Hexavalent Chromium Inhibited Zebrafish Embryo Development by Altering Apoptosis- and Antioxidant-Related Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Breeding and Embryo Collection
2.2. Chromium Exposure
2.3. Effect of Chromium on Zebrafish Embryo Development
2.4. Heart Rate and Body Length Evaluation
2.5. Quantitative Real-Time RT-PCR
2.5.1. Total RNA Extraction
2.5.2. Real Time RT-PCR Performance
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. Survival and Hatching Rate
3.2. Body Length
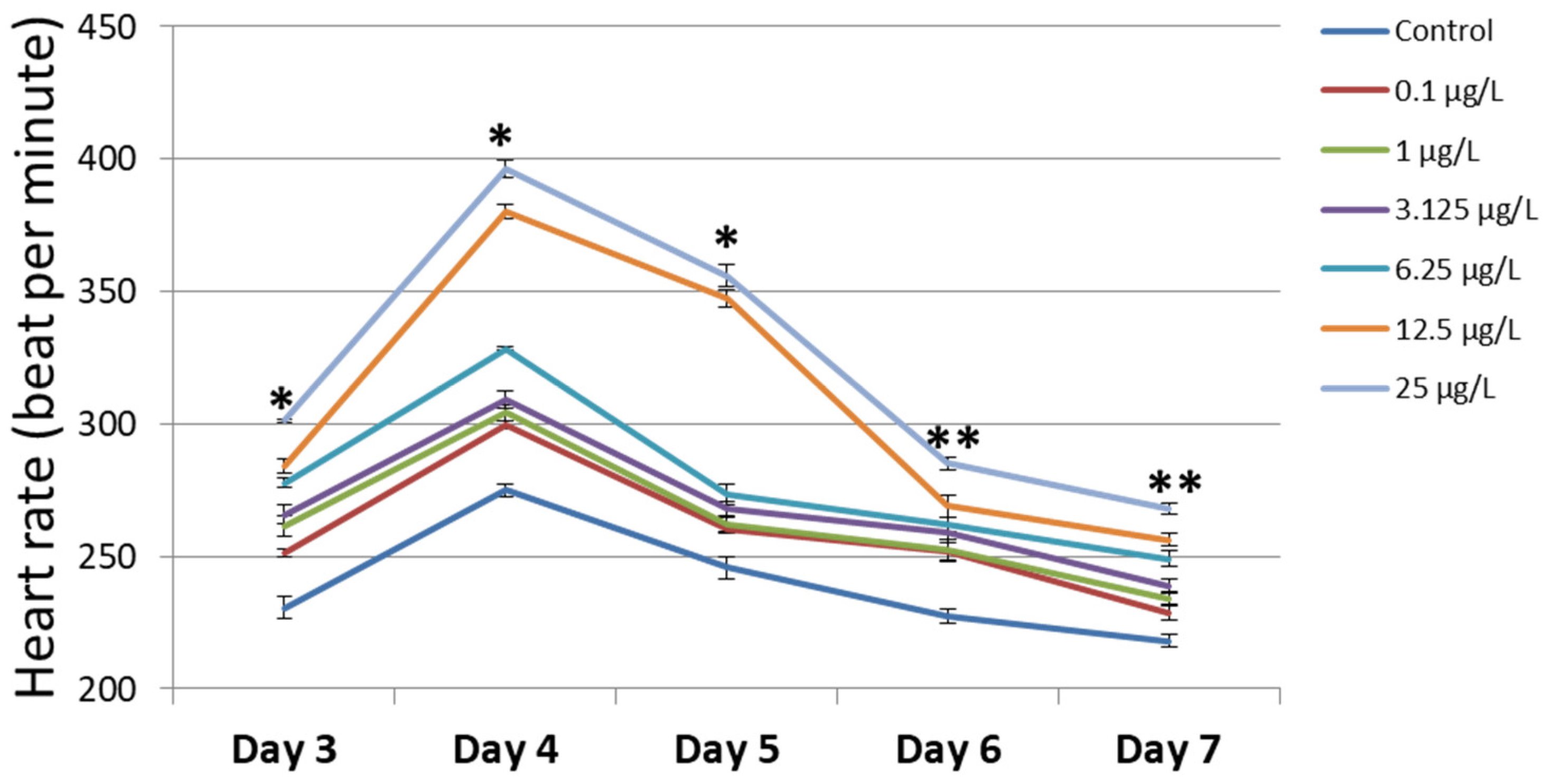
3.3. Heart Rate
3.4. Assessing the Transcript Expression of the Cell-Cycle-Related Genes, Oxidative-Stress-Related Genes, and Apoptosis-Related Genes
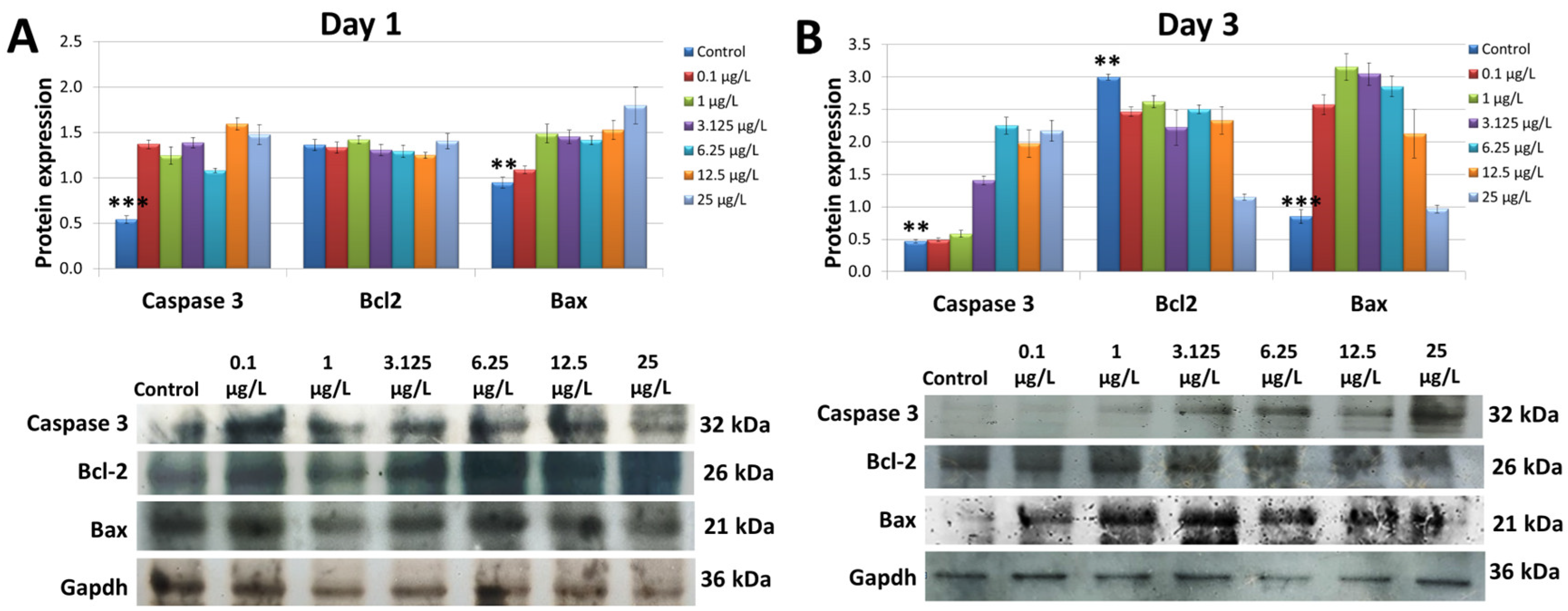
3.5. Assessing the Apoptosis-Related Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL2 | B-cell leukemia/lymphoma 2 protein. |
| CDKs | Cyclin-dependent kinases |
| Ct value | Cycle threshold value |
| ECL | Enhanced chemiluminescence |
| IgG | Immunoglobulin G |
| LDS | Lithium dodecyl sulfate |
| PCR | Polymerase-Chain-Reaction |
| PVDF | Polyvinylidene fluoride |
| ROS | Reactive oxygen species |
| RT-PCR | Reverse transcription PCR |
| RTAse | Reverse transcriptase |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SODs | Superoxide dismutase genes |
References
- Sánchez-Olivares, M.A.; Gaytán-Oyarzun, J.C.; Gordillo-Martínez, A.J.; Prieto-Garcia, F.; Cabrera-Cruz, R.B.E. Toxicity and teratogenicity in zebrafish Danio rerio embryos exposed to chromium. Lat. Am. J. Aquat. Res. 2021, 49, 289–298. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Toxicological Review of Hexavalent Chromium; External Review Draft; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Kim, B.; Nam, S.E.; Eom, H.J.; Lee, S.; Kim, K.; Rhee, J.S. Reductive transformation of hexavalent chromium in ice decreases chromium toxicity in aquatic animals. Environ. Sci. Technol. 2022, 56, 3503–3513. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Shi, X.L.; Lee, W.K.P.; Hill, R.; Wakeman, T.P.; Katz, A.; Xu, B.; Dalal, N.S.; Robertson, J.D.; Chen, C.; et al. Review of Chromium (VI) Apoptosis, Cell-Cycle-Arrest, and Carcinogenesis. J. Environ. Sci. Health Part C 2010, 28, 188–230. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, K.K.; Stanley, J.A.; Arosh, J.A.; Pepling, M.E.; Burghardt, R.C.; Banu, S.K. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring. Dev. Biol. 2014, 388, 22–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, J.; Kang, M.H.; Kim, E.; Kwon, D.N.; Choi, Y.J.; Kim, J.H. Hexavalent chromium induces apoptosis in male somatic and spermatogonial stem cells via redox imbalance. Sci. Rep. 2015, 5, 13921. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wuri, L.; Burghardt, R.C.; Arosh, J.A.; Long, C.R.; Banu, S.K. Hexavalent Chromium Disrupts Oocyte Development in Rats by Elevating Oxidative Stress, DNA Double-Strand Breaks, Microtubule Disruption, and Aberrant Segregation of Chromosomes. Int. J. Mol. Sci. 2023, 24, 10003. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Q.; Yuan, J.; Kneepkens, R.; Yue, Y.; Zhang, C. Direct embryotoxicity of chromium (III) exposure during preimplantation development. J. Reprod. Dev. 2021, 67, 283–291. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Taylor, R.J.; Burghardt, R.C. Chromium VI—Induced developmental toxicity of placenta is mediated through spatiotemporal dysregulation of cell survival and apoptotic proteins. Reprod. Toxicol. 2017, 68, 171–190. [Google Scholar] [CrossRef]
- Leite, F.D.; Carvalho, P.M.S.; Oliveira, R.G.; Lopes, M.C.; Domingues, I.; Correia, P.M.M.; Silva, A.L.M. Analysis of Zebrafish contamination with heavy metals using a FF-XRF imaging system based on a MPGD. Spectrochim. Acta Part B At. Spectrosc. 2022, 198, 106545. [Google Scholar] [CrossRef]
- Tye, M.T.; Montgomery, J.E.; Hobbs, M.R.; Vanpelt, K.T.; Masino, M.A. An adult zebrafish diet contaminated with chromium reduces the viability of progeny. Zebrafish 2018, 15, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Zhu, J.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Chromium induced neurotoxicity by altering metabolism in zebrafish larvae. Ecotoxicol. Environ. Saf. 2021, 228, 112983. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Xu, Y.; Zhu, Y.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Chromium exposure altered metabolome and microbiome-associated with neurotoxicity in zebrafish. J. Appl. Toxicol. 2023, 43, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhou, Q.; Liu, Z.; Li, Q. Hexavalent chromium amplifies the developmental toxicity of graphene oxide during zebrafish embryogenesis. Ecotoxicol. Environ. Saf. 2021, 208, 111487. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.Y.; Renquist, B.J. High Throughput Danio Rerio Energy Expenditure Assay. J. Vis. Exp. 2016, 107, e53297. [Google Scholar]
- Wang, R.F.; Zhu, L.M.; Zhang, J.; An, X.P.; Yang, Y.P.; Song, M.; Zhang, L. Developmental toxicity of copper in marine medaka (Oryzias melastigma) embryos and larvae. Chemosphere 2020, 247, 125923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Namdeo, M. Superoxide dismutase: A key enzyme for the survival of intracellular pathogens in host. In Reactive Oxygen Species; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Peritore, A.F. Combined toxicity of xenobiotics Bisphenol A and heavy metals on zebrafish embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Castro, P.L.; Ferraz, A.L.J.; Patil, J.G.; Ribeiro, R.P. Use of melatonin as an inhibitor of apoptotic process for cryopreservation of zebrafish (Danio rerio) embryos. Braz. J. Biol. 2021, 82, e241081. [Google Scholar] [CrossRef]
- Webster, K.A.; Henke, K.; Ingalls, D.M.; Nahrin, A.; Harris, M.P.; Siegfried, K.R. Cyclin-dependent kinase 21 is a novel regulator of proliferation and meiosis in the male germline of zebrafish. Reproduction 2019, 157, 383–398. [Google Scholar] [CrossRef]
- Papasani, M.R.; Robison, B.D.; Hardy, R.W.; Hill, R.A. Early developmental expression of two insulins in zebrafish (Danio rerio). Physiol. Genom. 2006, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, K.; Hoar, W.S.; Randall, D.J. Mechanisms of Hatching in Fish, Fish Physiology; Academic Press: San Diego, CA, USA, 1988; Volume XI, pp. 447–499. [Google Scholar]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Gierten, J.; Pylatiuk, C.; Hammouda, O.T.; Schock, C.; Stegmaier, J.; Wittbrodt, J.; Loosli, F. Automated high-throughput heartbeat quantification in medaka and zebrafish embryos under physiological conditions. Sci. Rep. 2020, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of heavy metals on early development, growth and reproduction of fish—A review. Toxicol. Rep. 2022, 9, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Carew, E.; Sloman, K.A. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat. Toxicol. 2007, 84, 431–438. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Hu, C.; Chen, Y.; Chen, Z.; Chen, Z.S.; Fang, S. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol. Cancer 2022, 21, 103. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Skotheim, J.M. Cyclin D-Cdk4, 6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol. Cell 2019, 74, 758–770. [Google Scholar] [CrossRef]
- Capriello, T.; Monteiro, S.M.; Félix, L.M.; Donizetti, A.; Aliperti, V.; Ferrandino, I. Apoptosis, oxidative stress and genotoxicity in developing zebrafish after aluminium exposure. Aquat. Toxicol. 2021, 236, 105872. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, J.; Shen, C.; Cui, Y.; Du, J.; Chen, X. The effects of cobalt on the development, oxidative stress, and apoptosis in zebrafish embryos. Biol. Trace Elem. Res. 2012, 150, 200–207. [Google Scholar] [CrossRef]
- Kratz, E.; Eimon, P.M.; Mukhyala, K.; Stern, H.; Zha, J.; Strasser, A.; Ashkenazi, A. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006, 13, 1631–1640. [Google Scholar] [CrossRef]
- Strasser, A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005, 5, 189–200. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, K.D.; Ho, C.N.Q.; Van, H.D.; Dinh, S.T.; Nguyen, Q.T.T.; Nguyen, T.T.T.; Kien, X.T.N.; Dao, T.V.; Nong, H.V.; Nguyen, M.T.; et al. Hexavalent Chromium Inhibited Zebrafish Embryo Development by Altering Apoptosis- and Antioxidant-Related Genes. Curr. Issues Mol. Biol. 2023, 45, 6916-6926. https://doi.org/10.3390/cimb45080436
Dang KD, Ho CNQ, Van HD, Dinh ST, Nguyen QTT, Nguyen TTT, Kien XTN, Dao TV, Nong HV, Nguyen MT, et al. Hexavalent Chromium Inhibited Zebrafish Embryo Development by Altering Apoptosis- and Antioxidant-Related Genes. Current Issues in Molecular Biology. 2023; 45(8):6916-6926. https://doi.org/10.3390/cimb45080436
Chicago/Turabian StyleDang, Khoa Dang, Chi Nguyen Quynh Ho, Huy Duc Van, Son Thanh Dinh, Quynh Thi Truc Nguyen, Tram Thi Thuy Nguyen, Xuyen Thi Ngoc Kien, Tuyet Van Dao, Hung Viet Nong, Minh Thai Nguyen, and et al. 2023. "Hexavalent Chromium Inhibited Zebrafish Embryo Development by Altering Apoptosis- and Antioxidant-Related Genes" Current Issues in Molecular Biology 45, no. 8: 6916-6926. https://doi.org/10.3390/cimb45080436
APA StyleDang, K. D., Ho, C. N. Q., Van, H. D., Dinh, S. T., Nguyen, Q. T. T., Nguyen, T. T. T., Kien, X. T. N., Dao, T. V., Nong, H. V., Nguyen, M. T., Doan, C. C., Hoang, S. N., Nguyen, T. T. P., & Le, L. T. (2023). Hexavalent Chromium Inhibited Zebrafish Embryo Development by Altering Apoptosis- and Antioxidant-Related Genes. Current Issues in Molecular Biology, 45(8), 6916-6926. https://doi.org/10.3390/cimb45080436






