The Identification of New c-FLIP Inhibitors for Restoring Apoptosis in TRAIL-Resistant Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Modeling
2.1.1. The Homology Modeling of DED2s
2.1.2. The Identification of the Binding Site
2.1.3. The Docking of Chemical Libraries
2.1.4. Consensus Scoring Function
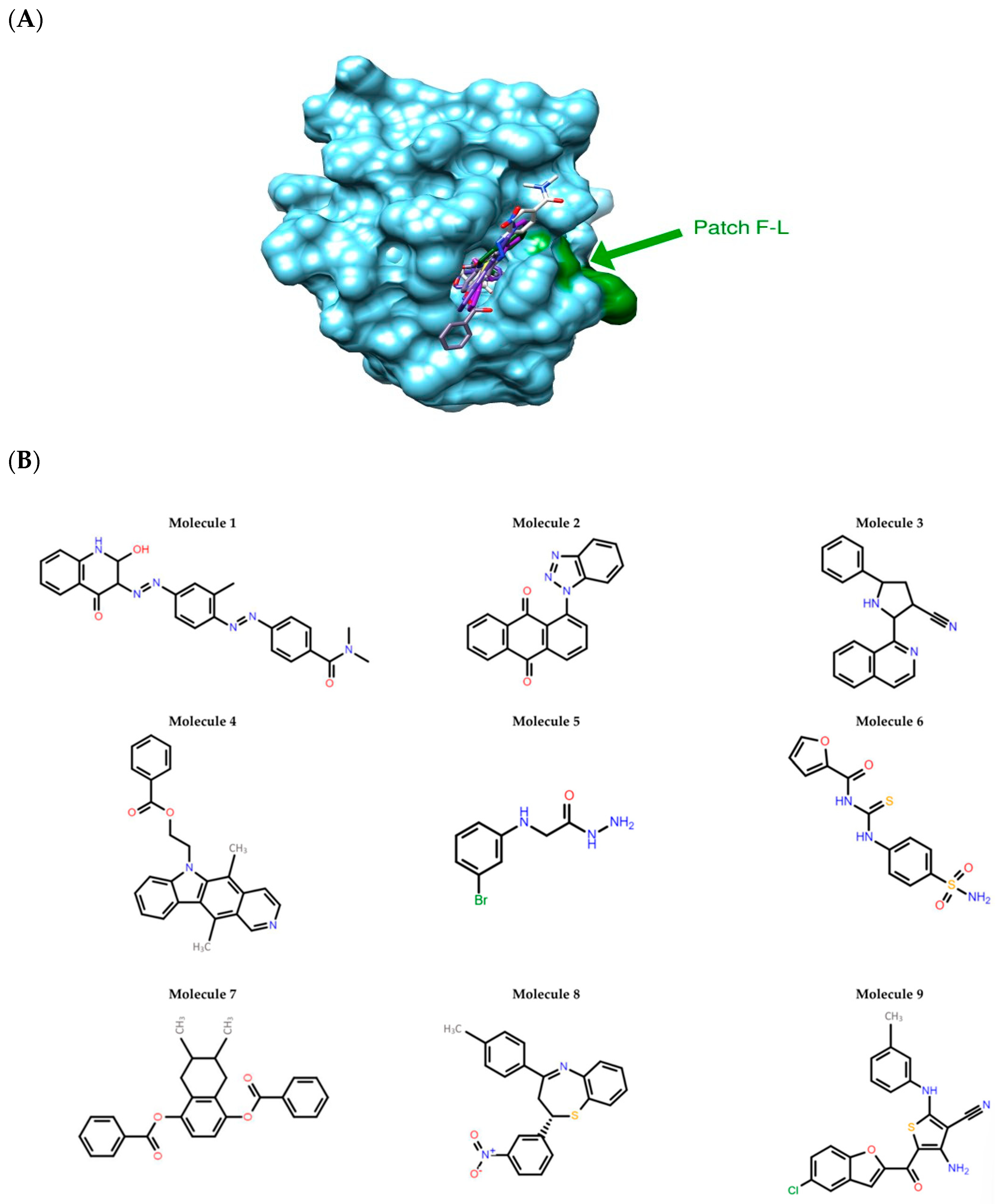
2.1.5. Hit Selection
2.2. Cell Culture
2.3. Reagents and Antibodies
2.4. Flow Cytometry Analysis
2.5. Recombinant Protein Production and Purification
2.6. Pull-Down Binding Assay
2.7. DISC Immunoprecipitation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Potential c-FLIP Inhibitors Selected by In Silico Screening
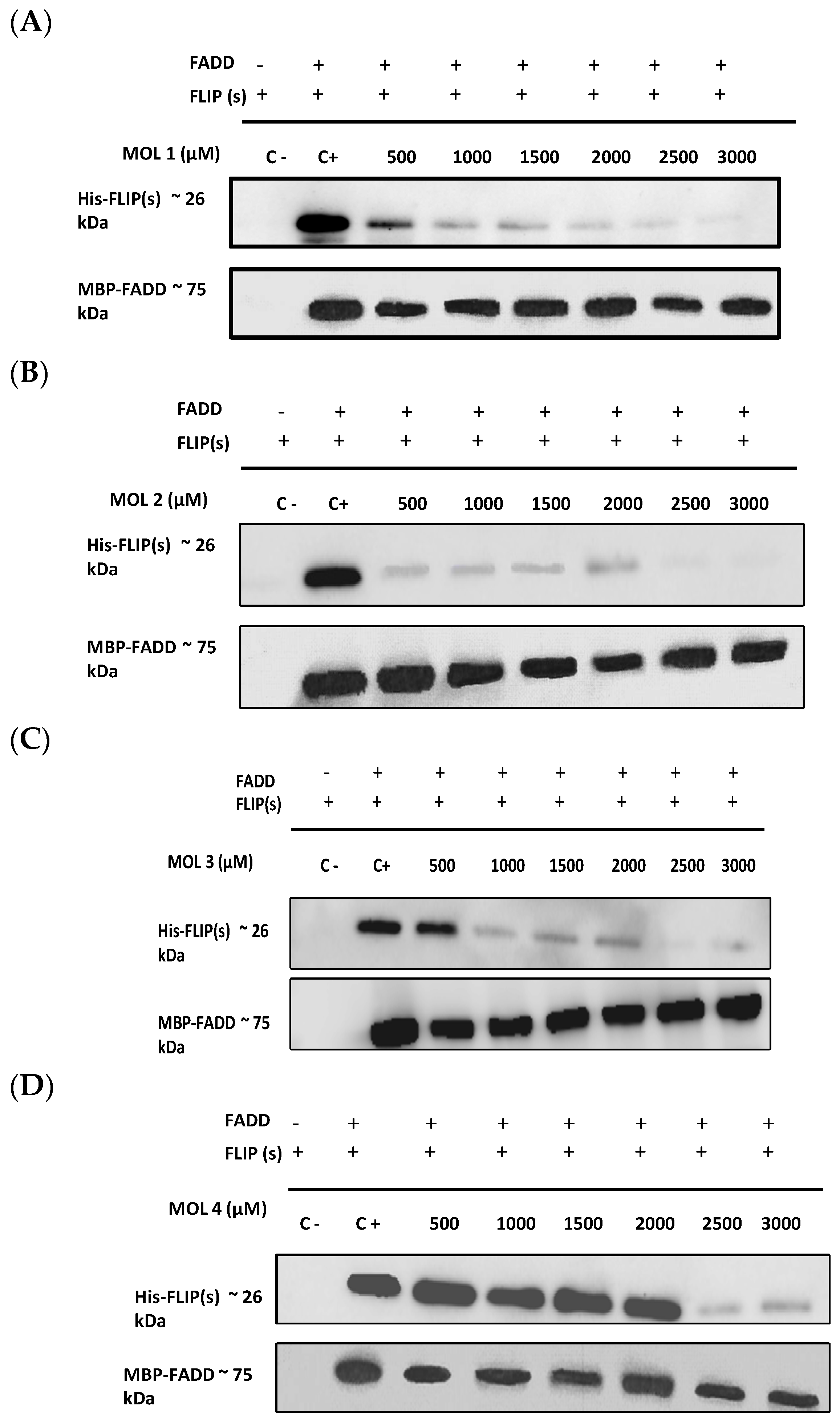
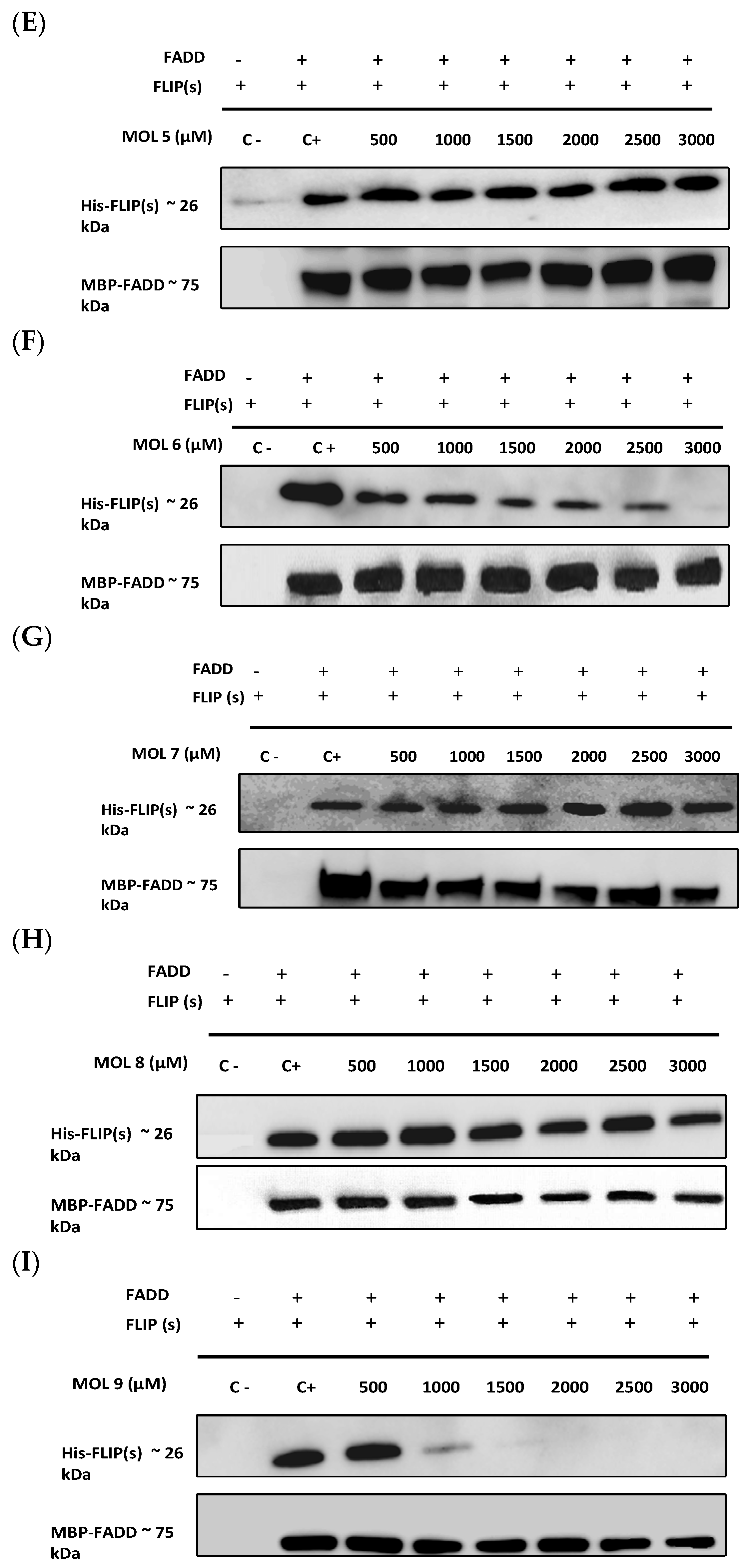
3.2. The Newly Identified c-FLIP Inhibitors Prevent DED–FADD/DED2–c-FLIP Interactions
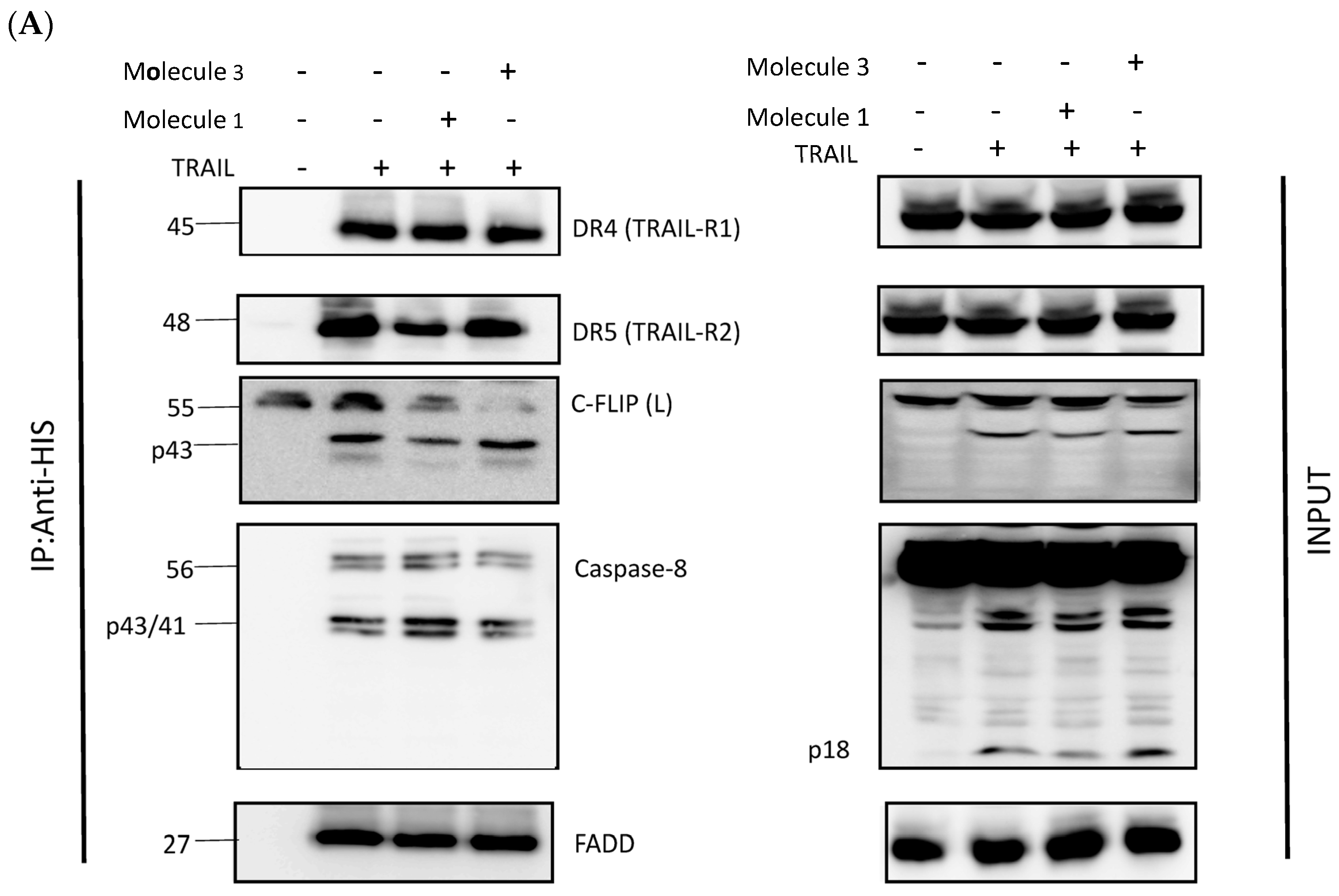
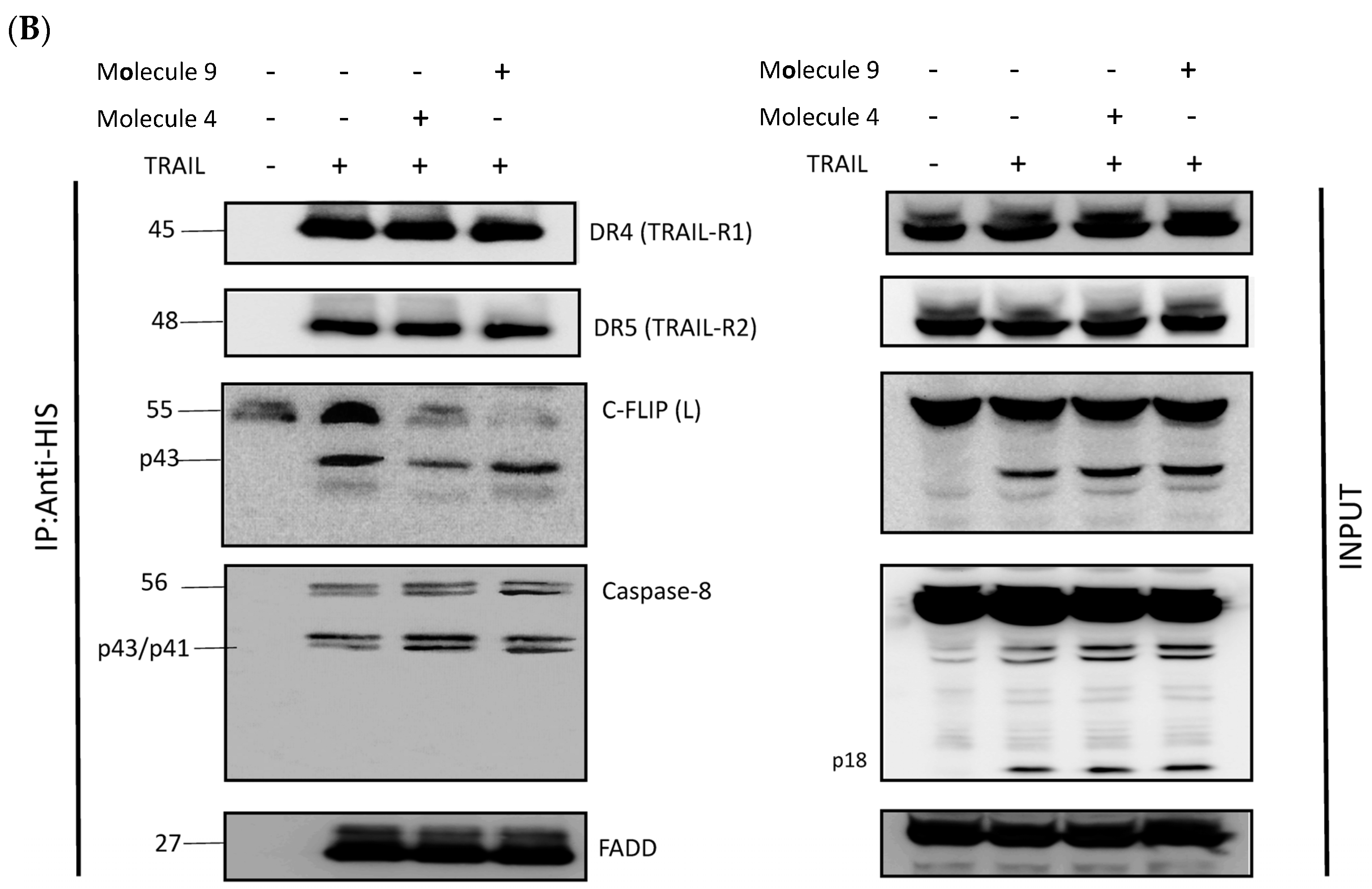
3.3. Newly Identified Molecules Inhibit c-FLIP Recruitment into the DISC by Preventing Its Interaction with the FADD
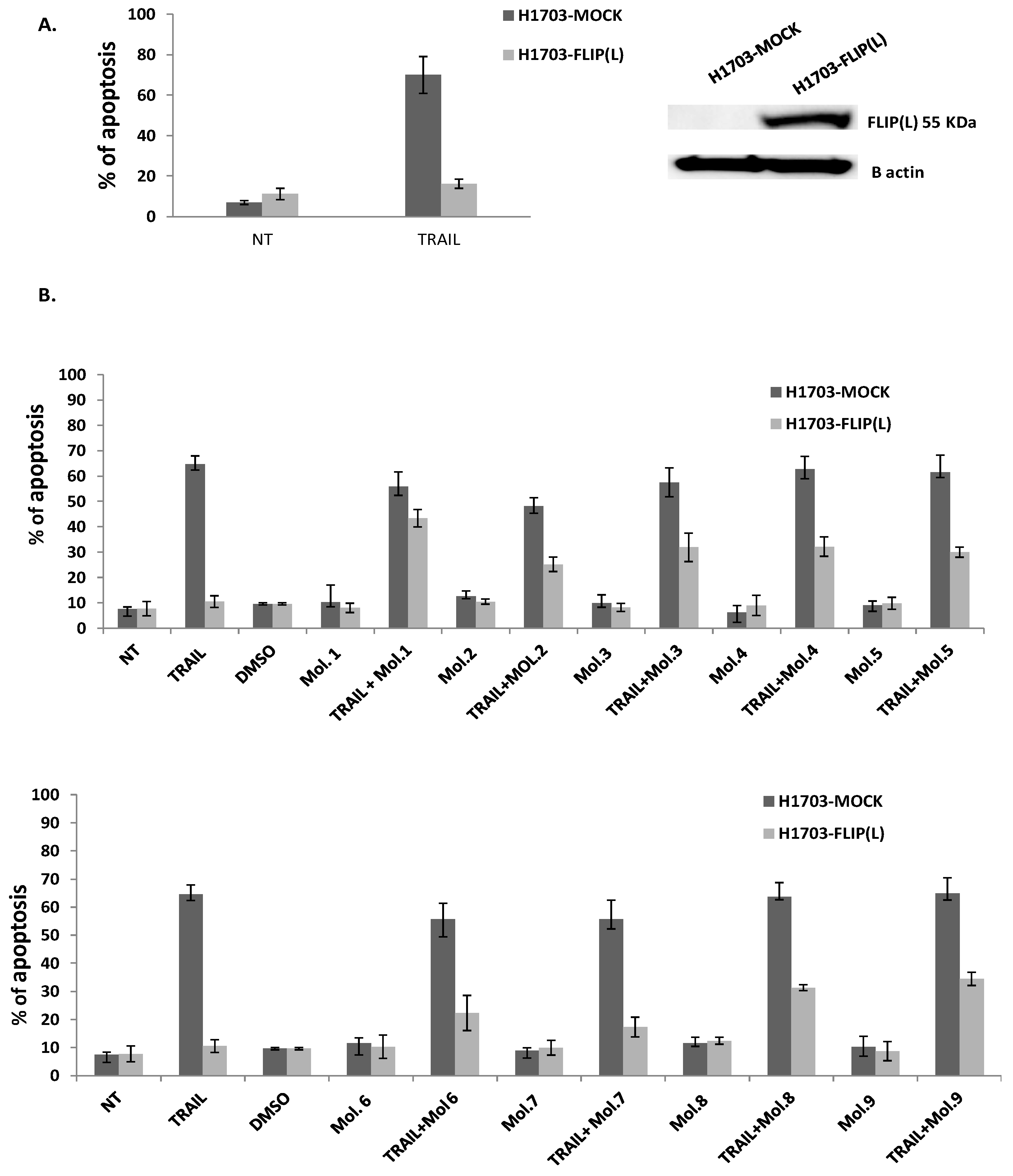
3.4. c-FLIP Inhibitors Rescue TRAIL-Mediated Apoptosis
3.5. A Combination Treatment of TRAIL with c-FLIP-Targeting Molecules Restores Caspases-Dependent Apoptosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberti, A.; La Sala, D.; Cinti1, C. Multiple Genetic and Epigenetic Interacting Mechanisms Contribute to Clonally Selection of Drug-Resistant Tumors: Current Views and New Therapeutic Prospective. J. Cell. Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef]
- Safa, A.R. Identification and characterization of the binding sites of P-glycoprotein for multidrug resistance-related drugs and modulators. Curr. Med. Chem. 2004, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stover, E.H.; Baco, M.B.; Cohen, O.; Li, Y.Y.; Christie, E.L.; Bagul, M.; Goodale, A.; Lee, Y.; Pantel, S.; Rees, M.G.; et al. Pooled genomic screens identify anti-apoptotic genes as targetable mediators of chemotherapy resistance in ovarian cancer. Mol. Cancer Res. 2019, 17, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.-Y.; Mihich, E.; Berleth, E.S. TNF Apoptosis Protection Fraction (TAPF) prevents apoptosis induced by TNF, but not by Fas or TRAIL, via NF-κB-induced increase in cFLIP. Cytokine 2015, 75, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mezzanzanica, D.; Balladore, E.; Turatti, F.; Luison, E.; Alberti, P.; Bagnoli, M.; Figini, M.; Mazzoni, A.; Raspagliesi, F.; Oggionni, M.; et al. CD95-mediated apoptosis is impaired at receptor level by cellular FLICE-inhibitory protein (long form) in wild-type p53 human ovarian carcinoma. Clin. Cancer Res. 2004, 10, 5202–5214. [Google Scholar] [CrossRef]
- Longley, D.B.; Wilson, T.R.; McEwan, M.; Allen, W.L.; McDermott, U.; Galligan, L.; Johnston, P.G. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 2006, 25, 838–848. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jung, G.A.; Hur, G.C.; Chung, H.Y.; Kim, W.H.; Seol, D.W.; Lee, B.L. Upregulation of FLIPs by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003, 94, 1066–1073. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, T.; Yang, H.; Dewolf, W.C.; Khosravi-far, R.; Olumi, A.F. Persistent c-FLIP (L) Expression Is Necessary and Sufficient to Maintain Resistance to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand—Mediated Apoptosis in Prostate Cancer Sensitivity of Prostate Cancer Cells to Recombinant Human. Cancer Res. 2004, 64, 7086–7091. [Google Scholar] [CrossRef]
- Brambilla, C.; Brambilla, E.; Gazzeri, S. E2F1 induces apoptosis and sensitizes human lung adenocarcinoma cells to death-receptor-mediated apoptosis through specific downregulation of c-FLIPshort. Cell Death Differ. 2006, 13, 260–272. [Google Scholar] [CrossRef]
- Wilson, N.S.; Dixit, V.; Ashkenazi, A. Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 2009, 10, 348–355. [Google Scholar] [CrossRef]
- Bullani, R.R.; Huard, B.; Viard-Leveugle, I.; Byers, H.R.; Irmler, M.; Saurat, J.H.; Tschopp, J.; French, L.E. Selective expression of FLIP in malignant melanocytic skin lesions. J. Investig. Dermatol. 2001, 117, 360–364. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.; Harper, N.; Snowden, R.T.; Dyer, M.J.S.; Barnett, G.A.; Pringle, J.H.; Cohen, G.M. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene 2002, 21, 6809–6818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathas, S.; Lietz, A.; Anagnostopoulos, I.; Hummel, F.; Wiesner, B.; Janz, M.; Jundt, F.; Hirsch, B.; Jöhrens-Leder, K.; Vornlocher, H.-P.; et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J. Exp. Med. 2004, 199, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O. Cellular FLICE-inhibitory protein: An attractive therapeutic target? Expert Opin. Ther. Targets 2003, 7, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Kamata, H.; Solinas, G.; Luo, J.-L.; Maeda, S.; Venuprasad, K.; Liu, Y.-C.; Karin, M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 2006, 124, 601–613. [Google Scholar] [CrossRef]
- Golks, A.; Brenner, D.; Fritsch, C.; Krammer, P.H.; Lavrik, I.N. c-FLIPR, a new regulator of death receptor-induced apoptosis. J. Biol. Chem. 2005, 280, 14507–14513. [Google Scholar] [CrossRef]
- Fu, Q.; Fu, T.-M.; Cruz, A.C.; Sengupta, P.; Thomas, S.K.; Wang, S.; Siegel, R.M.; Wu, H.; Chou, J.J. Structural Basis and Functional Role of Intramembrane Trimerization of the Fas/CD95 Death Receptor. Mol. Cell 2016, 61, 602–613. [Google Scholar] [CrossRef]
- Carrington, P.E.; Sandu, C.; Wei, Y.; Hill, J.M.; Morisawa, G.; Huang, T.; Gavathiotis, E.; Wei, Y.; Werner, M.H. The Structure of FADD and Its Mode of Interaction with Procaspase-8. Mol. Cell 2006, 22, 599–610. [Google Scholar] [CrossRef]
- Rasper, D.M.; Vaillancourt, J.P.; Hadano, S.; Houtzager, V.M.; Seiden, I.; Keen, S.L.; Tawa, P.; Xanthoudakis, S.; Nasir, J.; Martindale, D.; et al. Cell death attenuation by “Usurpin”, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998, 5, 271–288. [Google Scholar] [CrossRef]
- Scaffidi, C.; Schmitz, I.; Krammer, P.H.; Peter, M.E. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 1999, 274, 1541–1548. [Google Scholar] [CrossRef]
- Mahdizadeh, S.J.; Thomas, M.; Eriksson, L.A. Reconstruction of the Fas-Based Death-Inducing Signaling Complex (DISC) Using a Protein–Protein Docking Meta-Approach. J. Chem. Inf. Model. 2021, 61, 3543–3558. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, F.C.; Screaton, G.R. Following a TRAIL: Update on a ligand and its five receptors. Cell Res. 2004, 14, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Greer, Y.E.; Dine, J.L.; Lipkowitz, S. Targeting TRAIL Death Receptors in Triple-Negative Breast Cancers: Challenges and Strategies for Cancer Therapy. Cells 2022, 11, 3717. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.Z.; Holohan, C.; Sessler, T.; Fox, J.; Crawford, N.; Riley, J.S.; Khawaja, H.; Majkut, J.; Evergren, E.; Humphreys, L.M.; et al. The SCFSkp2 ubiquitin ligase complex modulates TRAIL-R2-induced apoptosis by regulating FLIP(L). Cell Death Differ. 2020, 27, 2726–2741. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.A.; Lawrence, D.A.; Ashkenazi, A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 2005, 280, 19401–19409. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Brooks, A.D.; Erickson, K.L.; Thomas, C.L.; Bokesch, H.R.; Tewary, P.; Thompson, C.R.; Pompei, R.J.; Gustafson, K.R.; McMahon, J.B.; et al. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell Death Dis. 2015, 6, e1666. [Google Scholar] [CrossRef]
- Abedini, M.R.; Muller, E.J.; Brun, J.; Bergeron, R.; Gray, D.A.; Tsang, B.K. Cisplatin Induces p53-Dependent FLICE-like Inhibitory Protein Ubiquitination in Ovarian Cancer Cells. Cancer Res. 2008, 68, 4511–4517. [Google Scholar] [CrossRef]
- Olsson, A.; Diaz, T.; Aguilar-Santelises, M.; Osterborg, A.; Celsing, F.; Jondal, M.; Osorio, L.M. Sensitization to TRAIL-induced apoptosis and modulation of FLICE-inhibitory protein in B chronic lymphocytic leukemia by actinomycin D. Leukemia 2001, 15, 1868–1877. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11753607 (accessed on 13 November 2016). [CrossRef]
- Riley, J.S.; Malik, A.; Holohan, C.; Longley, D.B. DED or alive: Assembly and regulation of the death effector domain complexes. Cell Death Dis. 2015, 6, e1866. [Google Scholar] [CrossRef]
- Hughes, M.A.; Powley, I.R.; Jukes-Jones, R.; Horn, S.; Feoktistova, M.; Fairall, L.; Schwabe, J.W.R.; Leverkus, M.; Cain, K.; MacFarlane, M. Co-operative and Hierarchical Binding of c-FLIP and Caspase-8: A Unified Model Defines How c-FLIP Isoforms Differentially Control Cell Fate. Mol. Cell 2016, 61, 834–849. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Jeong, M.S.; Park, S.Y.; Jang, S.B. Evidence of complex formation between FADD and c-FLIP death effector domains for the death inducing signaling complex. BMB Rep. 2014, 47, 488–493. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24355299 (accessed on 6 March 2017). [CrossRef]
- Majkut, J.; Sgobba, M.; Holohan, C.; Crawford, N.; Logan, A.E.; Kerr, E.; Higgins, C.A.; Redmond, K.L.; Riley, J.S.; Stasik, I.; et al. Differential affinity of FLIP and procaspase 8 for FADD’s DED binding surfaces regulates DISC assembly. Nat. Commun. 2014, 5, 3350. [Google Scholar] [CrossRef] [PubMed]
- Morlé, A.; Garrido, C.; Micheau, O. Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion. Cell Death Dis. 2015, 6, e1633. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Komazawa-Sakon, S.; Nishina, T.; Koike, M.; Piao, J.-H.; Ehlken, H.; Kurihara, H.; Hara, M.; Van Rooijen, N.; Schütz, G.; et al. c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci. Signal. 2012, 5, ra93. [Google Scholar] [CrossRef] [PubMed]
- Travert, M.; Ame-Thomas, P.; Pangault, C.; Morizot, A.; Micheau, O.; Semana, G.; Lamy, T.; Fest, T.; Tarte, K.; Guillaudeux, T. CD40 ligand protects from TRAIL-induced apoptosis in follicular lymphomas through NF-kappaB activation and up-regulation of c-FLIP and Bcl-xL. J. Immunol. 2008, 181, 1001–1011. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18606651 (accessed on 30 November 2016). [CrossRef] [PubMed]
- Day, T.W.; Safa, A.R. RNA interference in cancer: Targeting the anti-apoptotic protein c-FLIP for drug discovery. Mini Rev. Med. Chem. 2009, 9, 741–748. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19519499 (accessed on 30 November 2016). [CrossRef] [PubMed]
- El-Zawahry, A.; McKillop, J.; Voelkel-Johnson, C. Doxorubicin increases the effectiveness of Apo2L/TRAIL for tumor growth inhibition of prostate cancer xenografts. BMC Cancer 2005, 5, 2. [Google Scholar] [CrossRef]
- Logue, S.E.; Martin, S.J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. 2008, 36, 1–9. [Google Scholar] [CrossRef]
- Shall, S.; de Murcia, G. Poly(ADP-ribose) polymerase-1: What have we learned from the deficient mouse model? Mutat. Res. 2000, 460, 1–15. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10856830 (accessed on 7 March 2017). [CrossRef]
- Pop, C.; Oberst, A.; Drag, M.; Van Raam, B.J.; Riedl, S.J.; Green, D.R.; Salvesen, G.S. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 2011, 433, 447–457. [Google Scholar] [CrossRef]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Gores, G.J. TRAIL resistance results in cancer progression: A TRAIL to perdition? Oncogene 2006, 25, 7333–7335. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.R.; Pollok, K.E. Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy. Cancers 2011, 3, 1639–1671. [Google Scholar] [CrossRef] [PubMed]
- Ullenhag, G.J.; Mukherjee, A.; Watson, N.F.S.; Al-Attar, A.H.; Scholefield, J.H.; Durrant, L.G. Overexpression of FLIPL Is an Independent Marker of Poor Prognosis in Colorectal Cancer Patients. Clin. Cancer Res. 2007, 13, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Galligan, L.; Longley, D.B.; McEwan, M.; Wilson, T.R.; McLaughlin, K.; Johnston, P.G. Chemotherapy and TRAIL-mediated colon cancer cell death: The roles of p53, TRAIL receptors, and c-FLIP. Mol. Cancer Ther. 2005, 4, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Shiraki, K.; Inoue, H.; Kawakita, T.; Yamanaka, T.; Deguchi, M.; Sugimoto, K.; Sakai, T.; Ohmori, S.; Fujikawa, K.; et al. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab. Investig. 2003, 83, 1033–1043. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12861043 (accessed on 28 February 2017). [CrossRef] [PubMed]
- Yang, J.K.; Wang, L.; Zheng, L.; Wan, F.; Ahmed, M.; Lenardo, M.J.; Wu, H. Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol. Cell 2005, 20, 939–949. [Google Scholar] [CrossRef]
- Scott, F.L.; Stec, B.; Pop, C.; Dobaczewska, M.K.; Lee, J.J.; Monosov, E.; Robinson, H.; Salvesen, G.S.; Schwarzenbacher, R.; Riedl, S.J. The Fas–FADD death domain complex structure unravels signalling by receptor clustering. Nature 2009, 457, 1019–1022. [Google Scholar] [CrossRef]
- Berglund, H.; Olerenshaw, D.; Sankar, A.; Federwisch, M.; McDonald, N.Q.; Driscoll, P.C. The three-dimensional solution structure and dynamic properties of the human FADD death domain. J. Mol. Biol. 2000, 302, 171–188. [Google Scholar] [CrossRef]
- Riley, J.S.; Hutchinson, R.; McArt, D.G.; Crawford, N.; Holohan, C.; Paul, I.; Van Schaeybroeck, S.; Salto-Tellez, M.; Johnston, P.G.; Fennell, D.A.; et al. Prognostic and therapeutic relevance of FLIP and procaspase-8 overexpression in non-small cell lung cancer. Cell Death Dis. 2013, 4, e951. [Google Scholar] [CrossRef]
- Shirley, S.; Micheau, O. Targeting c-FLIP in cancer. Cancer Lett. 2013, 332, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12887920 (accessed on 1 March 2017). [CrossRef] [PubMed]
- Zou, W.; Chen, S.; Liu, X.; Yue, P.; Sporn, M.B.; Khuri, F.R.; Sun, S.-Y. c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) in human lung cancer cells. Cancer Biol. Ther. 2007, 6, 1614–1620. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18253090 (accessed on 1 March 2017). [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacoub, K.; Pedeux, R.; Lafite, P.; Jarry, U.; Aci-Sèche, S.; Bonnet, P.; Daniellou, R.; Guillaudeux, T. The Identification of New c-FLIP Inhibitors for Restoring Apoptosis in TRAIL-Resistant Cancer Cells. Curr. Issues Mol. Biol. 2024, 46, 710-728. https://doi.org/10.3390/cimb46010046
Yaacoub K, Pedeux R, Lafite P, Jarry U, Aci-Sèche S, Bonnet P, Daniellou R, Guillaudeux T. The Identification of New c-FLIP Inhibitors for Restoring Apoptosis in TRAIL-Resistant Cancer Cells. Current Issues in Molecular Biology. 2024; 46(1):710-728. https://doi.org/10.3390/cimb46010046
Chicago/Turabian StyleYaacoub, Katherine, Rémy Pedeux, Pierre Lafite, Ulrich Jarry, Samia Aci-Sèche, Pascal Bonnet, Richard Daniellou, and Thierry Guillaudeux. 2024. "The Identification of New c-FLIP Inhibitors for Restoring Apoptosis in TRAIL-Resistant Cancer Cells" Current Issues in Molecular Biology 46, no. 1: 710-728. https://doi.org/10.3390/cimb46010046
APA StyleYaacoub, K., Pedeux, R., Lafite, P., Jarry, U., Aci-Sèche, S., Bonnet, P., Daniellou, R., & Guillaudeux, T. (2024). The Identification of New c-FLIP Inhibitors for Restoring Apoptosis in TRAIL-Resistant Cancer Cells. Current Issues in Molecular Biology, 46(1), 710-728. https://doi.org/10.3390/cimb46010046








