Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model
Abstract
1. Introduction
2. Materials and Methods
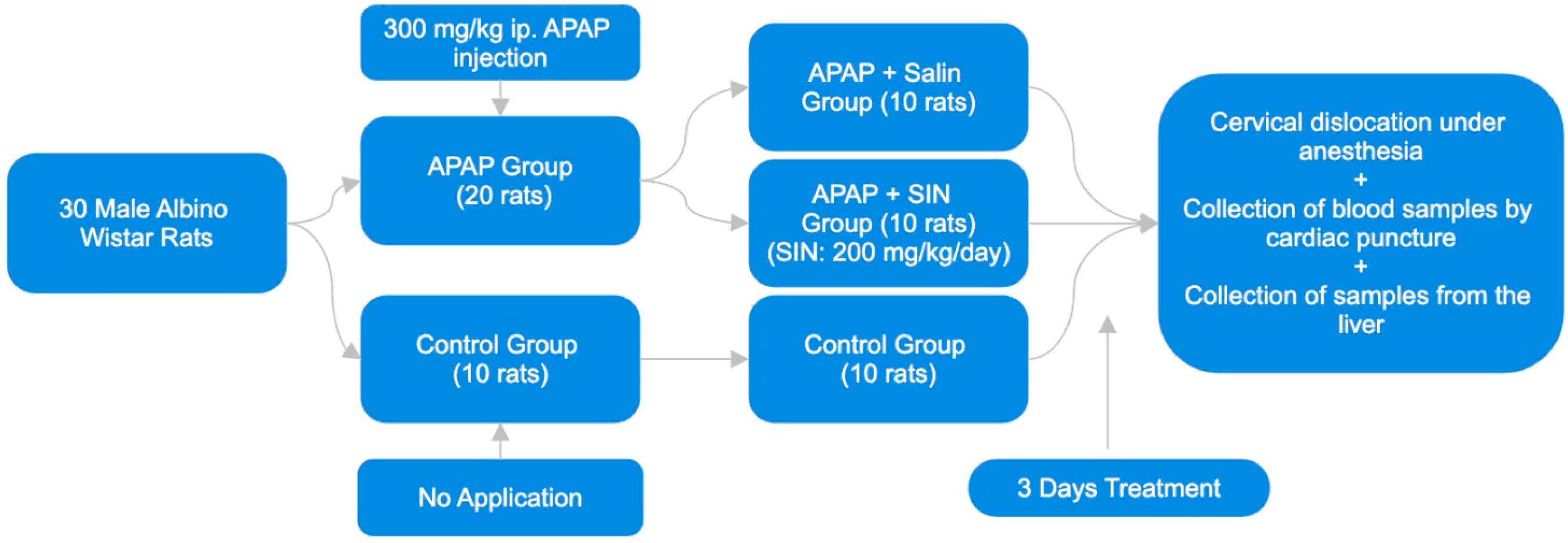
2.1. Animals
2.2. Experimental Design
2.3. Histopathological Studies of the Liver
2.4. Determination of Plasma ALT Level
2.5. Liver Biochemical Analysis
2.6. Assessment of Hepatic Lipid Peroxidation
2.7. Quantification of Hepatic Glutathione (GSH) Concentrations
2.8. Statistical Analysis
3. Results
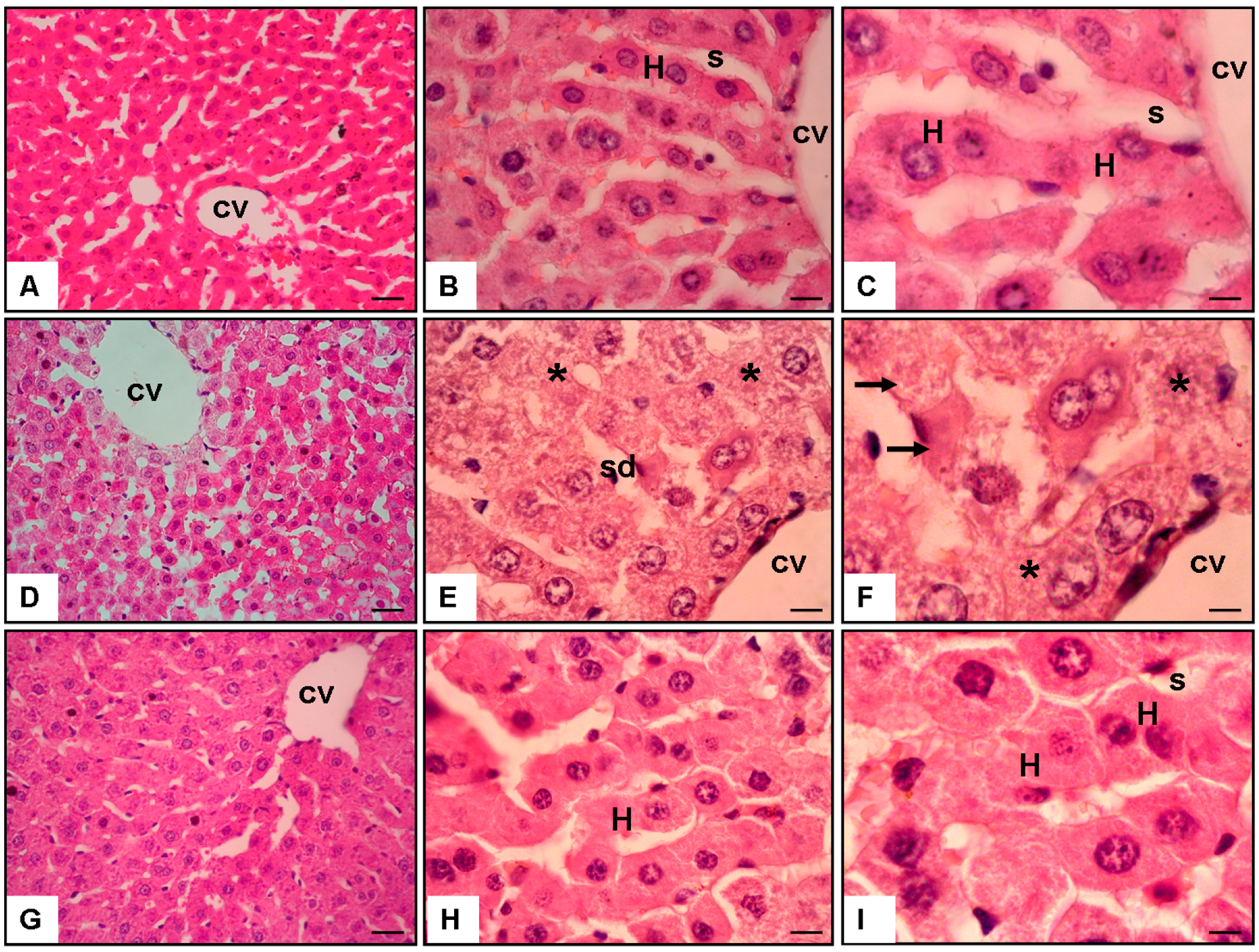
3.1. Analysis of Histopathology Evaluation
3.1.1. Normal Group (Figure 2A–C)
3.1.2. APAP + Saline Group (Figure 2D–F)
3.1.3. APAP + 200 mg/kg/day of SIN Group (Figure 2G–I)
3.2. Biochemical Indicators of Liver Damage
3.2.1. Alanine Aminotransferase (ALT)
3.2.2. Lipid Peroxidation (MDA Level)
3.2.3. Glutathione (GSH) Level
3.2.4. Sirtuin 1 (SIRT1) Level
3.2.5. Bone Morphogenetic Protein-7 (BMP-7) Level
3.2.6. Damaged Hepatocytes Percentage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, N.J.; Royer, A.; John, S. Acute Liver Failure; StatPearls: Treasure Island, FL, USA, 2023; p. 7. [Google Scholar]
- Ingawale, D.K.; Mandlik, S.K.; Naik, S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): A critical discussion. Environ. Toxicol. Pharmacol. 2014, 31, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of Acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar]
- Schilling, A.; Corey, R.; Leonard, M.; Eghtesad, B. Acetaminophen: Old drug, new warnings. Clevel. Clin. J. Med. 2010, 77, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.G.; Eastham, W.N. Acute liver necrosis following overdose of paracetamol. Br. Med. J. 1966, 2, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, M.J.; Knapp, G.L.; Kulig, K.W.; Rumack, B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 1988, 319, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef]
- Saad, M.A.; Rastanawi, A.A.; El-Yamany, M.F. Alogliptin abates memory injuries of hepatic encephalopathy induced by acute APAPintoxication via switching-off autophagy-related apoptosis. Life Sci. 2018, 215, 11–21. [Google Scholar] [CrossRef]
- Sener, G.; Sehirli, O.; Cetinel, S.; Yeğen, B.G.; Gedik, N.; Ayanoğlu-Dülger, G. Protective effects of MESNA (2-mercaptoethane sulphonate) against acetaminophen-induced hepatorenal oxidative damage in mice. J. Appl. Toxicol. 2005, 25, 20–29. [Google Scholar] [CrossRef]
- Moyer, A.M.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.J.; Pelleymounter, L.L.; Kalari, K.R.; Ji, Y.; Chai, Y.; Nordgren, K.K.; Weinshilboum, R.M. Acetaminophen-NAPQI hepatotoxicity: A cell line model system genome-wide association study. Toxicol. Sci. 2011, 120, 33–41. [Google Scholar] [CrossRef]
- Feng, H.; Yamaki, K.; Takano, H.; Inoue, K.; Yanagisawa, R.; Yoshino, S. Suppression of Th1 and Th2 immune responses in mice by Sinomenine, an alkaloid extracted from the chinese medicinal plant Sinomenium acutum. Planta Med. 2006, 72, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Yao, R.B.; Zhao, L.J.; Shen, S.Y.; Zhao, Z.M.; Cai, H. Sinomenine decreases MyD88 expression and improves inflammation-induced joint damage progression and symptoms in rat adjuvant-induced arthritis. Inflammation 2013, 36, 1136–1144. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.X.; Luo, J.F.; Cheng, C.S.; Leung, E.L.; Li, Y.; Su, X.H.; Liu, Z.Q.; Chen, T.B.; Duan, F.G.; et al. Suppressing mPGES-1 expression by Sinomenine ameliorates inflammation and arthritis. Biochem. Pharmacol. 2017, 142, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Huang, W.; Zhou, X.; Wang, M.; Zhong, B.; Peng, D. Effect of Sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J. Ethnopharmacol. 2005, 98, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Pharmacology of Sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med. Okayama. 1976, 30, 1–20. [Google Scholar]
- Qian, L.; Xu, Z.; Zhang, W.; Wilson, B.; Hong, J.S.; Flood, P.M. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J. Neuroinflammation 2007, 4, 23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Li, R. [Study progress in Sinomenium acutum (Thunb.) Rehd. et Wils]. Zhong Yao Cai 2002, 25, 209–211. [Google Scholar]
- Wang, Q.; Li, X.K. Immunosuppressive and anti-inflammatory activities of Sinomenine. Int. Immunopharmacol. 2011, 11, 373–376. [Google Scholar] [CrossRef]
- Kondo, Y.; Takano, F.; Yoshida, K.; Hojo, H. Protection by Sinomenine against endotoxin-induced fulminant hepatitis in galactosamine-sensitized mice. Biochem. Pharmacol. 1994, 48, 1050–1052. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Aluganti Narasimhulu, C.; Singla, D.K. The Role of Bone Morphogenetic Protein 7 (BMP-7) in Inflammation in Heart Diseases. Cells 2020, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Yang, C.; LeBleu, V.S.; Soubasakos, M.A.; Giraldo, M.; Zeisberg, M.; Kalluri, R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007, 21, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N. Liver regeneration: From laboratory to clinic. Liver Transpl. 2001, 7, 835–844. [Google Scholar] [CrossRef]
- Wheatcroft, S.B.; Kearney, M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: Implications for metabolic homeostasis. Trends Endocrinol. Metab. 2009, 20, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Salacinski, H.J.; Yang, W.; Davidson, B.R.; Seifalian, A.M. The current role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: A review. Liver Transpl. 2005, 11, 1031–1047. [Google Scholar] [CrossRef]
- Halladin, N.L. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan. Med. J. 2015, 62, B5054. [Google Scholar]
- Mobasher, M.A.; Valverde, Á.M. Signaling pathways involved in paracetamol-induced hepatotoxicity: New insights on the role of protein tyrosine phosphatase 1B. Arch. Physiol. Biochem. 2014, 120, 51–63. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.L.; Ding, B.X. Inhibitory effect of Sinomenine on H2O2-induced apoptosis in neonatal rat cardiomyocytes. Zhongguo Zhong Yao Za Zhi 2008, 33, 939–941. [Google Scholar] [PubMed]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Tashakori-Miyanroudi, M.; Nourabadi, D.; Nazari-Serenjeh, M.; Roghani, M.; Baluchnejadmojarad, T. Neuroprotective and anticonvulsant effects of Sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 2020, 108, 101800. [Google Scholar] [CrossRef] [PubMed]
- Lin, F. Protective effect of sinomenine on ischemia-reperfusion injury during orthotopic liver transplantation in rats. Acad. J. Second. Mil. Med. Univ. 2010, 1433–1437. [Google Scholar]
- Li, Q.; Chen, F.; Wang, F. The immunological mechanisms and therapeutic potential in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Cell Biosci. 2022, 12, 187. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Q.; Li, M.; Su, J.; Wang, P.; Wang, X.; Yin, Y.; Wang, X.; Zhao, M. Sinomenine Suppresses Development of Hepatocellular Carcinoma Cells via Inhibiting MARCH1 and AMPK/STAT3 Signaling Pathway. Front. Mol. Biosci. 2021, 8, 684262. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, J.; Shen, X.; Zhu, H.; Sun, X.; Wen, X.; Bian, J.; Hu, H.; Yuan, L.; Tao, J.; et al. Sinomenine hydrochloride enhancement of the inhibitory effects of anti-transferrin receptor antibody-dependent on the COX-2 pathway in human hepatoma cells. Cancer Immunol. Immunother. 2013, 62, 447–454. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Jiao, F.Z.; Yang, F.; Li, X.; Wang, L.W. Sinomenine Attenuates Acetaminophen-Induced Acute Liver Injury by Decreasing Oxidative Stress and Inflammatory Response via Regulating TGF-β/Smad Pathway in vitro and in vivo. Drug Des. Dev. Ther. 2020, 17, 2393–2403. [Google Scholar] [CrossRef]
- Sharma, A.; Gautam, V.; Costantini, S.; Paladino, A.; Colonna, G. Interactomic and pharmacological insights on human sirt-1. Front. Pharmacol. 2012, 23, 40. [Google Scholar] [CrossRef]
- El-Sheikh, M.M.; Abdel-Naby, D.H.; El-Hazek, R.M.; El-Ghazaly, M.A. Regulation of radiation-induced liver damage by modulation of SIRT-1 activity: In vivo rat model. Cell Biochem. Funct. 2023, 41, 67–77. [Google Scholar] [CrossRef]
- Biel, T.G.; Lee, S.; Flores-Toro, J.A.; Dean, J.W.; Go, K.L.; Lee, M.H.; Law, B.K.; Law, M.E.; Dunn, W.A., Jr.; Zendejas, I.; et al. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. 2016, 23, 279–290. [Google Scholar] [CrossRef]
- Yan, T.; Huang, J.; Nisar, M.F.; Wan, C.; Huang, W. The Beneficial Roles of SIRT1 in Drug-Induced Liver Injury. Oxid. Med. Cell Longev. 2019, 1, 8506195. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Salem, A.A.; Eassawy, M.M. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J. Photochem. Photobiol. B 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Dong, J.Z.; Xiong, L.J.; Shi, K.Q.; Zou, Z.L.; Zhang, S.N.; Cao, S.T.; Lin, Z.; Chen, Y.P. BMP-7 attenuates liver fibrosis via regulation of epidermal growth factor receptor. Int. J. Clin. Exp. Pathol. 2014, 7, 3537–3547. [Google Scholar] [PubMed]
- Cao, H.; Shu, X.; Chen, L.B.; Zhang, K.; Xu, Q.H.; Li, G. The relationship of expression of BMP-7 in the liver and hepatic inflammation and fibrosis in patients with chronic HBV infection. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2010, 24, 101–103. [Google Scholar]
- Zou, G.L.; Zuo, S.; Lu, S.; Hu, R.H.; Lu, Y.Y.; Yang, J.; Deng, K.S.; Wu, Y.T.; Mu, M.; Zhu, J.J.; et al. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J. Gastroenterol. 2019, 25, 4222–4234. [Google Scholar] [CrossRef]
| Normal Group | APAP + Saline | APAP + 200 mg/kg/day of Sinomenine | |
|---|---|---|---|
| ALT (U/L) | 36.7 ± 1.9 | 183.5 ± 5.7 ** | 62.7 ± 6.4 ## |
| Liver MDA Level (nmol/mg protein) | 1.25 ± 0.2 | 4.7 ± 0.1 * | 1.8 ± 0.2 ## |
| Liver GSH Level (nmol/mg protein) | 6.9 ± 0.2 | 1.4 ± 0.2 ** | 4.7 ± 0.1 ## |
| Liver SIRT1 Level (pg/g protein) | 2.1 ± 0.2 | 1.4 ± 0.1 * | 1.9 ± 0.1 # |
| Liver BMP-7 Level (pg/g protein) | 55.6 ± 2.8 | 50.8 ± 4.4 | 73.6 ± 3.5 # |
| Damaged hepatocytes (percent) | 2.7 ± 0.1 | 74.1 ± 6.9 ** | 13.3 ± 2.8 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayalı, A.; Bora, E.S.; Acar, H.; Erbaş, O. Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model. Curr. Issues Mol. Biol. 2024, 46, 923-933. https://doi.org/10.3390/cimb46010059
Kayalı A, Bora ES, Acar H, Erbaş O. Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model. Current Issues in Molecular Biology. 2024; 46(1):923-933. https://doi.org/10.3390/cimb46010059
Chicago/Turabian StyleKayalı, Ahmet, Ejder Saylav Bora, Hüseyin Acar, and Oytun Erbaş. 2024. "Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model" Current Issues in Molecular Biology 46, no. 1: 923-933. https://doi.org/10.3390/cimb46010059
APA StyleKayalı, A., Bora, E. S., Acar, H., & Erbaş, O. (2024). Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model. Current Issues in Molecular Biology, 46(1), 923-933. https://doi.org/10.3390/cimb46010059






