, C. Association between Exposure to Ambient Air Pollution and Occurrence of Inflammatory Acne in the Adult Population. BMC Public Health 2021, 21, 1664. [Google Scholar] [CrossRef]
- Krutmann, J.; Moyal, D.; Liu, W.; Kandahari, S.; Lee, G.-S.; Nopadon, N.; Xiang, L.F.; Seité, S. Pollution and Acne: Is There a Link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental Pollutants and Skin Cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef]
- Siddens, L.K.; Larkin, A.; Krueger, S.K.; Bradfield, C.A.; Waters, K.M.; Tilton, S.C.; Pereira, C.B.; Löhr, C.V.; Arlt, V.M.; Phillips, D.H.; et al. Baird. Polycyclic Aromatic Hydrocarbons as Skin Carcinogens: Comparison of Benzo[a]Pyrene, Dibenzo[Def,p]Chrysene and Three Environmental Mixtures in the FVB/N Mouse. Toxicol. Appl. Pharmacol. 2012, 264, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Cervellati, F.; Benedusi, M.; Manarini, F.; Woodby, B.; Russo, M.; Valacchi, G.; Pietrogrande, M.C. Proinflammatory Properties and Oxidative Effects of Atmospheric Particle Components in Human Keratinocytes. Chemosphere 2020, 240, 124746. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The Impact of Airborne Pollution on Skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the Impact of Ambient Air Pollution on Skin Health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef]
- Dean, J.R.; Elom, N.I.; Entwistle, J.A. Use of Simulated Epithelial Lung Fluid in Assessing the Human Health Risk of Pb in Urban Street Dust. Sci. Total Environ. 2017, 579, 387–395. [Google Scholar] [CrossRef]
- Schultz, E.S.; Litonjua, A.A.; Melén, E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr. Allergy Asthma Rep. 2017, 17, 41. [Google Scholar] [CrossRef]
- Zhu, R.-X.; Nie, X.-H.; Liu, X.-F.; Zhang, Y.-X.; Chen, J.; Liu, X.-J.; Hui, X.-J. Short-Term Effect of Particulate Matter on Lung Function and Impulse Oscillometry System (IOS) Parameters of Chronic Obstructive Pulmonary Disease (COPD) in Beijing, China. BMC Public Health 2023, 23, 1417. [Google Scholar] [CrossRef]
- Nel, A.E.; Diaz-Sanchez, D.; Li, N. The Role of Particulate Pollutants in Pulmonary Inflammation and Asthma: Evidence for the Involvement of Organic Chemicals and Oxidative Stress. Curr. Opin. Pulm. Med. 2001, 7, 20–26. [Google Scholar] [CrossRef]
- Schichlein, K.D.; Smith, G.J.; Jaspers, I. Protective Effects of Inhaled Antioxidants against Air Pollution-Induced Pathological Responses. Respir. Res. 2023, 24, 187. [Google Scholar] [CrossRef]
- Li, N.; Hao, M.; Phalen, R.F.; Hinds, W.C.; Nel, A.E. Particulate Air Pollutants and Asthma. A Paradigm for the Role of Oxidative Stress in PM-Induced Adverse Health Effects. Clin. Immunol. 2003, 109, 250–265. [Google Scholar] [CrossRef]
- An, Z.; Jin, Y.; Li, J.; Li, W.; Wu, W. Impact of Particulate Air Pollution on Cardiovascular Health. Curr. Allergy Asthma Rep. 2018, 18, 15. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Shin, T.H.; Park, C.B.; Lee, W.-S.; Kim, J.; Lee, G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials 2022, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Perz, S.; Döring, A.; Stieber, J.; Koenig, W.; Wichmann, H.E. Increases in Heart Rate during an Air Pollution Episode. Am. J. Epidemiol. 1999, 150, 1094–1098. [Google Scholar] [CrossRef]
- Riediker, M.; Franc, Y.; Bochud, M.; Meier, R.; Rousson, V. Exposure to Fine Particulate Matter Leads to Rapid Heart Rate Variability Changes. Front. Environ. Sci. 2018, 6, 2. [Google Scholar] [CrossRef]
- Barthelemy, J.; Sanchez, K.; Miller, M.R.; Khreis, H. New Opportunities to Mitigate the Burden of Disease Caused by Traffic Related Air Pollution: Antioxidant-Rich Diets and Supplements. Int. J. Environ. Res. Public Health 2020, 17, 630. [Google Scholar] [CrossRef]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and Respiratory Disease: Can Diet or Supplements Help? A Review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef]
- Romieu, I.; Castro-Giner, F.; Kunzli, N.; Sunyer, J. Air Pollution, Oxidative Stress and Dietary Supplementation: A Review. Eur. Respir. J. 2008, 31, 179–197. [Google Scholar] [CrossRef]
- Wilhelm Filho, D.; Avila, S.; Possamai, F.P.; Parisotto, E.B.; Moratelli, A.M.; Garlet, T.R.; Inácio, D.B.; Torres, M.A.; Colepicolo, P.; Dal-Pizzol, F. Antioxidant Therapy Attenuates Oxidative Stress in the Blood of Subjects Exposed to Occupational Airborne Contamination from Coal Mining Extraction and Incineration of Hospital Residues. Ecotoxicology 2010, 19, 1193–1200. [Google Scholar] [CrossRef]
- Péter, S.; Holguin, F.; Wood, L.G.; Clougherty, J.E.; Raederstorff, D.; Antal, M.; Weber, P.; Eggersdorfer, M. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients 2015, 7, 10398–10416. [Google Scholar] [CrossRef]
- Chen, L.; Mo, H.; Zhao, L.; Gao, W.; Wang, S.; Cromie, M.M.; Lu, C.; Wang, J.-S.; Shen, C.-L. Therapeutic Properties of Green Tea against Environmental Insults. J. Nutr. Biochem. 2017, 40, 1–13. [Google Scholar] [CrossRef]
- Jin, X.; Su, R.; Li, R.; Song, L.; Chen, M.; Cheng, L.; Li, Z. Amelioration of Particulate Matter-Induced Oxidative Damage by Vitamin c and Quercetin in Human Bronchial Epithelial Cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, L.; Wang, H.; Huang, M.; Sun, X.; Kan, J.; Du, J.; Li, Y. Protective Effect of Supplementation with Ginseng, Lilii Bulbus and Poria against PM2.5 in Air Pollution-Induced Cardiopulmonary Damage among Adults. Phytother. Res. 2021, 35, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural Compounds Protect the Skin from Airborne Particulate Matter by Attenuating Oxidative Stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Schiano, I.; Peral, A.; Giardina, S.; Spartà, E.; Caturla, N. Antioxidant and Reduced Skin-Ageing Effects of a Polyphenol-Enriched Dietary Supplement in Response to Air Pollution: A Randomized, Double-Blind, Placebo-Controlled Study. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Rodriguez, K.J.; Wong, H.-K.; Oddos, T.; Southall, M.; Frei, B.; Kaur, S. A Purified Feverfew Extract Protects from Oxidative Damage by Inducing DNA Repair in Skin Cells via a PI3-Kinase-Dependent Nrf2/ARE Pathway. J. Dermatol. Sci. 2013, 72, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Huang, Y.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. Resveratrol Protects against PM2.5-Induced Heart Defects in Zebrafish Embryos as an Antioxidant Rather than as an AHR Antagonist. Toxicol. Appl. Pharmacol. 2020, 398, 115029. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and Other Interactions in Phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-E.; Lee, G.-H.; Hyun, K.-Y. Anti-Oxidant and Anti-Pollution Composition Containing the Extract of Nypa Fruticans Wurmb, Saussurea Neoserrata, Codium Fragile and Enteromorpha Compressa. Biomed. Sci. Lett. 2020, 26, 157–163. [Google Scholar] [CrossRef]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis Canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.I.; Jang, H.-J.; Kwon, O.-K.; Kim, J.-H.; Oh, J.-H.; Kim, S.-H.; Oh, S.-R.; Han, S.-B.; Ahn, K.-S.; Park, J.-W. Quercetin Attenuates the Production of Pro-Inflammatory Cytokines in H292 Human Lung Epithelial Cells Infected with Pseudomonas Aeruginosa by Modulating ExoS Production. J. Microbiol. Biotechnol. 2023, 33, 430–440. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Plasmati, E.; Viappiani, S.; Hernandez, A.; Colombo, C.; Sala, A. Olive Phenol Hydroxytyrosol Prevents Passive Smoking-Induced Oxidative Stress. Circulation 2000, 102, 2169–2171. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Patatian, A.; Delestre-Delacour, C.; Percoco, G.; Ramdani, Y.; Di Giovanni, M.; Peno-Mazzarino, L.; Bader, T.; Bénard, M.; Driouich, A.; Lati, E.; et al. Skin Biological Responses to Urban Pollution in an Ex Vivo Model. Toxicol. Lett. 2021, 348, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Goldner, J. A Modification of the Masson Trichrome Technique for Routine Laboratory Purposes. Am. J. Pathol. 1938, 14, 237–243. [Google Scholar] [PubMed]
- MTT Assay Protocol 17. Available online: https://jeodpp.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/17_P_MTT%20Assay.pdf (accessed on 2 February 2024).
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M. Beyond the Zebrafish: Diverse Fish Species for Modeling Human Disease. Dis. Model. Mech. 2014, 7, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Taniguchi, Y.; Okada, T.; Takeda, S.; Mori, K. Vertebrate Unfolded Protein Response: Mammalian Signaling Pathways Are Conserved in Medaka Fish. Cell Struct. Funct. 2011, 36, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 655/2013 of 10 July 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013R0655 (accessed on 1 February 2024).
- Iwamatsu, T. Stages of Normal Development in the Medaka Oryzias Latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Yonekura, M.; Kondoh, N.; Han, C.; Toyama, Y.; Ohba, T.; Ono, K.; Itagaki, S.; Tomita, H.; Murakami, M. Medaka as a Model for ECG Analysis and the Effect of Verapamil. J. Pharmacol. Sci. 2018, 137, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT System in Skin Homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The Role of Metallothionein in Oxidative Stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Cosman, D. A Family of Ligands for the TNF Receptor Superfamily. Stem Cells 1994, 12, 440–455. [Google Scholar] [CrossRef]
- Korsmeyer, S.J. BCL-2 Gene Family and the Regulation of Programmed Cell Death. Cancer Res. 1999, 59 (Suppl. S7), 1693s–1700s. [Google Scholar] [CrossRef] [PubMed]
- Dagher, Z.; Garçon, G.; Billet, S.; Gosset, P.; Ledoux, F.; Courcot, D.; Aboukais, A.; Shirali, P. Activation of Different Pathways of Apoptosis by Air Pollution Particulate Matter (PM2.5) in Human Epithelial Lung Cells (L132) in Culture. Toxicology 2006, 225, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate Matter Toxicity Is Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2020, 33, 1110–1120. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol Prevents PM2.5-Induced Adiposity and Insulin Resistance by Restraining Oxidative Stress Related NF-ΚB Pathway and Modulation of Gut Microbiota in a Murine Model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Mikhal’chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular Mechanisms Underlying Wound Healing and Anti-Inflammatory Properties of Naturally Occurring Biotechnologically Produced Phenylpropanoid Glycosides. Cell Mol. Biol. 2007, 53, 84–91. [Google Scholar] [PubMed]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside Protects Endothelial Cells against Free Radical-Induced Oxidative Stress†. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of Acteoside on Lipopolysaccharide-Induced Inflammation in Acute Lung Injury via Regulation of NF-ΚB Pathway in Vivo and in Vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef]
- Khorashadizadeh, N.; Neamati, A.; Moshiri, M.; Etemad, L. Verbascoside Inhibits Paraquate-Induced Pulmonary Toxicity via Modulating Oxidative Stress, Inflammation, Apoptosis and DNA Damage in A549 Cell. Drug Chem. Toxicol. 2022, 45, 2212–2220. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Futur. Sci. OA 2018, 4. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Hoskin, R.; Pambianchi, E.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Valacchi, G.; Lila, M.A. Novel Spray Dried Algae-Rosemary Particles Attenuate Pollution-Induced Skin Damage. Molecules 2021, 26, 3781. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and Traditional Uses, Phytochemistry, and Pharmacology of Sophora japonica L.: A Review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Shin, S.; Park, D.; Byun, S.; Jung, E. Protective Effects of Camellia Japonica Flower Extract against Urban Air Pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef]
- Durga, M.; Vijayakumar, M.; Priya, K.; Vidhya Kanagarajan, S.; Banu, B.B.; Abraham, V.S.M.; Devasena, T.; Abdelaziz, M.A.; Mohideen, A.P.; Bahakim, N.O.; et al. Effects of Quercetin on Ultrafine Petrol Exhaust Nanoparticles Induced DNA Damage, Oxidative Stress and Inflammation in Different Sections of Rat Brain. J. King Saud Univ.—Sci. 2022, 34, 101813. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; González-Acedo, A.; Illescas-Montes, R.; García-Recio, E.; Ramos-Torrecillas, J.; Costela-Ruiz, V.J.; García-Martínez, O. Biological Effects of the Olive Tree and Its Derivatives on the Skin. Food Funct. 2022, 13, 11410–11424. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Watanabe, G.; Taya, K.; Sagai, M. Inhibitory Effects of Foods and Polyphenols on Activation of Aryl Hydrocarbon Receptor Induced by Diesel Exhaust Particles. Environ. Sci. 2007, 14, 149–156. [Google Scholar] [PubMed]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant Polyphenols Differentially Modulate Inflammatory Responses of Human Keratinocytes by Interfering with Activation of Transcription Factors NFκB and AhR and EGFR-ERK Pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Guttenplan, J.B.; Kochhar, A.; Zhao, Z.-L.; Kosinska, W.; Subbaramaiah, K.; Dannenberg, A.J. Carnosol, a Constituent of Zyflamend, Inhibits Aryl Hydrocarbon Receptor-Mediated Activation of CYP1A1 and CYP1B1 Transcription and Mutagenesis. Cancer Prev. Res. 2012, 5, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.-R.; Hsu, J.-Y.; Tseng, C.-Y.; Chen, J.-H.; Lin, H.-H. The Inhibitory Effect of Quercetin-3-Glucuronide on Pulmonary Injury in Vitro and in Vivo. J. Food Drug Anal. 2023, 31, 254–277. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Tool, T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Sharma, K.; Lee, H.-H.; Gong, D.-S.; Park, S.-H.; Yi, E.; Schini-Kerth, V.; Oak, M.-H. Fine Air Pollution Particles Induce Endothelial Senescence via Redox-Sensitive Activation of Local Angiotensin System. Environ. Pollut. 2019, 252, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic Treatment with N-Acetyl-Cystein Delays Cellular Senescence in Endothelial Cells Isolated from a Subgroup of Atherosclerotic Patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liang, J.; Wang, J.; Yin, B.; Zhang, F.; Li, X.; Zhu, S.; Tian, H.; Cui, Q.; Song, J.; et al. Vascular Benefits of Vitamin C Supplementation against Fine Particulate Air Pollution in Healthy Adults: A Double-Blind Randomised Crossover Trial. Ecotoxicol. Environ. Saf. 2022, 241, 113735. [Google Scholar] [CrossRef]
- Fu, H.; Wang, L.; Wang, J.; Bennett, B.D.; Li, J.-L.; Zhao, B.; Hu, G. Dioxin and AHR Impairs Mesoderm Gene Expression and Cardiac Differentiation in Human Embryonic Stem Cells. Sci. Total Environ. 2019, 651, 1038–1046. [Google Scholar] [CrossRef]
- Lanham, K.A.; Plavicki, J.; Peterson, R.E.; Heideman, W. Cardiac Myocyte-Specific AHR Activation Phenocopies TCDD-Induced Toxicity in Zebrafish. Toxicol. Sci. 2014, 141, 141–154. [Google Scholar] [CrossRef]
- Liang, J.; Wu, M.; Chen, C.; Mai, M.; Huang, J.; Zhu, P.; Agnetti, G. Roles of Reactive Oxygen Species in Cardiac Differentiation, Reprogramming, and Regenerative Therapies. Oxid. Med. Cell. Longev. 2020, 2020, 2102841. [Google Scholar] [CrossRef]
- Ren, F.; Ji, C.; Huang, Y.; Aniagu, S.; Jiang, Y.; Chen, T. AHR-Mediated ROS Production Contributes to the Cardiac Developmental Toxicity of PM2.5 in Zebrafish Embryos. Sci. Total Environ. 2020, 719, 135097. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Zou, H.; Aniagu, S.; Jiang, Y.; Chen, T. Synergistic Protective Effects of Folic Acid and Resveratrol against Fine Particulate Matter-Induced Heart Malformations in Zebrafish Embryos. Ecotoxicol. Environ. Saf. 2022, 241, 113825. [Google Scholar] [CrossRef]
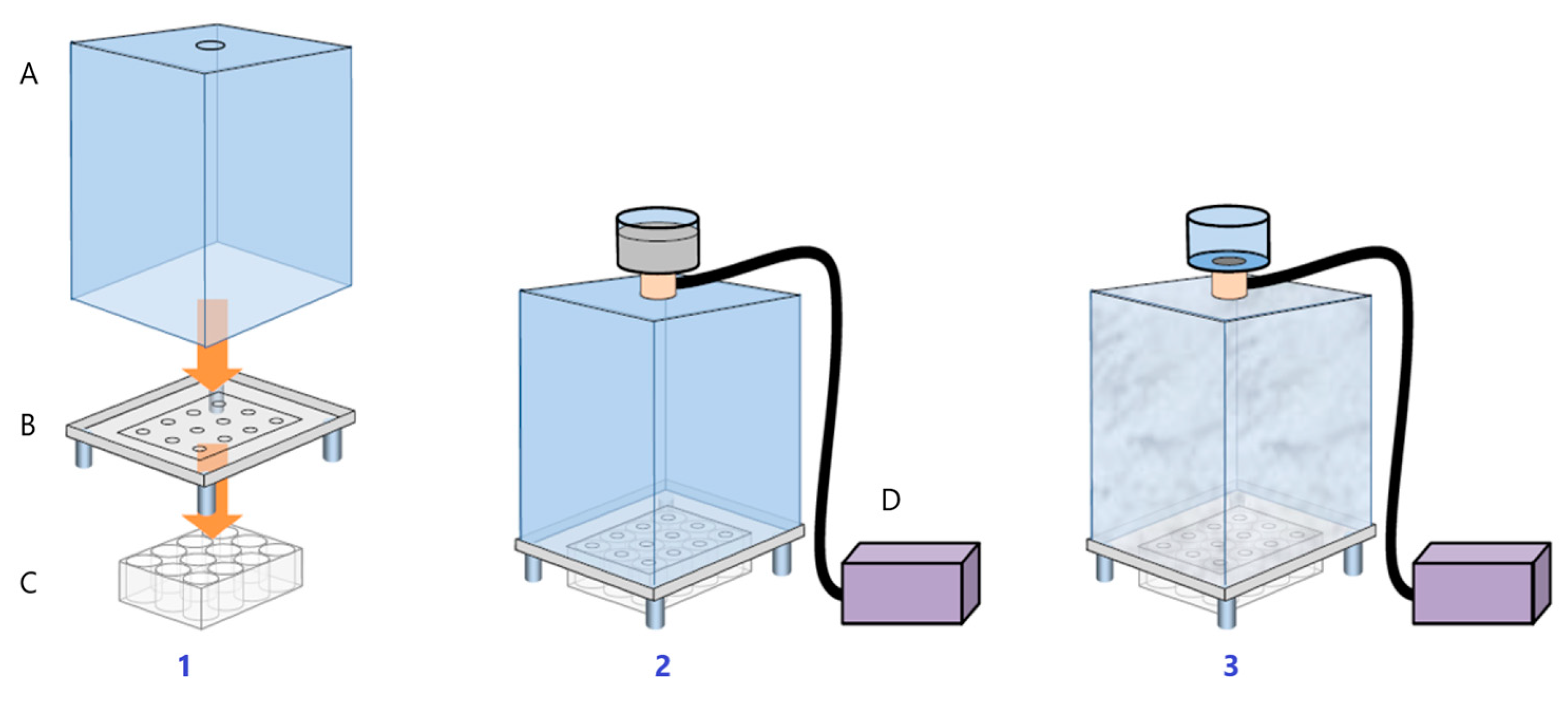



| Batch | Treatment |
Pollutants Exposure |
Number of Explants |
Sampling Time |
|---|---|---|---|---|
| T0 | Untreated control | − | 3 | Day 0 |
| T | Untreated control | − | 4 | Day 3 |
| ZP | ZP treatment | − | 4 | Day 3 |
| P | Pollution treatment | + | 4 | Day 3 |
| PZP | Pollution + ZP | + | 4 | Day 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peno-Mazzarino, L.; Radionov, N.; Merino, M.; González, S.; Mullor, J.L.; Jones, J.; Caturla, N. Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study. Curr. Issues Mol. Biol. 2024, 46, 1530-1555. https://doi.org/10.3390/cimb46020099
Peno-Mazzarino L, Radionov N, Merino M, González S, Mullor JL, Jones J, Caturla N. Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study. Current Issues in Molecular Biology. 2024; 46(2):1530-1555. https://doi.org/10.3390/cimb46020099
Chicago/Turabian StylePeno-Mazzarino, Laurent, Nikita Radionov, Marián Merino, Sonia González, José L. Mullor, Jonathan Jones, and Nuria Caturla. 2024. "Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study" Current Issues in Molecular Biology 46, no. 2: 1530-1555. https://doi.org/10.3390/cimb46020099
APA StylePeno-Mazzarino, L., Radionov, N., Merino, M., González, S., Mullor, J. L., Jones, J., & Caturla, N. (2024). Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study. Current Issues in Molecular Biology, 46(2), 1530-1555. https://doi.org/10.3390/cimb46020099







