Quantitative Analysis and Molecular Docking Simulation of Flavonols from Eruca sativa Mill. and Their Effect on Skin Barrier Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction and Fractionation of E. sativa
2.3. Antioxidant Activity of 2,2-Diphenyl-1-Picrylhydrazyl
2.4. Total Polyphenol and Flavonoid Content Assay
2.5. Cell Culture
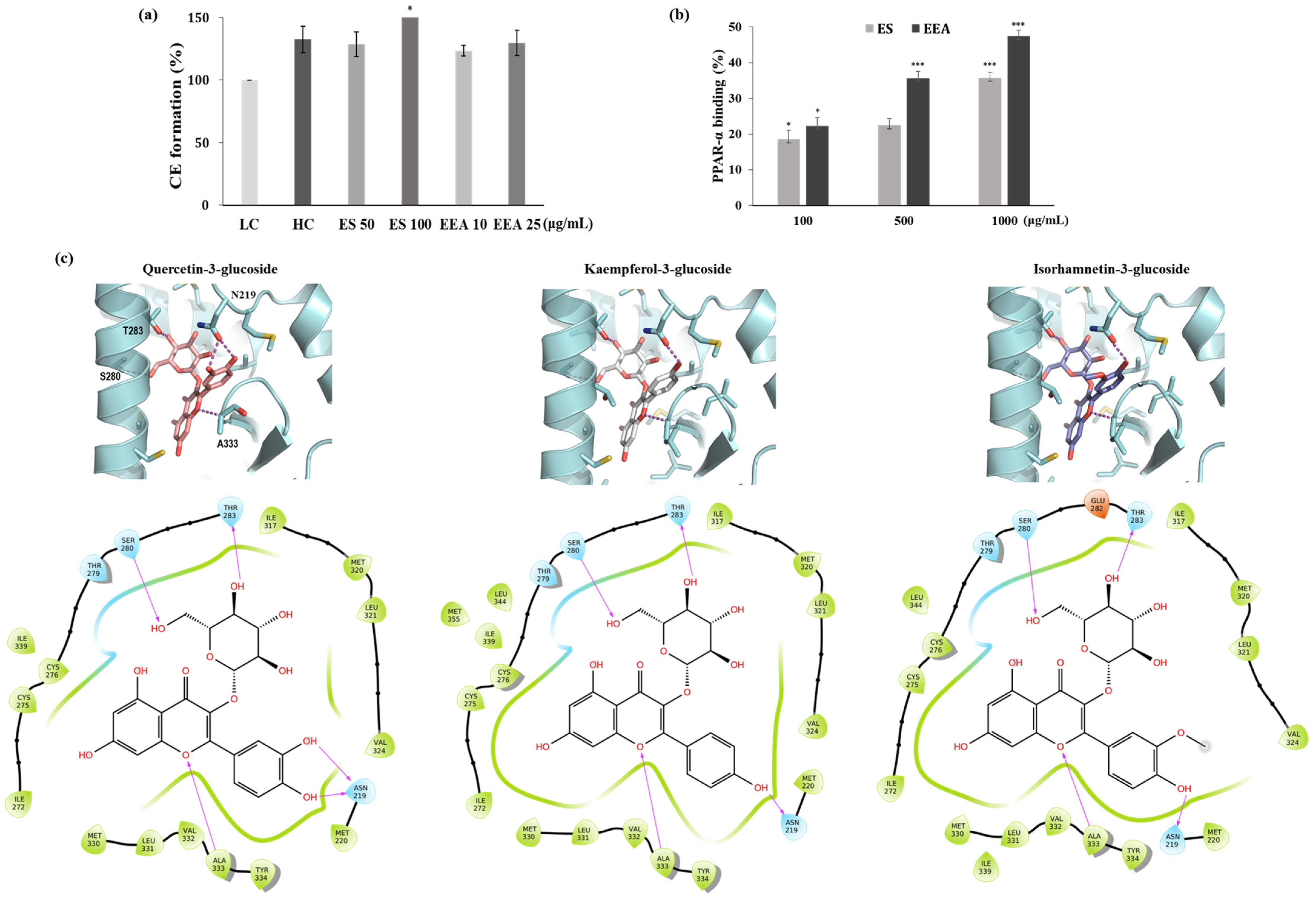
2.6. Cornified Envelope Formation
2.7. PPAR-α Binding Assay
2.8. Docking Study
2.9. Measurement of Intracellular Reactive Oxygen Species
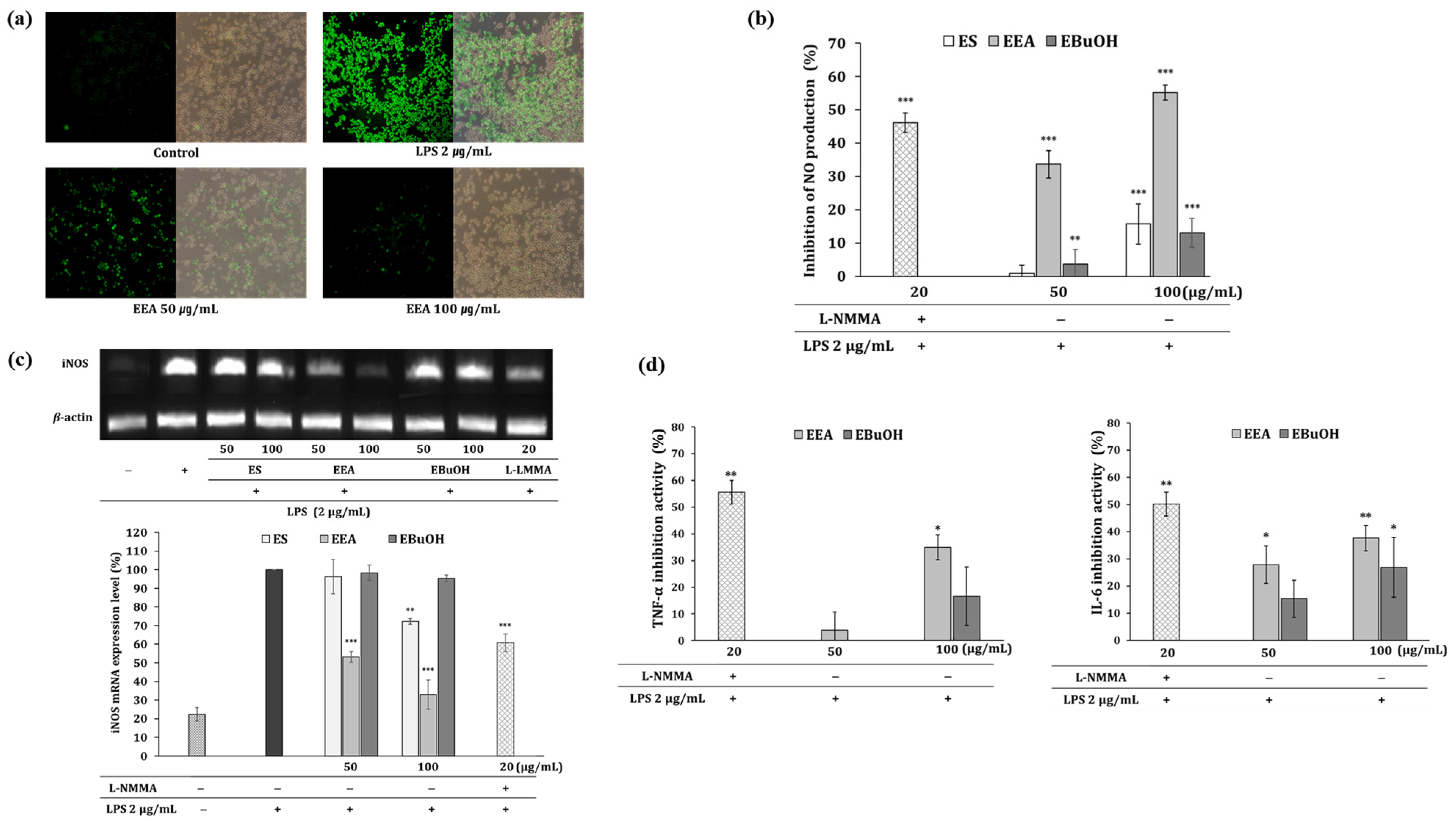
2.10. Measurement of Nitric Oxide, IL-6 and TNF-α Production
2.11. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.12. Analysis of Flavonols
2.13. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Determination of Total Phenolic and Flavonoid Content
3.3. Anti-Inflammatory Effects
3.4. CE Formation and PPAR-α Binding Assay
3.5. Binding Model Prediction between PPAR-α and Flavonols
3.6. Analysis of Flavonols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haftek, M.; Roy, D.C.; Liao, I.C. ARTICLE: Evolution of Skin Barrier Science for Healthy and Compromised Skin. J. Drugs Dermatol. 2021, 20, s3–s9. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Honda, T.; Mostafa, A.; Kabashima, K. Stratum corneum as polymer sheet: Concept and cornification processes. Trends Mol. Med. 2022, 28, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Furue, K.; Mitoma, C.; Tsuji, G.; Furue, M. Protective role of peroxisome proliferator-activated receptor α agonists in skin barrier and inflammation. Immunobiology 2018, 223, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.; Jiang, Y.; Crumrine, D.; Bass, N.M.; Appel, R.; Elias, P.M.; Williams, M.L.; Feingold, K.R. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J. Clin. Investig. 1997, 100, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Wallmeyer, L.; Lehnen, D.; Eger, N.; Sochorová, M.; Opálka, L.; Kováčik, A.; Vávrová, K.; Hedtrich, S. Stimulation of PPARα normalizes the skin lipid ratio and improves the skin barrier of normal and filaggrin deficient reconstructed skin. J. Dermatol. Sci. 2015, 80, 102–110. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Fowler, A.J.; Schmuth, M.; Lau, P.; Chang, S.; Brown, B.E.; Moser, A.H.; Michalik, L.; Desvergne, B.; Wahli, W.; et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J. Investig. Dermatol. 2004, 123, 305–312. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.E.; Kim, H.S. Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phytother. Res. 2014, 28, 1359–1366. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Yehuda, H.; Khatib, S.; Sussan, I.; Musa, R.; Vaya, J.; Tamir, S. Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (MTBI) isolated from rocket (Eruca sativa) seeds. Biofactors 2009, 35, 295–305. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.O.; Adnan, M.; Siddiqui, A.J.; Mahmood, D.; Azad, Z.R.A.A.; Patel, M.; Mehmood, K.; Danciu, C.; et al. Anti-Adhesion and Antibiofilm Activity of Eruca sativa Miller Extract Targeting Cell Adhesion Proteins of Food-Borne Bacteria as a Potential Mechanism: Combined In Vitro-In Silico Approach. Plants 2022, 11, 610. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.E.O.; Adnan, M.; Siddiqui, A.J.; Snoussi, M.; Khan, M.I.; Azad, Z.R.A.A.; Patel, M.; Ashraf, S.A. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules 2022, 27, 1409. [Google Scholar] [CrossRef]
- Ikram, J.; Alamgeer; Muhammad Irfan, H.; Akram, M.; Hussain Asim, M.; Hadal Alotaibi, N.; Saad Alharbi, K.; Abbas Bukhari, S.N.; Qasim, S.; Niazi, Z.R. Ethyl-acetate extract of tara mira (Eruca sativa) alleviates the inflammation and rheumatoid arthritis in rats. Pak. J. Pharm. Sci. 2021, 34, 1897–1902. [Google Scholar] [PubMed]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Dubrac, S.; Schmuth, M. PPAR-alpha in cutaneous inflammation. Dermatoendocrinol 2011, 3, 23–26. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, S.R.; Kim, M.K.; Shin, C.Y.; Lee, D.H.; Chung, J.H. Activation of Peroxisome Proliferator-Activated Receptor Alpha Improves Aged and UV-Irradiated Skin by Catalase Induction. PLoS ONE 2016, 11, e0162628. [Google Scholar] [CrossRef]
- Zhou, L.L.; Lin, Z.X.; Fung, K.P.; Che, C.T.; Zhao, M.; Cheng, C.H.; Zuo, Z. Ethyl acetate fraction of Radix rubiae inhibits cell growth and promotes terminal differentiation in cultured human keratinocytes. J. Ethnopharmacol. 2012, 142, 241–247. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Kim, S.O.; Han, Y.; Ahn, S.; An, S.; Shin, J.C.; Choi, H.; Kim, H.J.; Park, N.H.; Kim, Y.J.; Jin, S.H.; et al. Kojyl cinnamate esters are peroxisome proliferator-activated receptor α/γ dual agonists. Bioorg. Med. Chem. 2018, 26, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Perez-Sanchez, H.; Lightstone, F.C. A Comprehensive Docking and MM/GBSA Rescoring Study of Ligand Recognition upon Binding Antithrombin. Curr. Top. Med. Chem. 2017, 17, 1631–1639. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Chen, D.; Oezguen, N.; Urvil, P.; Ferguson, C.; Dann, S.M.; Savidge, T.C. Regulation of protein-ligand binding affinity by hydrogen bond pairing. Sci. Adv. 2016, 2, e1501240. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca sativa Mill. and Diplotaxis tenuifolia) Grown in Hydroponics. Plants 2019, 8, 68. [Google Scholar] [CrossRef]
- Cuellar, M.; Baroni, V.; Pfaffen, V.; Griboff, J.; Ortiz, P.; Monferrán, M.V. Uptake and accumulation of Cr in edible parts of Eruca sativa from irrigation water. Effects on polyphenol profile and antioxidant capacity. Heliyon 2021, 7, e06086. [Google Scholar] [CrossRef]
| Sample | SC50 (µg/mL) | GAE (mg/g) | QE (mg/g) |
|---|---|---|---|
| ES | 610.40 | 32.22 ± 2.70 | 38.46 ± 15.55 |
| EEA | 229.38 *** | 104.99 ± 5.88 *** | 74.24 ± 1.67 ** |
| EBuOH | 494.93 ** | 88.42 ± 2.17 *** | 53.41 ± 0.33 |
| ECHCl3 | 650.19 | 44.35 ± 3.55 | 39.30 ± 8.29 |
| EDW | 1993.50 | 16.58 ± 0.89 | 27.43 ± 8.18 |
| EHEX | 2078.39 | 8.52 ± 1.00 | 44.73 ± 14.31 |
| L-ascorbic acid | 23.86 *** | - | - |
| Sample | IC50 (μM) |
|---|---|
| Quercetin-3-glucoside | 1.81 |
| Kaempferol-3-glucoside | 58.33 |
| Isorhamnetin-3-glucoside | - |
| Quercetin | 72.27 |
| Kaempferol | 89.38 |
| Isorhamnetin | 442.02 |
| No. | TR (min) | Formula | Ion | Experimental m/z | Calculated m/z | Fragment | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 6.2 | C27H30O17 | [M-H]- | 625.25 | 625.14 | 463, 301 | Quercetin 3,4′-diglucoside |
| 2 | 6.9 | C27H30O16 | [M-H]- | 609.25 | 609.15 | 477, 315 | Kaempferol 3,4′-diglucoside * |
| 3 | 7.9 | C28H32O17 | [M-H]- | 639.25 | 639.16 | 447, 285 | Isorhamnetin 3,4′-diglucoside * |
| 4 | 11.4 | C21H20O12 | [M-H]- | 463.17 | 463.09 | 301 | Quercetin-3-glucoside |
| 5 | 14.6 | C21H20O11 | [M-H]- | 447.17 | 447.09 | 284, 255, 327 | Kaempferol 3-glucoside |
| 6 | 15.4 | C22H22O12 | [M-H]- | 477.17 | 477.10 | 314, 357, 285 | Isorhamnetin 3-glucoside |
| 7 | 25.1 | C15H10O7 | [M-H]- | 301.08 | 301.03 | 179, 151, 273 | Quercetin |
| 8 | 32.6 | C15H10O6 | [M-H]- | 285.08 | 285.03 | 151, 257, 229, 185 | Kaempferol |
| 9 | 33.9 | C16H12O7 | [M-H]- | 315.07 | 315.05 | 300 | Isorhamnetin |
| Flavonols Detected | Crude Extract (by 70%EtOH) | Hexane Fraction | CHCl3 Fraction | Ethyl Acetate Fraction | Butanol Fraction |
|---|---|---|---|---|---|
| Quercetin 3,4′-diglucoside | 0.062 ± 0.002 | 0.049 ± 0.004 | N.D. | 0.25 ± 0.02 | 1.05 ± 0.009 |
| Kaempferol 3,4′-diglucoside | 0.22 ± 0.006 | 0.19 ± 0.002 | 0.010 ± 0.0009 | 1.26 ± 0.05 | 5.70 ± 0.05 |
| Isorhamnetin 3,4′-diglucoside | 0.048 ± 0.003 | 0.020 ± 0.002 | N.D. | 0.12 ± 0.004 | 0.37 ± 0.02 |
| Quercetin 3-glucoside | 0.0055 ± 0.0003 | 0.0045 ± 0.0003 | 0.0021 ± 0.0002 | 2.27 ± 0.07 | 0.20 ± 0.003 |
| Kaempferol 3-glucoside | 0.018 ± 0.0004 | 0.016 ± 0.0007 | N.D. | 7.37 ± 0.3 | 0.31 ± 0.004 |
| Isorhamnetin 3-glucoside | 0.0072 ± 0.0003 | 0.0031 ± 0.0001 | N.D. | 1.44 ± 0.08 | 0.099 ± 0.001 |
| Quercetin | N.D. | N.D. | 0.0016 ± 0.00007 | 0.093 ± 0.008 | 0.0014 ± 0.00009 |
| Kaempferol | 0.00071 ± 0.00004 | N.D. | 0.0042 ± 0.0002 | 0.32 ± 0.02 | N.D. |
| Isorhamnetin | N.D. | N.D. | 0.010 ± 0.0005 | 0.13 ± 0.006 | N.D. |
| Sum | 0.36 | 0.28 | 0.03 | 13.2 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Choi, W.; Kim, J.; Kim, H.W.; Lee, J.-Y.; Lee, J.; Kim, B. Quantitative Analysis and Molecular Docking Simulation of Flavonols from Eruca sativa Mill. and Their Effect on Skin Barrier Function. Curr. Issues Mol. Biol. 2024, 46, 398-408. https://doi.org/10.3390/cimb46010025
Park J, Choi W, Kim J, Kim HW, Lee J-Y, Lee J, Kim B. Quantitative Analysis and Molecular Docking Simulation of Flavonols from Eruca sativa Mill. and Their Effect on Skin Barrier Function. Current Issues in Molecular Biology. 2024; 46(1):398-408. https://doi.org/10.3390/cimb46010025
Chicago/Turabian StylePark, Jihye, Wonchul Choi, Jayoung Kim, Hye Won Kim, Jee-Young Lee, Jongsung Lee, and Bora Kim. 2024. "Quantitative Analysis and Molecular Docking Simulation of Flavonols from Eruca sativa Mill. and Their Effect on Skin Barrier Function" Current Issues in Molecular Biology 46, no. 1: 398-408. https://doi.org/10.3390/cimb46010025
APA StylePark, J., Choi, W., Kim, J., Kim, H. W., Lee, J.-Y., Lee, J., & Kim, B. (2024). Quantitative Analysis and Molecular Docking Simulation of Flavonols from Eruca sativa Mill. and Their Effect on Skin Barrier Function. Current Issues in Molecular Biology, 46(1), 398-408. https://doi.org/10.3390/cimb46010025








