Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω6(S)-Lipoxygenase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
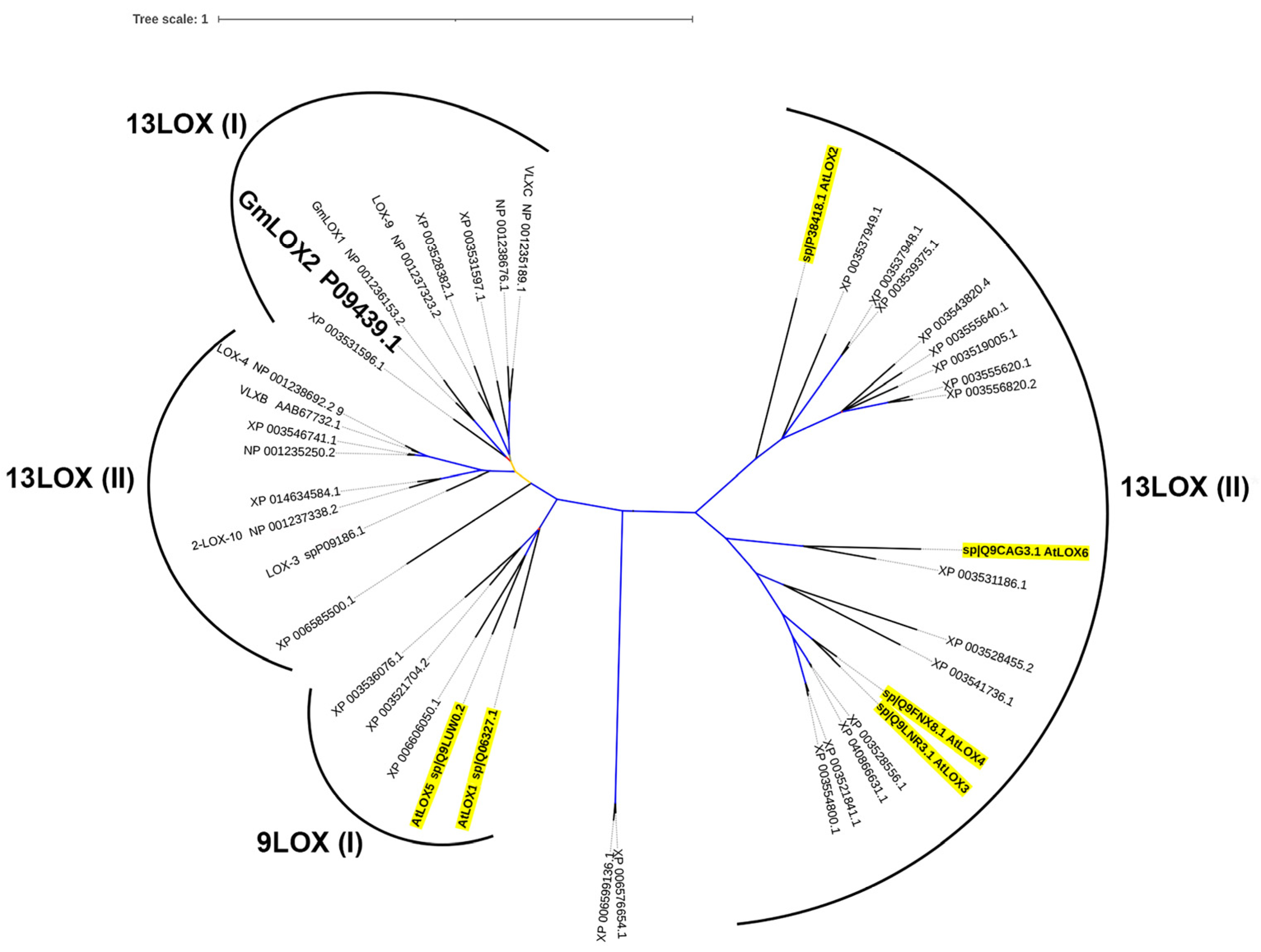
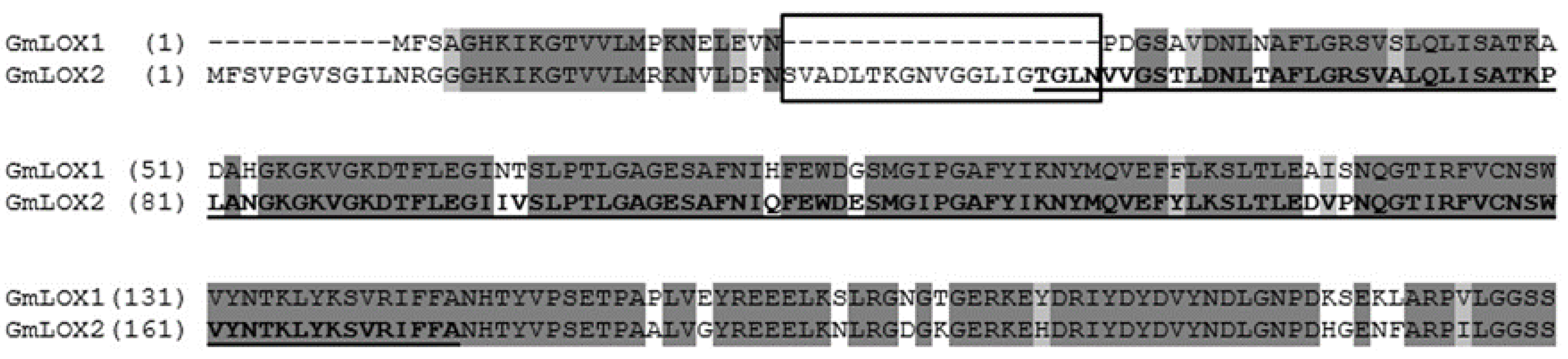
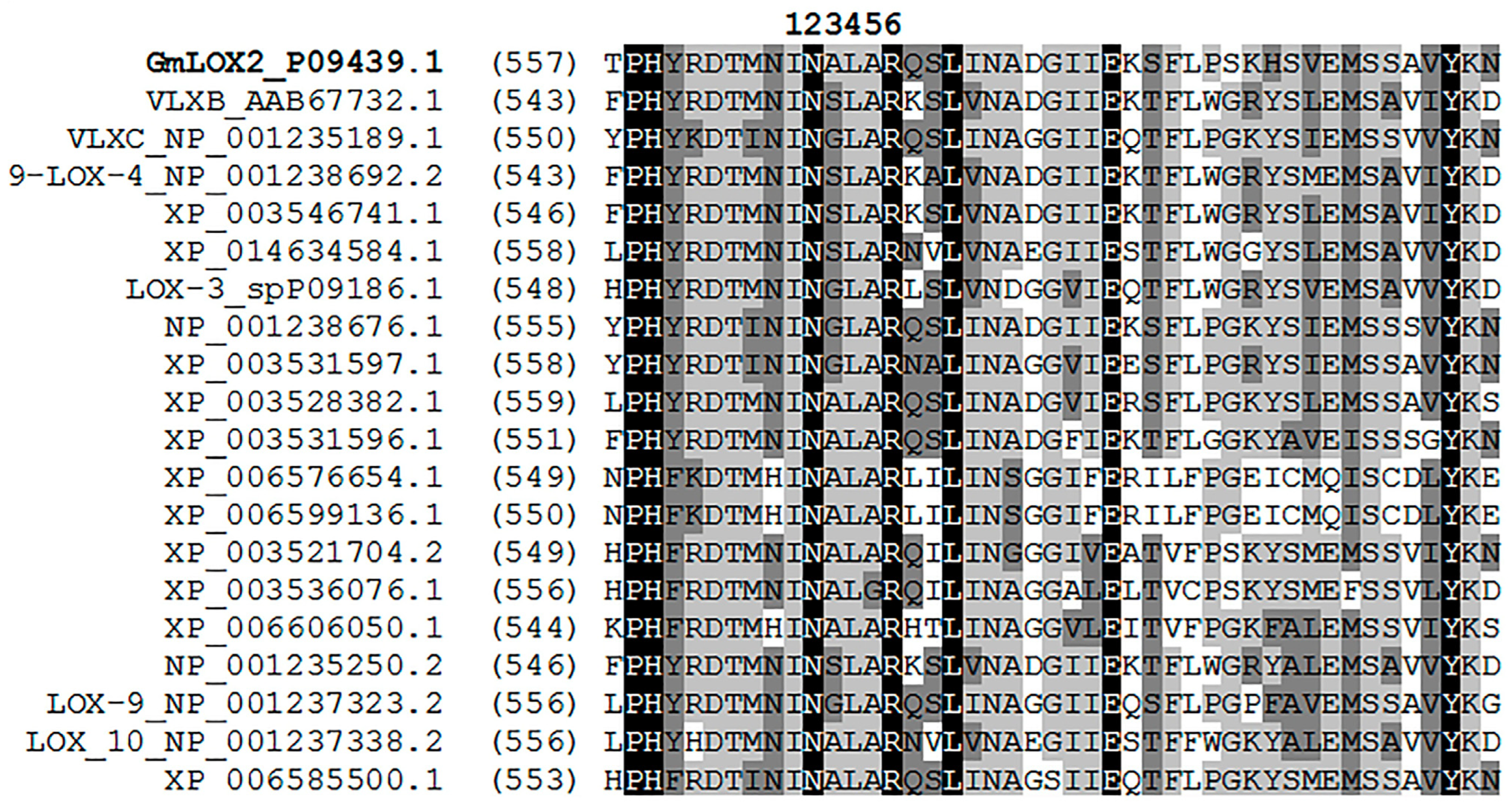
2.2. Bioinformatics Studies
2.3. Cloning and Expression of Recombinant GmLOX2
2.4. Purification of the Recombinant GmLOX2
2.5. Studies of the Substrate Specificity and pH Optimum
2.6. Incubations of Free Fatty Acids with Recombinant GmLOX2
2.7. HPLC analysis of Products
2.8. Methods of Spectral Analyses
3. Results
3.1. Preparation of the Recombinant GmLOX2 and Studies of Its Kinetics and Substrate Specificity
3.2. Products of C18 Fatty Acids Conversions by Recombinant GmLOX2
3.3. Products of C20 Fatty Acids Conversions by Recombinant GmLOX2
3.4. Products of Hexadecatrienoic Acid (C16) Conversion by the Recombinant GmLOX2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The Lipoxygenase Pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Oliw, E.H. Plant and Fungal Lipoxygenases. Prostaglandins Other Lipid Mediat. 2002, 68, 313–323. [Google Scholar] [CrossRef]
- Vliegenthart, J.F.G.; Veldink, G.A. Lipoxygenases, Nonheme Iron-Containing Enzymes. Adv. Inorg. Biochem. 1984, 6, 139–161. [Google Scholar]
- Grechkin, A. Recent Developments in Biochemistry of the Plant Lipoxygenase Pathway. Prog. Lipid Res. 1998, 37, 317–352. [Google Scholar] [CrossRef]
- Blée, E. Impact of Phyto-Oxylipins in Plant Defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar]
- Fuller, M.A.; Weichert, H.; Fischer, A.M.; Feussner, I.; Grimes, H.D. Activity of Soybean Lipoxygenase Isoforms against Esterified Fatty Acids Indicates Functional Specificity. Arch. Biochem. Biophys. 2001, 388, 146–154. [Google Scholar] [CrossRef]
- Fischer, A.M.; Dubbs, W.E.; Baker, R.A.; Fuller, M.A.; Stephenson, L.C.; Grimes, H.D. Protein Dynamics, Activity and Cellular Localization of Soybean Lipoxygenases Indicate Distinct Functional Roles for Individual Isoforms. Plant J. 1999, 19, 543–554. [Google Scholar] [CrossRef]
- Shibata, D.; Steczko, J.; Dixon, J.E.; Andrews, P.C.; Hermodson, M.; Axelrod, B. Primary Structure of Soybean Lipoxygenase L-2. J. Biol. Chem. 1988, 263, 6816–6821. [Google Scholar] [CrossRef]
- Coffa, G.; Imber, A.N.; Maguire, B.C.; Laxmikanthan, G.; Schneider, C.; Gaffney, B.J.; Brash, A.R. On the Relationships of Substrate Orientation, Hydrogen Abstraction, and Product Stereochemistry in Single and Double Dioxygenations by Soybean Lipoxygenase-1 and Its Ala542Gly Mutant. J. Biol. Chem. 2005, 280, 38756–38766. [Google Scholar] [CrossRef]
- Saravitz, D.M.; Siedow, J.N. The Differential Expression of Wound-Inducible Lipoxygenase Genes in Soybean Leaves. Plant Physiol. 1996, 110, 287–299. [Google Scholar] [CrossRef]
- Bunker, T.W.; Koetje, D.S.; Stephenson, L.C.; Creelman, R.A.; Mullet, J.E.; Grimes, H.D. Sink Limitation Induces the Expression of Multiple Soybean Vegetative Lipoxygenase MRNAs While the Endogenous Jasmonic Acid Level Remains Low. Plant Cell 1995, 7, 1319–1331. [Google Scholar] [PubMed]
- Shibata, D.; Steczko, J.; Dixon, J.E.; Hermodson, M.; Yazdanparast, R.; Axelrod, B. Primary Structure of Soybean Lipoxygenase-1. J. Biol. Chem. 1987, 262, 10080–10085. [Google Scholar] [CrossRef] [PubMed]
- Yenofsky, R.L.; Fine, M.; Liu, C. Isolation and characterization of a soybean (Glycine max) lipoxygenase-3 gene. Mol. Gen. Genet 1988, 211, 215–222. [Google Scholar] [CrossRef]
- Fukushige, H.; Wang, C.; Simpson, T.D.; Gardner, H.W.; Hildebrand, D.F. Purification and Identification of Linoleic Acid Hydroperoxides Generated by Soybean Seed Lipoxygenases 2 and 3. J. Agric. Food Chem. 2005, 53, 5691–5694. [Google Scholar] [CrossRef]
- Maccarrone, M.; van Aarle, P.G.M.; Veldink, G.A.; Vliegenthart, J.F.G. In Vitro Oxygenation of Soybean Biomembranes by Lipoxygenase-2. Biochim. Biophys. Acta (BBA) Biomembr. 1994, 1190, 164–169. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. [53] Lipoxygenase from Soybeans: EC 1.13.11.12 Linoleate: Oxygen Oxidoreductase. In Methods in Enzymology; Lipids Part C; Academic Press: Cambridge, MA, USA, 1981; Volume 71, pp. 441–451. [Google Scholar]
- Van Os, C.P.A.; Rijke-Schilder, G.P.M.; Vliegenthart, J.F.G. 9-LR-Linoleyl Hydroperoxide, a Novel Product from the Oxygenation of Linoleic Acid by Type-2 Lbpoxygenases from Soybeans and Peas. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1979, 575, 479–484. [Google Scholar]
- Christopher, J.; Pistorius, E.; Axelrod, B. Isolation of an Isozyme of Soybean Lipoxygenase. Biochim. Biophys. Acta (BBA) Enzymol. 1970, 198, 12–19. [Google Scholar] [CrossRef]
- Feiters, M.C.; Veldink, G.A.; Vliegenthart, J.F.G. Heterogeneity of Soybean Lipoxygenase 2. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1986, 870, 367–371. [Google Scholar] [CrossRef]
- Andrawis, A.; Pinsky, A.; Grossman, S. Isolation of Soybean Lipoxygenase-2 by Affinity Chromatography. Phytochemistry 1982, 21, 1523–1525. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Tarchevsky, I.A.; Egorova, A.M. Proteomic Analysis of Cycloheximide Influence on Pea Roots. Russ. J. Plant Physiol. 2015, 62, 883–895. [Google Scholar] [CrossRef]
- Hamberg, M.; Hughes, M.A. Fatty Acid Allene Oxides. III. Albumin-Induced Cyclization of 12,13(S)-Epoxy-9(Z),11-Octadecadienoic Acid. Lipids 1988, 23, 469–475. [Google Scholar] [CrossRef]
- Grechkin, A.N.; Mukhtarova, L.S.; Hamberg, M. Thermal Conversions of Trimethylsilyl Peroxides of Linoleic and Linolenic Acids. Chem. Phys. Lipids 2005, 138, 93–101. [Google Scholar] [CrossRef]
- Vliegenthart, J.F.G.; Verhagen, J.; Bouman, A.A.; Boldingh, J. Conversion of 9-D-and 13-L-hydroperoxylinoleic acids by soybean lipoxygenase-1 under anaerobic conditions. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1977, 486, 114–120. [Google Scholar]
- Joshi, N.; Hoobler, E.K.; Perry, S.; Diaz, G.; Fox, B.; Holman, T.R. Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2. Biochemistry 2013, 52, 8026–8035. [Google Scholar] [CrossRef][Green Version]
- Chechetkin, I.R.; Mukhitova, F.K.; Gogolev, V.; Grechkin, A.N. Regio- and stereospecificity of recombinant soybean lipoxygenase-2. Dokl. Biochem. Biophys. 2007, 415, 225–227. [Google Scholar] [CrossRef]
- Youn, B.; Sellhorn, G.E.; Mirchel, R.J.; Gaffney, B.J.; Grimes, H.D.; Kang, C. Crystal Structures of Vegetative Soybean Lipoxygenase VLX-B and VLX-D, and Comparisons with Seed Isoforms LOX-1 and LOX-3. Proteins Struct. Funct. Genet. 2006, 65, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef]
- Islas-Flores, I.; Corrales-Villamar, S.; Bearer, E.; Raya, J.C.; Villanueva, M.A. Isolation of Lipoxygenase Isoforms from Glycine Max Embryo Axes Based on Apparent Cross-Reactivity with Anti-Myosin Antibodies. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1571, 64–70. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, C.H.; Choi, Y.; Park, K.-M.; Chang, P.-S. Catalytic Characterization of Heterodimeric Linoleate 13S-Lipoxygenase from Black Soybean (Glycine Max (L.) Merr.). Enzym. Microb. Technol. 2020, 139, 109595. [Google Scholar] [CrossRef]
- Steczko, J.; Muchmore, C.R.; Smith, J.L.; Axelrod, B. Crystallization and Preliminary X-Ray Investigation of Lipoxygenase 1 from Soybeans. J. Biol. Chem. 1990, 265, 11352–11354. [Google Scholar] [CrossRef]
- Eskin, N.A.; Grossman, S.; Pinsky, A. Biochemistry of lipoxygenase in relation to food quality. CRC Crit. Rev. Food Sci. Nutr. 1977, 9, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview. Molecules 2018, 23, 3244. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Zhang, C.; Kong, X.; Chen, Y.; Hua, Y. Formation Mechanism of Hexanal and (E)-2-Hexenal during Soybean [Glycine Max (L.) Merr] Processing Based on the Subcellular and Molecular Levels. J. Agric. Food Chem. 2022, 70, 289–300. [Google Scholar] [CrossRef]
- Skrzypczak-Jankun, E.; Borbulevych, O.Y.; Jankun, J. Soybean Lipoxygenase-3 in Complex with 4-nitrocatechol. Acta Cryst. D 2004, 60, 613–615. [Google Scholar] [CrossRef]
- Boyington, J.C.; Gaffney, B.J.; Mario Amzel, L. The Three-Dimensional Structure of Soybean Lipoxygenase-1: An Arachidonic Acid 15-Lipoxygenase. In Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury 2: Part A; Honn, K.V., Nigam, S., Marnett, L.J., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1997; pp. 133–138. ISBN 978-1-4615-5325-0. [Google Scholar]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef]
- Kramer, J.A.; Johnson, K.R.; Dunham, W.R.; Sands, R.H.; Funk, M.O., Jr. Position 713 is critical for catalysis but not iron binding in soybean lipoxygenase 3. Biochemistry 1994, 33, 15017–15022. [Google Scholar] [CrossRef]
- Solomon, E.I.; Zhou, J.; Neese, F.; Pavel, E.G. New Insights from Spectroscopy into the Structure/Function Relationships of Lipoxygenases. Chem. Biol. 1997, 4, 795–808. [Google Scholar] [CrossRef]
- Gaffney, B.J.; Boyington, J.C.; Amzel, L.M.; Doctor, K.S.; Prigge, S.T.; Yuan, S.M. Lipoxygenase structure and mechanism. Adv. Prostaglandin Thromboxane Leukot. 1995, 23, 11–16. [Google Scholar]
- Chohany, L.E.; Bishop, K.A.; Camic, H.; Sup, S.J.; Findeis, P.M.; Clapp, C.H. Cationic Substrates of Soybean Lipoxygenase-1. Bioorganic Chem. 2011, 39, 94–100. [Google Scholar] [CrossRef]
- Gaffney, B.J. Connecting lipoxygenase function to structure by electron paramagnetic resonance. Acc. Chem. Res. 2014, 47, 3588–3595. [Google Scholar] [CrossRef]
- Ngin, P.; Cho, K.; Han, O. Immobilization of Soybean Lipoxygenase on Nanoporous Rice Husk Silica by Adsorption: Retention of Enzyme Function and Catalytic Potential. Molecules 2021, 26, 291. [Google Scholar] [CrossRef] [PubMed]
- Klinman, J.P. Importance of Protein Dynamics during Enzymatic C–H Bond Cleavage Catalysis. Biochemistry 2013, 52, 2068–2077. [Google Scholar] [CrossRef]
- Shin, J.H.; Van, K.; Kim, D.H.; Kim, K.D.; Jang, Y.E.; Choi, B.-S.; Kim, M.Y.; Lee, S.-H. The Lipoxygenase Gene Family: A Genomic Fossil of Shared Polyploidy between Glycine Max and Medicago Truncatula. BMC Plant Biol. 2008, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, P.; Li, C.; Han, S.; Lopez-Baltazar, J.; Zhang, X.; Wang, X. Identification of Lipoxygenase (LOX) Genes from Legumes and Their Responses in Wild Type and Cultivated Peanut upon Aspergillus Flavus Infection. Sci. Rep. 2016, 6, 35245. [Google Scholar] [CrossRef] [PubMed]
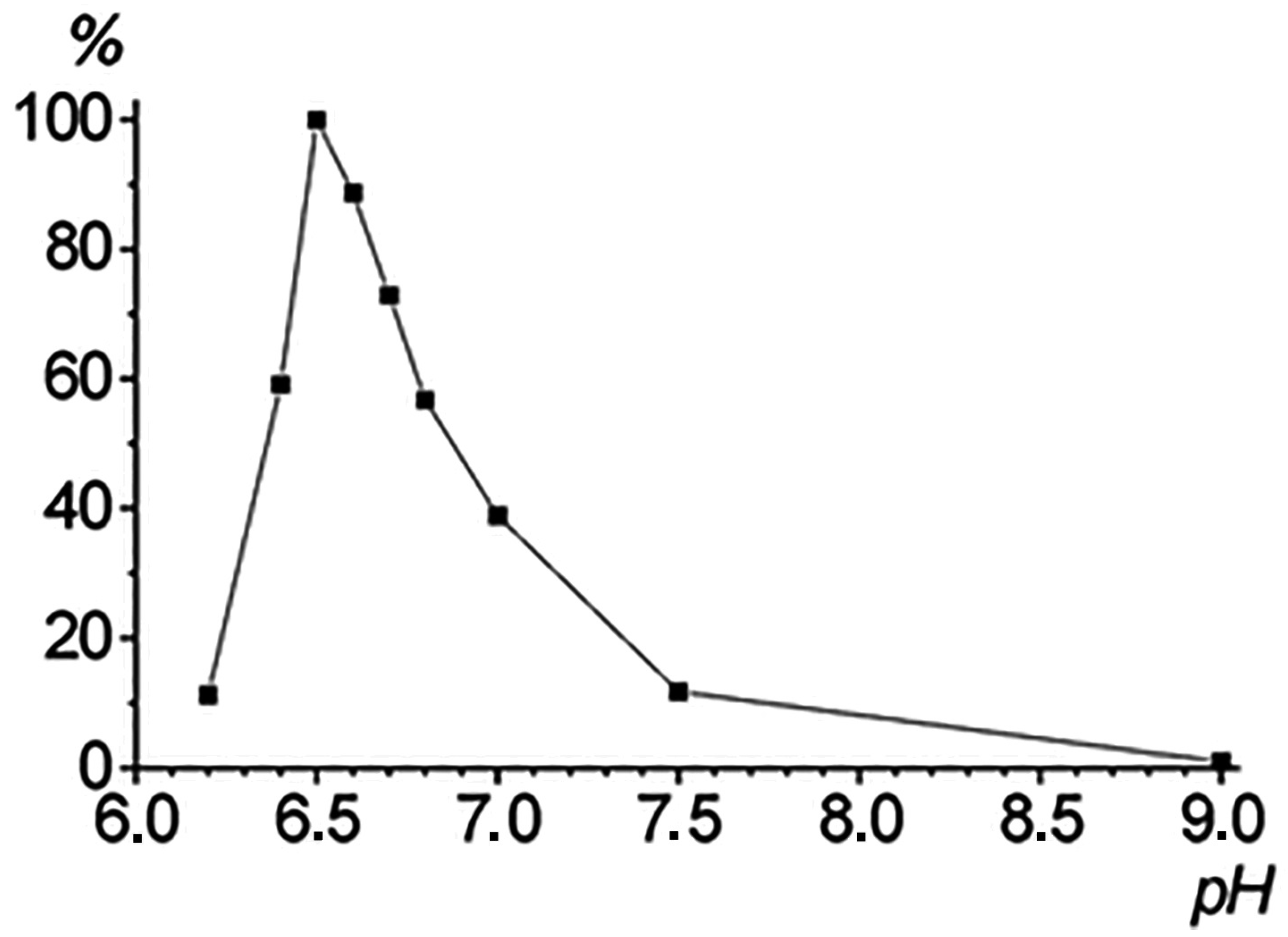
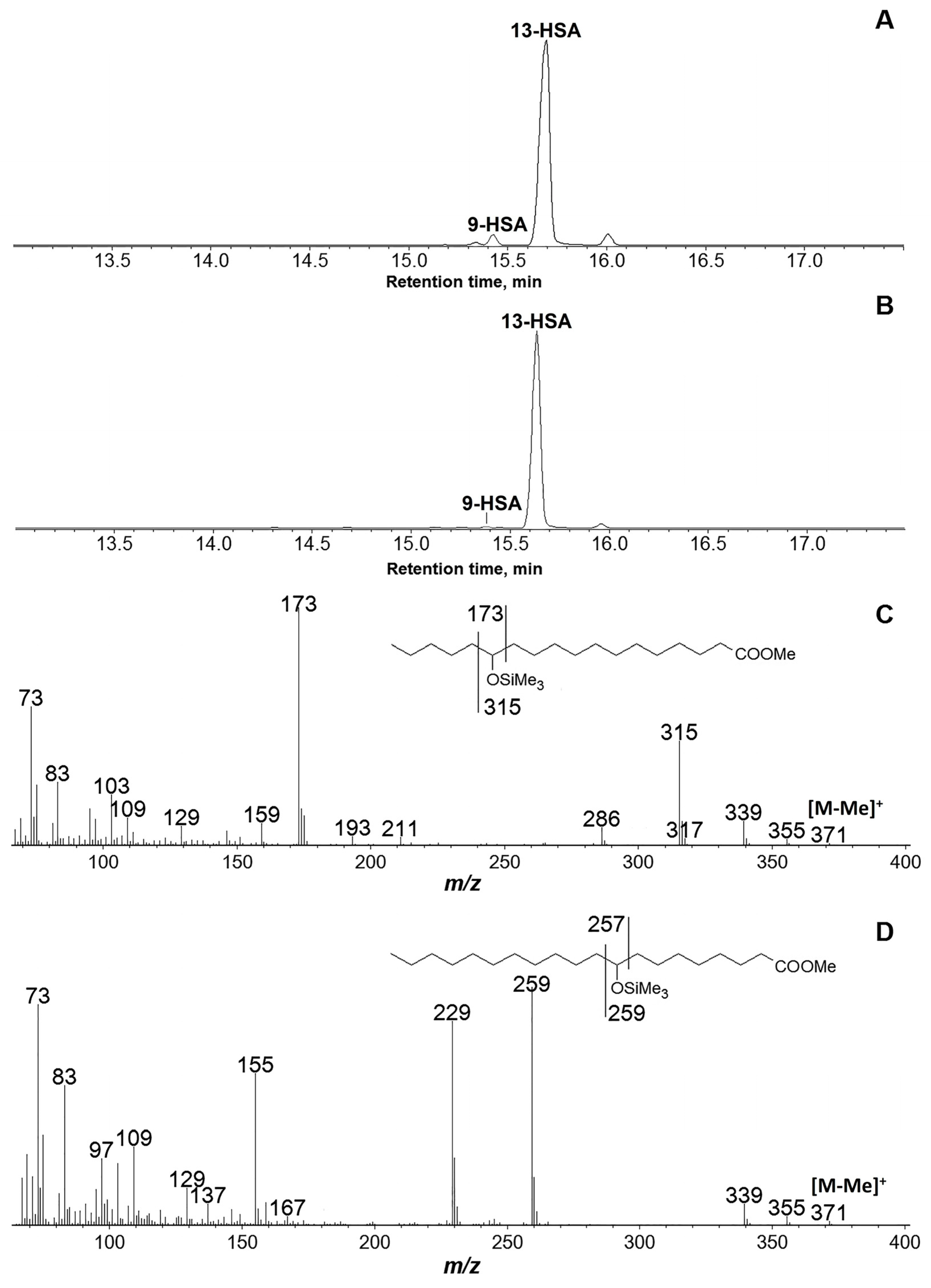
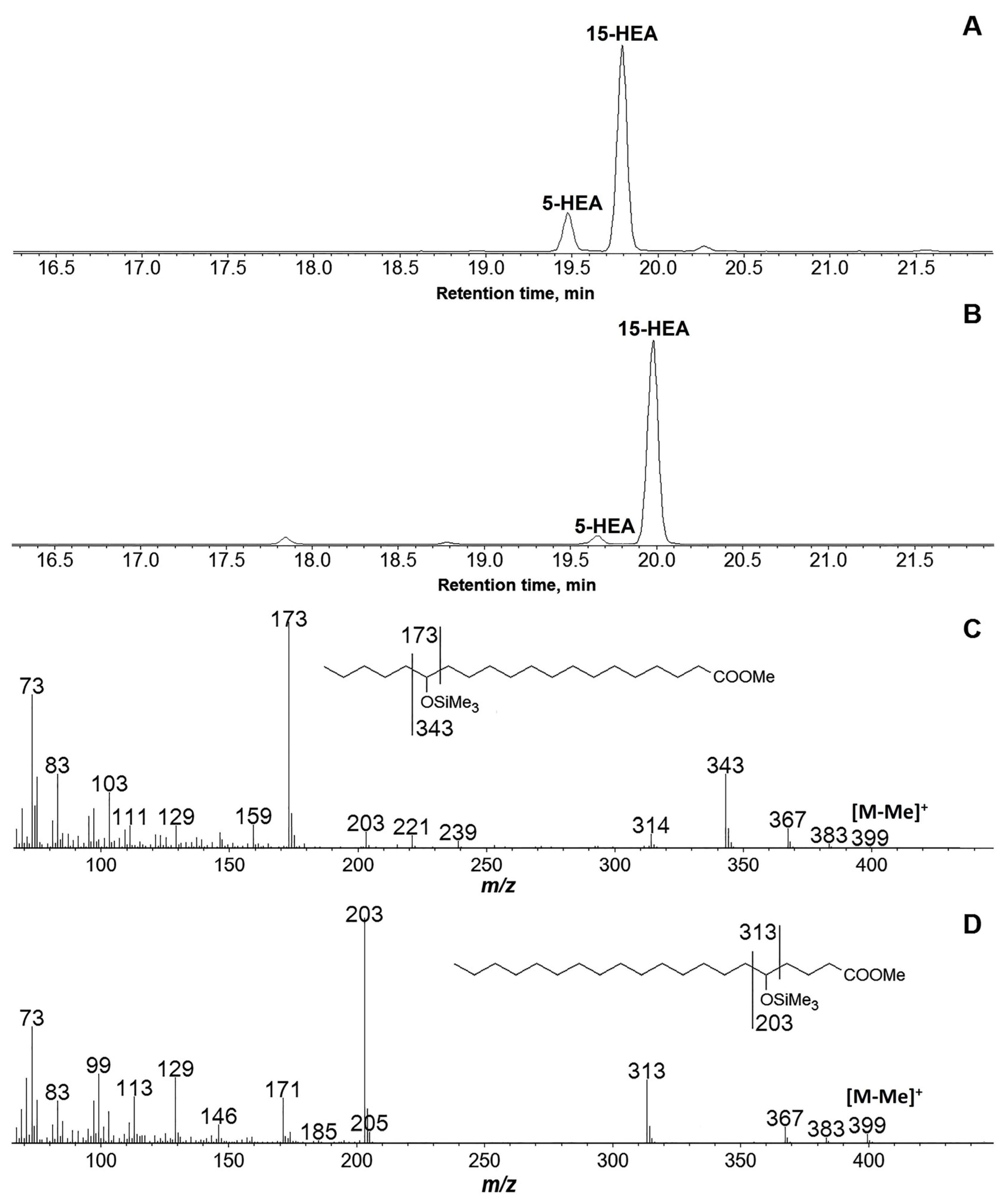
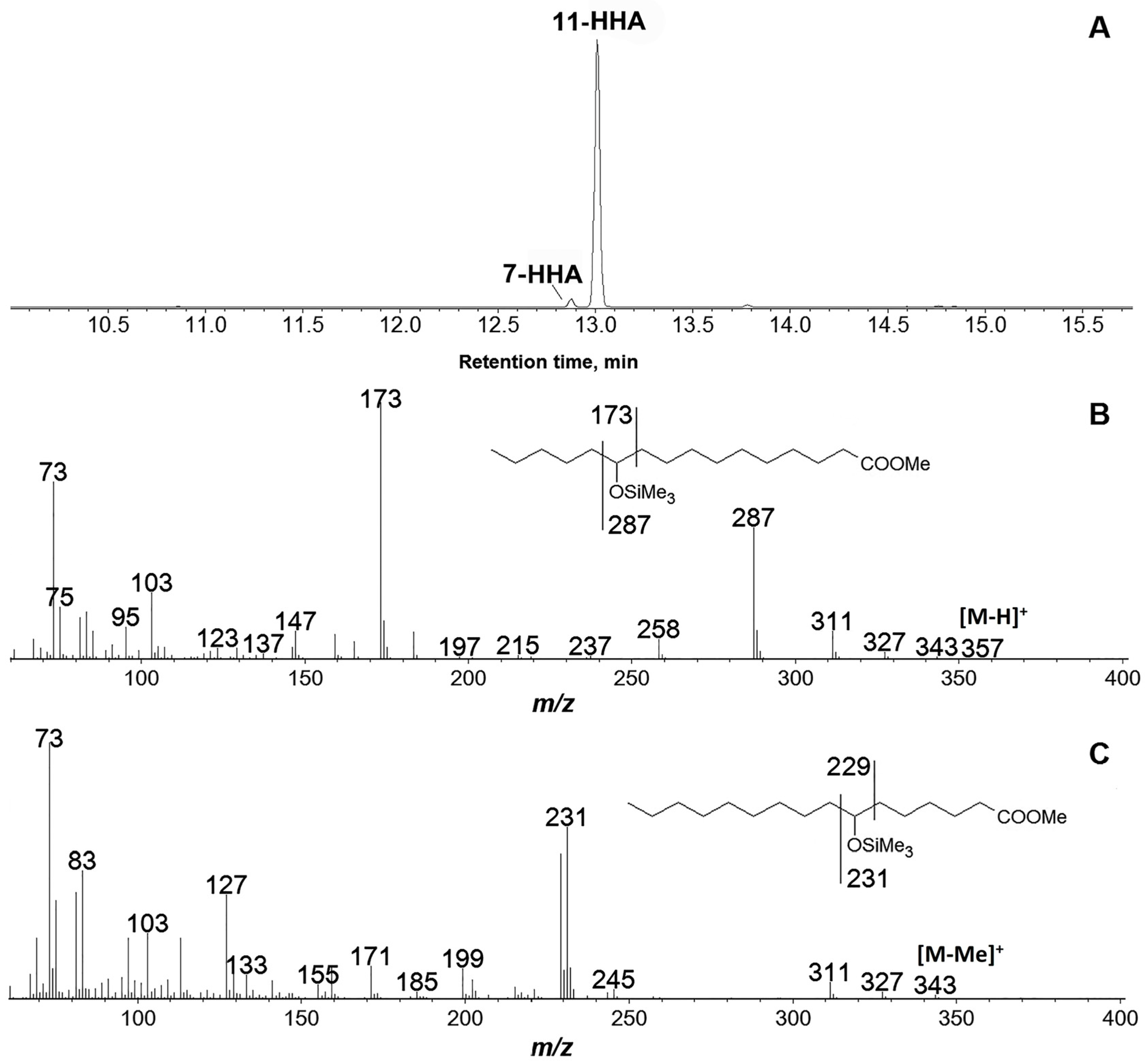
| Substrate | kcat (s−1) | KM (μM) | kcat/KM (μM−1∙s−1) | Specificity, % |
|---|---|---|---|---|
| 18:2, linoleic acid | 61.9 ± 2.8 | 10.6 ± 1.2 | 5.8 | 100 |
| 18:3, α-linolenic acid | 66.6 ± 3.8 | 20.8 ± 1.5 | 3.2 | 55.17 |
| pH | 13-HPOD/9-HPOD | 13S-/13R-HPOD | 9S-/9R-HPOD |
|---|---|---|---|
| 6.5 | 94:6 | 95:5 | 52:48 |
| 9.0 | 87:13 | 95:5 | 52:48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, E.O.; Egorova, A.M.; Lantsova, N.V.; Chechetkin, I.R.; Toporkova, Y.Y.; Grechkin, A.N. Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω6(S)-Lipoxygenase. Curr. Issues Mol. Biol. 2023, 45, 6283-6295. https://doi.org/10.3390/cimb45080396
Smirnova EO, Egorova AM, Lantsova NV, Chechetkin IR, Toporkova YY, Grechkin AN. Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω6(S)-Lipoxygenase. Current Issues in Molecular Biology. 2023; 45(8):6283-6295. https://doi.org/10.3390/cimb45080396
Chicago/Turabian StyleSmirnova, Elena O., Alevtina M. Egorova, Natalia V. Lantsova, Ivan R. Chechetkin, Yana Y. Toporkova, and Alexander N. Grechkin. 2023. "Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω6(S)-Lipoxygenase" Current Issues in Molecular Biology 45, no. 8: 6283-6295. https://doi.org/10.3390/cimb45080396
APA StyleSmirnova, E. O., Egorova, A. M., Lantsova, N. V., Chechetkin, I. R., Toporkova, Y. Y., & Grechkin, A. N. (2023). Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω6(S)-Lipoxygenase. Current Issues in Molecular Biology, 45(8), 6283-6295. https://doi.org/10.3390/cimb45080396


_Kim.png)



