Avermectin B1a Shows Potential Anti-Proliferative and Anticancer Effects in HCT-116 Cells via Enhancing the Stability of Microtubules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Preparation of Solutions
2.2. Methods
2.2.1. Cell Culture
2.2.2. Cell Viability Assay
2.2.3. Polymerization Assay of Mammalian Tubulin
2.2.4. Cell Apoptosis and Flow Cytometry Analysis
2.2.5. Wound-Healing Assay
2.2.6. Statistical Analysis
3. Results
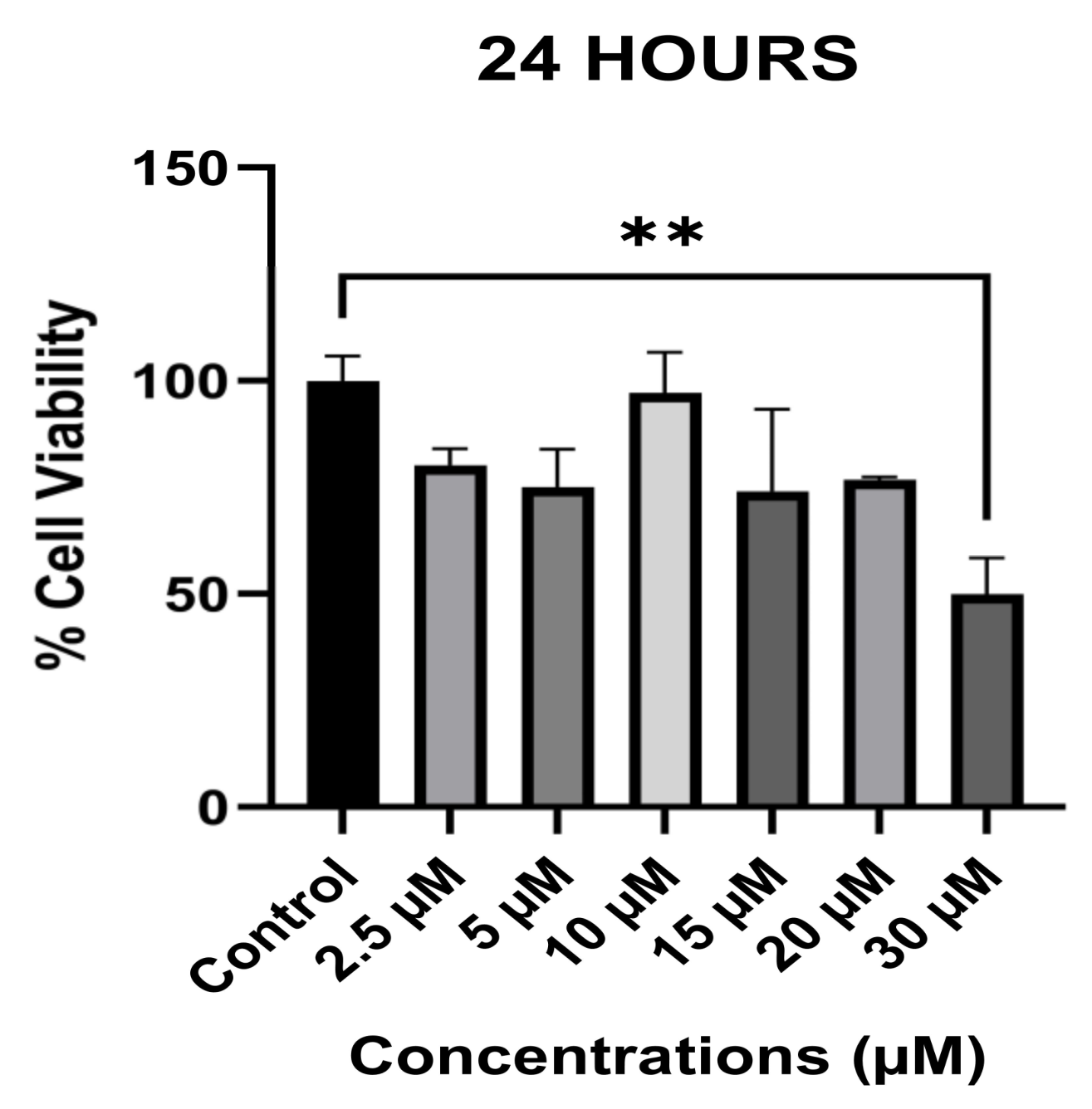
3.1. The Anti-Proliferative Effect of Avermectin B1a on HCT-116
3.2. The Effect of Avermectin B1a on Tubulin Polymerization
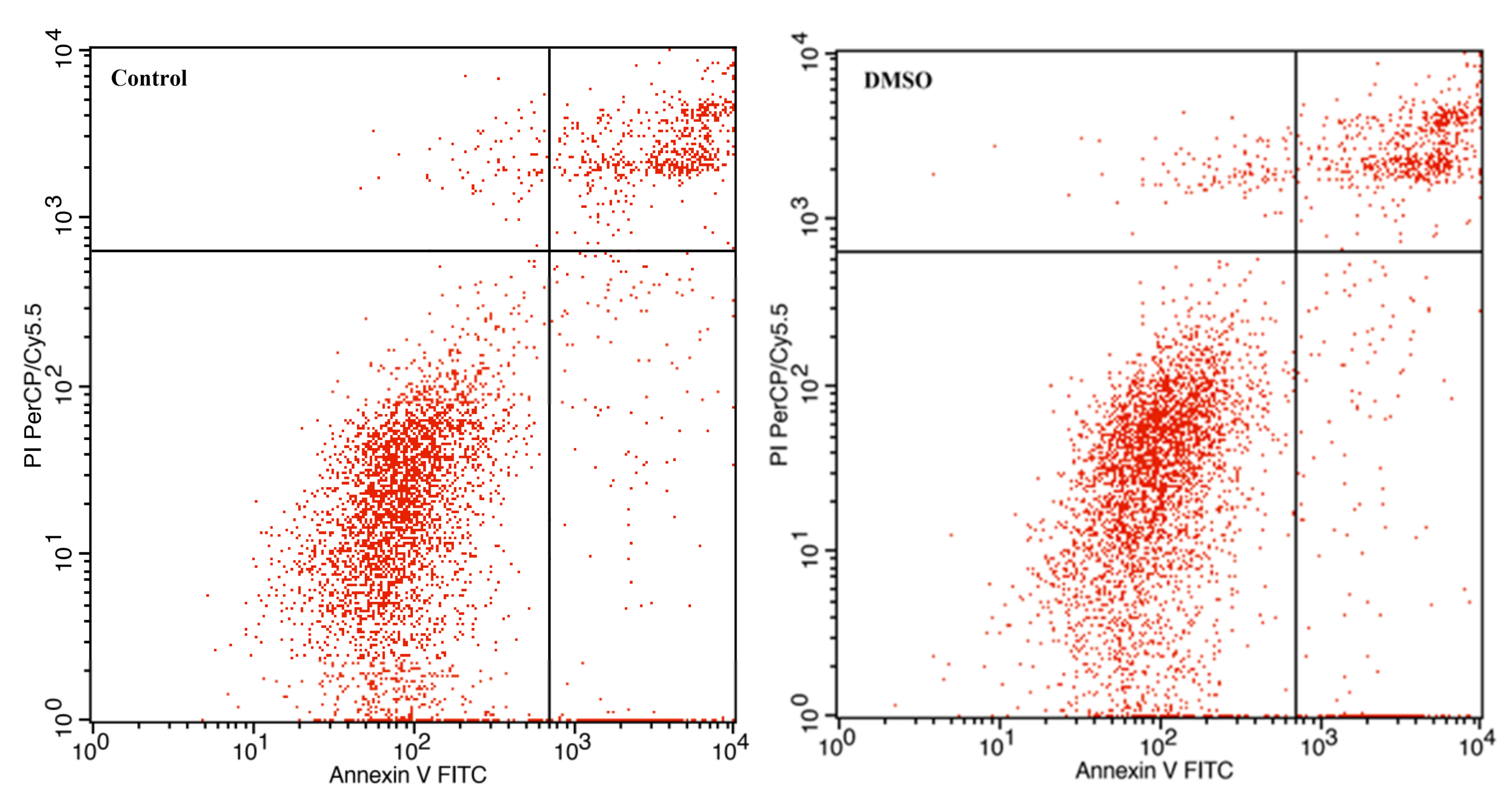
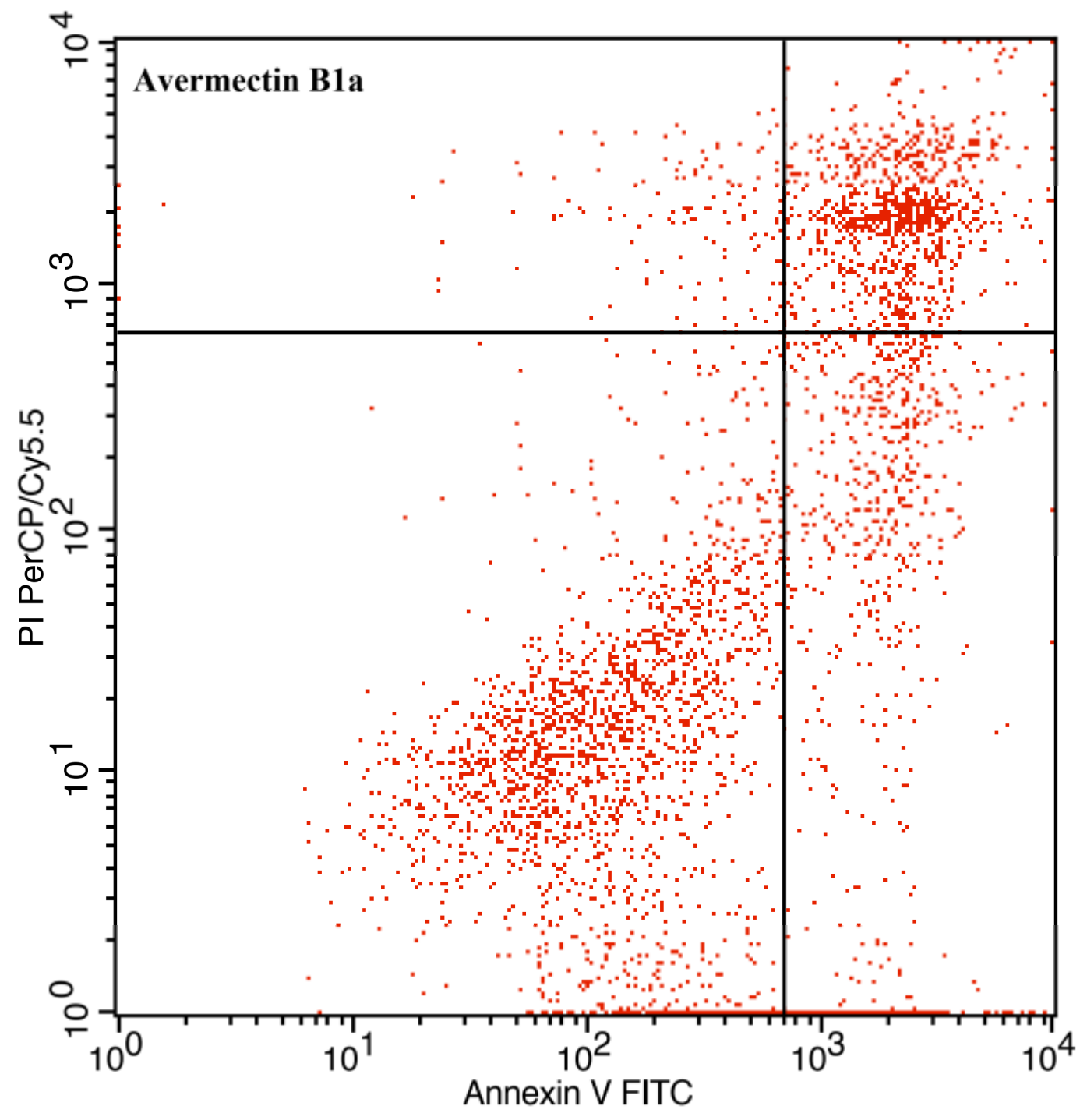
3.3. The Effect of Avermectin B1a on Apoptosis
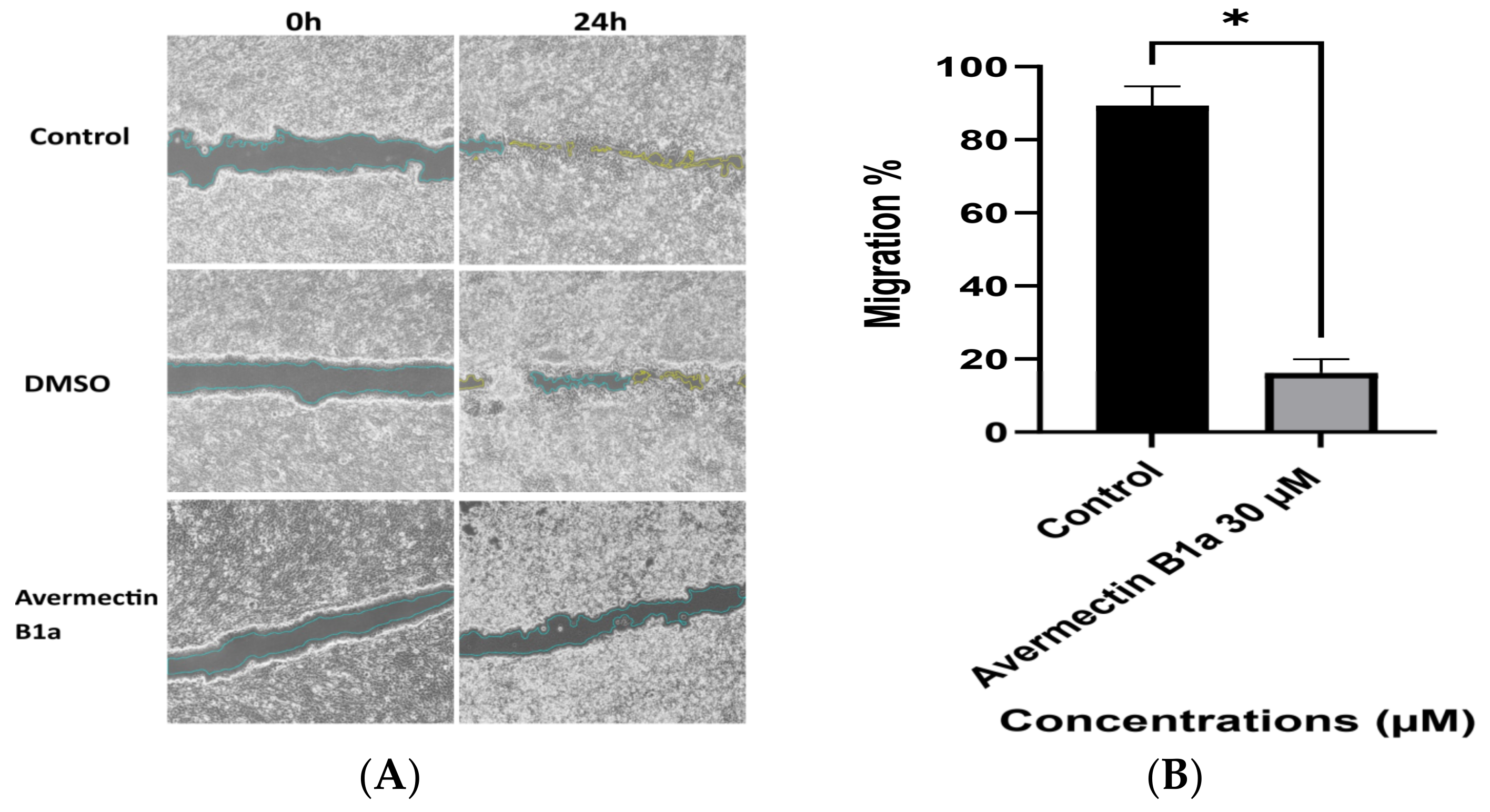
3.4. The Effect of Avermectin B1a on Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, W.C. History of Avermectin and Ivermectin, with Notes on the History of Other Macrocyclic Lactone Antiparasitic Agents. Curr. Pharm. Biotechnol. 2012, 13, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; You, Y.; Chen, Z.; Li, J.; Liu, G.; Wen, Y. Avermectin B1a production in Streptomyces avermitilis is enhanced by engineering aveC and precursor supply genes. Appl. Microbiol. Biotechnol. 2022, 106, 2191–2205. [Google Scholar] [CrossRef]
- Yamashita, S.; Hayashi, D.; Nakano, A.; Hayashi, Y.; Hirama, M. Total synthesis of avermectin B1a revisited. J. Antibiot. 2016, 69, 31–50. [Google Scholar] [CrossRef]
- Campbell, W.C.; Burg, R.W.; Fisher, M.H.; Dybas, R.A. The Discovery of Ivermectin and Other Avermectins. ACS Symp. Ser. 1984, 255, 5–20. [Google Scholar] [CrossRef]
- Khalil, M.S. Abamectin and Azadirachtin as Eco-friendly Promising Biorational Tools in Integrated Nematodes Management Programs. J. Plant Pathol. Microbiol. 2013, 4, 1000174. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, S.-H.; Li, D.; Wang, S.-Y.; Liu, X.; Song, J.; Wang, Y.-T.; Zhang, S.-Y. Progress of tubulin polymerization activity detection methods. Bioorganic Med. Chem. Lett. 2021, 37, 127698. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Sana, S.; Tokala, R.; Bajaj, D.M.; Nagesh, N.; Bokara, K.K.; Kiranmai, G.; Lakshmi, U.J.; Vadlamani, S.; Talla, V.; Shankaraiah, N. Design and synthesis of substituted dihydropyrimidinone derivatives as cytotoxic and tubulin polymerization inhibitors. Bioorganic Chem. 2019, 93, 103317. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. Structural Insights into Microtubule Function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Self-Assembly and Dynamic Structure of Cytoskeletal Filaments. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26862/ (accessed on 10 October 2022).
- Battaje, R.R.; Panda, D. Lessons from bacterial homolog of tubulin, FtsZ for microtubule dynamics. Endocr. Relat. Cancer 2017, 24, T1–T21. [Google Scholar] [CrossRef] [PubMed]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rosel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef] [PubMed]
- Prassanawar, S.S.; Panda, D. Tubulin heterogeneity regulates functions and dynamics of microtubules and plays a role in the development of drug resistance in cancer. Biochem. J. 2019, 476, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Tangutur, A.D.; Kumar, D.; Krishna, K.V.; Kantevari, S. Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents. Curr. Top. Med. Chem. 2017, 17, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Chatterjee, A.; Ghosh, H.; Kapoor, S.; Ray, S. Phytochemicals as Anticancer Drugs: Targeting the Microtubular Network of Cancer Cells. In Phytochemistry: An In-Silico and In-Vitro Update; Kumar, S., Egbuna, C., Eds.; Springer: Singapore, 2019; pp. 57–67. [Google Scholar] [CrossRef]
- Jadala, C.; Sathish, M.; Anchi, P.; Tokala, R.; Lakshmi, U.J.; Reddy, V.G.; Shankaraiah, N.; Godugu, C.; Kamal, A. Synthesis of Combretastatin-A4 Carboxamidest that Mimic Sulfonyl Piperazines by a Molecular Hybridization Approach: In vitro Cytotoxicity Evaluation and Inhibition of Tubulin Polymerization. Chemmedchem 2019, 14, 2052–2060. [Google Scholar] [CrossRef]
- Muñoz, J.; Ballester, M.R.; Antonijoan, R.M.; Gich, I.; Rodríguez, M.; Colli, E.; Gold, S.; Krolewiecki, A.J. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLOS Neglected Trop. Dis. 2018, 12, e0006020. [Google Scholar] [CrossRef]
- Ashraf, S.; Mani, T.; Beech, R.; Prichard, R. Macrocyclic lactones and their relationship to the SNPs related to benzimidazole resistance. Mol. Biochem. Parasitol. 2015, 201, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Prichard, R. Ivermectin exhibits potent anti-mitotic activity. Veter. Parasitol. 2016, 226, 1–4. [Google Scholar] [CrossRef]
- Ma, J.; Sun, X.; Wang, Y.; Chen, B.; Qian, L.; Wang, Y. Fibroblast-derived CXCL12 regulates PTEN expression and is associated with the proliferation and invasion of colon cancer cells via PI3k/Akt signaling. Cell Commun. Signal. 2019, 17, 119. [Google Scholar] [CrossRef]
- Ning, H.; Lu, W.; Jia, Q.; Wang, J.; Yao, T.; Lv, S.; Li, Y.; Wen, H. Discovery of oxyepiberberine as a novel tubulin polymerization inhibitor and an anti-colon cancer agent against LS-1034 cells. Investig. New Drugs 2021, 39, 386–393. [Google Scholar] [CrossRef]
- Gurba, A.; Taciak, P.; Sacharczuk, M.; Młynarczuk-Biały, I.; Bujalska-Zadrożny, M.; Fichna, J. Gold (III) Derivatives in Colon Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 724. [Google Scholar] [CrossRef]
- Genc, S.; Taghizadehghalehjoughi, A.; Yeni, Y.; Jafarizad, A.; Hacimuftuoglu, A.; Nikitovic, D.; Docea, A.O.; Mezhuev, Y.; Tsatsakis, A. Fe3O4 Nanoparticles in Combination with 5-FU Exert Antitumor Effects Superior to Those of the Active Drug in a Colon Cancer Cell Model. Pharmaceutics 2023, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, Q.; Wang, M. Anti-colon-cancer effects of polysaccharides: A mini-review of the mechanisms. Int. J. Biol. Macromol. 2018, 114, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, F. Analysis of Microtubule Assembly Kinetics Using Turbidimetry. Methods Mol. Biol. 2011, 777, 99–105. [Google Scholar] [CrossRef]
- Yunes, S.A.; Willoughby, J.L.S.; Kwan, J.H.; Biagi, J.M.; Pokharel, N.; Chin, H.G.; York, E.A.; Su, K.-C.; George, K.; Shah, J.V.; et al. Factor quinolinone inhibitors disrupt spindles and multiple LSF (TFCP2)-protein interactions in mitosis, including with microtubule-associated proteins. PLoS ONE 2022, 17, e0268857. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, Y.; Shuai, W.; Xu, F.; Yao, H.; Liu, J.; Cheng, K.; Xu, J.; Zhu, Z.; Xu, S. Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain. Bioorganic Chem. 2019, 83, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Design, synthesis, biological evaluation and molecular docking studies of new chalcone derivatives containing diaryl ether moiety as potential anticancer agents and tubulin polymerization inhibitors. Bioorganic Chem. 2020, 95, 103565. [Google Scholar] [CrossRef]
- Ganguly, A.; Yang, H.; Sharma, R.; Patel, K.D.; Cabral, F. The Role of Microtubules and Their Dynamics in Cell Migration. J. Biol. Chem. 2012, 287, 43359–43369. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Beshbishy, A.M. Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Dominguez-Gomez, G.; Chavez-Blanco, A.; Medina-Franco, J.L.; Saldivar-Gonzalez, F.; Flores-Torrontegui, Y.; Juarez, M.; Díaz-Chávez, J.; Gonzalez-Fierro, A.; Dueñas-González, A. Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med. Rep. 2018, 17, 3397–3403. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt Axis in breast cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef]
- Markowska, A.; Kaysiewicz, J.; Huczyński, A. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorganic Med. Chem. Lett. 2019, 29, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Personalizing Colon Cancer Adjuvant Therapy: Selecting Optimal Treatments for Individual Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, Y.; Alnaggar, M. Silver nanoparticles induce cell death of colon cancer cells through impairing cytoskeleton and membrane nanostructure. Micron 2019, 126, 102750. [Google Scholar] [CrossRef] [PubMed]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, W.C.; Bloukh, S.H.; Edis, Z.; Du, W.; He, Y.; Hu, H.Y.; Hagen, T.L.; Falahati, M. An overview on the exploring the interaction of inorganic nanoparticles with microtubules for the advancement of cancer therapeutics. Int. J. Biol. Macromol. 2022, 212, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Q.; Lu, Y.; Zhang, W.; Yu, P.; Liu, Z.; Sun, X. Anti-tubulin agent vinorelbine inhibits metastasis of cancer cells by regulating epithelial-mesenchymal transition. Eur. J. Med. Chem. 2020, 200, 112332. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, C.; Hu, Z.; Chen, W.; Qi, W.; Li, A. Genistein induces apoptosis of colon cancer cells by reversal of epitheli-al-to-mesenchymal via a Notch1/NF-ΚB/slug/E-cadherin pathway. BMC Cancer 2017, 17, 813. [Google Scholar] [CrossRef]
- Luan, N.; Mu, Y.; Mu, J.; Chen, Y.; Ye, X.; Zhou, Q.; Xu, M.; Deng, Q.; Hu, Y.; Tang, Z.; et al. Dicer1 Promotes Colon Cancer Cell Invasion and Migration Through Modulation of tRF-20-MEJB5Y13 Expression Under Hypoxia. Front. Genet. 2021, 12, 638244. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoti, Q.; Rustem, D.G.; Dalmizrak, O. Avermectin B1a Shows Potential Anti-Proliferative and Anticancer Effects in HCT-116 Cells via Enhancing the Stability of Microtubules. Curr. Issues Mol. Biol. 2023, 45, 6272-6282. https://doi.org/10.3390/cimb45080395
Hoti Q, Rustem DG, Dalmizrak O. Avermectin B1a Shows Potential Anti-Proliferative and Anticancer Effects in HCT-116 Cells via Enhancing the Stability of Microtubules. Current Issues in Molecular Biology. 2023; 45(8):6272-6282. https://doi.org/10.3390/cimb45080395
Chicago/Turabian StyleHoti, Qendresa, Duygu Gencalp Rustem, and Ozlem Dalmizrak. 2023. "Avermectin B1a Shows Potential Anti-Proliferative and Anticancer Effects in HCT-116 Cells via Enhancing the Stability of Microtubules" Current Issues in Molecular Biology 45, no. 8: 6272-6282. https://doi.org/10.3390/cimb45080395
APA StyleHoti, Q., Rustem, D. G., & Dalmizrak, O. (2023). Avermectin B1a Shows Potential Anti-Proliferative and Anticancer Effects in HCT-116 Cells via Enhancing the Stability of Microtubules. Current Issues in Molecular Biology, 45(8), 6272-6282. https://doi.org/10.3390/cimb45080395





