Clinicopathological Characteristics and Mutational Landscape of APC, HOXB13, and KRAS among Rwandan Patients with Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
2.2. Patients
2.3. DNA Extraction
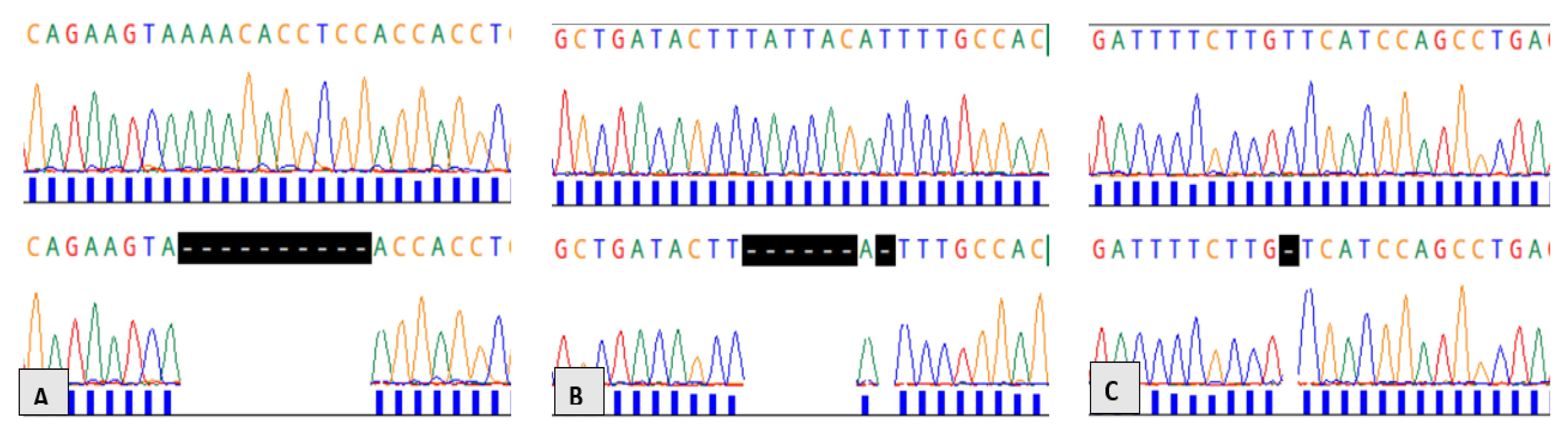
2.4. DNA Amplification and Sanger Sequencing
2.5. TA Cloning and Plasmid Sequence Analysis for Insertion/Deletion Cases
2.5.1. Ligation Reaction Using pGEM®-T Easy Vector System
2.5.2. Transformation Using Highly Competent DH5α E. coli Cells
2.5.3. Screening for Transformants followed by Sanger Sequencing of Positive Subclones
2.6. Mutation Detection, Annotation, and In Silico Analysis
3. Results
3.1. Clinicopathological Characteristics of Patients
3.2. Characteristics of APC Genetic Variants
3.3. Characteristics of HOXB13 Genetic Variants
3.4. Characteristics of Genetic Variants in the KRAS Gene
4. Discussion
4.1. New Mutations in the APC Gene
4.2. Variants in the HOXB13 Gene
4.3. KRAS Missense Mutations
4.4. Smoking, Alcohol Consumption, Diet, and Other Environmental Risk Factors for CRC
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALFA | Allele frequency aggregator |
| APC | Adenomatous polyposis coli |
| CHUK | University Teaching Hospital of Kigali |
| CMHS | College of Medicine and health sciences |
| COSMIC | Catalogue of somatic mutation in cancer |
| DNA | Deoxyribonucleic acid |
| HOXB13 | Homeobox B13 |
| IRB | Institution review board |
| KRAS | Kirsten rat sarcoma |
| LMICs | Low- and middle-income countries |
| SNP | Single-nucleotide polymorphism |
| WHO | World health organization |
References
- Kayamba, V.; Mutale, W.; Cassell, H.; Heimburger, D.C.; Shu, X.-O. Systematic Review of Cancer Research Output From Africa, With Zambia as an Example. JCO Glob. Oncol. 2021, 7, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, Y.; Abdeljaoued-Tej, I.; Zatchi, A.A.; Abdelhak, S.; Boubaker, S.; Brown, J.S.; Benkahla, A. Cancer in Africa: The Untold Story. Front. Oncol. 2021, 11, 650117. [Google Scholar] [CrossRef] [PubMed]
- Arhin, N.; Ssentongo, P.; Taylor, M.; Olecki, E.J.; Pameijer, C.; Shen, C.; Oh, J.; Eng, C. Age-standardised incidence rate and epidemiology of colorectal cancer in Africa: A systematic review and meta-analysis. BMJ Open 2022, 12, e052376. [Google Scholar] [CrossRef] [PubMed]
- Odedina, F.T.; Rotimi, S. Promoting cancer genomics research in Africa: A roadmap. Nat. Rev. Cancer 2021, 21, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, S.O.; Rotimi, O.A.; Salhia, B. A Review of Cancer Genetics and Genomics Studies in Africa. Front. Oncol. 2021, 10, 606400. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Awedew, A.F.; Asefa, Z.; Belay, W.B. Burden and trend of colorectal cancer in 54 countries of Africa 2010-2019: A systematic examination for Global Burden of Disease. BMC Gastroenterol. 2022, 22, 204. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- National Institute of Statistics of Rwanda (NISR). The Fifth Rwanda Population and Housing Census, Main Indicators Report, February 2023; National Institute of Statistics of Rwanda (NISR): Kigali, Rwanda, 2023; p. 3. ISBN 978-99977-43-09-1.
- Habyarimana, T.; Bakri, Y.; Mugenzi, P.; Mazarati, J.B.; Attaleb, M.; El Mzibri, M. Association between glutathione peroxidase 1 codon 198 variant and the occurrence of breast cancer in Rwanda. Mol. Genet. Genom. Med. 2018, 6, 268–275. [Google Scholar] [CrossRef]
- Habyarimana, T.; Attaleb, M.; Mugenzi, P.; Mazarati, J.B.; Bakri, Y.; El Mzibri, M. Association of p53 Codon 72 Polymorphism with Breast Cancer in a Rwandese Population. Pathobiology 2018, 85, 186–191. [Google Scholar] [CrossRef]
- Habyarimana, T.; Attaleb, M.; Mugenzi, P.; Mazarati, J.B.; Bakri, Y.; El Mzibri, M. CHEK2 Germ Line Mutations are Lacking among Familial and Sporadic Breast Cancer Patients in Rwanda. Asian Pac. J. Cancer Prev. 2018, 19, 375–379. [Google Scholar] [CrossRef]
- Uyisenga, J.P.; Debit, A.; Poulet, C.; Frères, P.; Poncin, A.; Thiry, J.; Mutesa, L.; Jerusalem, G.; Bours, V.; Josse, C. Differences in plasma microRNA content impair microRNA-based signature for breast cancer diagnosis in cohorts recruited from heterogeneous environmental sites. Sci. Rep. 2021, 11, 11698:1–11698:15. [Google Scholar] [CrossRef]
- Uyisenga, J.P.; Segers, K.; Lumaka, A.Z.; Mugenzi, P.; Fasquelle, C.; Boujemila, B.; Josse, C.; Mutesa, L.; Bours, V. Screening of germline mutations in young Rwandan patients with breast cancers. Mol. Genet. Genom. Med. 2020, 8, e1500. [Google Scholar] [CrossRef]
- Brand, M.; Gaylard, P.; Ramos, J. Colorectal cancer in South Africa: An assessment of disease presentation, treatment pathways and 5-year survival. S. Afr. Med. J. 2018, 108, 118–122. [Google Scholar] [CrossRef]
- Alatise, O.I.; Knapp, G.C.; Sharma, A.; Chatila, W.K.; Arowolo, O.A.; Olasehinde, O.; Famurewa, O.C.; Omisore, A.D.; Komolafe, A.O.; Olaofe, O.O.; et al. Molecular and phenotypic profiling of colorectal cancer patients in West Africa reveals biological insights. Nat. Commun. 2021, 12, 6821. [Google Scholar] [CrossRef]
- Uwamariya, D.; Ruhangaza, D.; Rugwizangoga, B. Pathological Characteristics, Prognostic Determinants and the Outcome of Patients Diagnosed with Colorectal Adenocarcinoma at the University Teaching Hospital of Kigali. Can. J. Gastroenterol. Hepatol. 2022, 2022, 6608870. [Google Scholar] [CrossRef]
- Fadelu, T.; Sebahungu, F.; Diasti, K.; Nguyen, C.; Yeh, T.; Shyirambere, C.; Nkusi, E.; Nsabimana, N.; Ruhangaza, D.; DeBoer, R.J.; et al. Patient characteristics and outcomes of colorectal cancer (CRC) at Butaro Cancer Center of Excellence (BCCOE): Results from a retrospective cohort. J. Clin. Oncol. 2020, 38 (Suppl. 15), e16081. [Google Scholar] [CrossRef]
- Rubagumya, F.; Costas-Chavarri, A.; Manirakiza, A.; Murenzi, G.; Uwinkindi, F.; Ntizimira, C.; Rukundo, I.; Mugenzi, P.; Rugwizangoga, B.; Shyirambere, C.; et al. State of Cancer Control in Rwanda: Past, Present, and Future Opportunities. JCO Glob. Oncol. 2020, 6, 1171–1177. [Google Scholar] [CrossRef]
- Cancedda, C.; Cotton, P.; Shema, J.; Rulisa, S.; Riviello, R.; Adams, L.V.; Farmer, P.E.; Kagwiza, J.N.; Kyamanywa, P.; Mukamana, D.; et al. Health Professional Training and Capacity Strengthening Through International Academic Partnerships: The First Five Years of the Human Resources for Health Program in Rwanda. Int. J. Health Policy Manag. 2018, 7, 1024–1039. [Google Scholar] [CrossRef]
- Stulac, S.; Binagwaho, A.; Tapela, N.M.; Wagner, C.M.; Muhimpundu, M.A.; Ngabo, F.; Nsanzimana, S.; Kayonde, L.; Bigirimana, J.B.; Lessard, A.J.; et al. Capacity building for oncology programmes in sub-Saharan Africa: The Rwanda experience. Lancet. Oncol. 2015, 16, e405–e413. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Graff, R.E.; Möller, S.; Passarelli, M.N.; Witte, J.S.; Skytthe, A.; Christensen, K.; Tan, Q.; Adami, H.-O.; Czene, K.; Harris, J.R.; et al. Familial Risk and Heritability of Colorectal Cancer in the Nordic Twin Study of Cancer. Clin. Gastroenterol. Hepatol. 2017, 15, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Irabor, D.O.; Oluwasola, O.A.; Ogunbiyi, O.J.; Ogun, O.G.; Okolo, C.A.; Melas, M.; Gruber, S.B.; Shi, C.; Raskin, L. Microsatellite Instability Is Common in Colorectal Cancer in Native Nigerians. Anticancer Res. 2017, 37, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.A.; Lee, J.K.; Madison, R.W.; Pradhan, K.; Newberg, J.Y.; Isasi, C.R.; Klempner, S.J.; Frampton, G.M.; Ross, J.S.; Venstrom, J.M.; et al. The Genomics of Colorectal Cancer in Populations with African and European Ancestry. Cancer Discov. 2022, 12, 1282–1293. [Google Scholar] [CrossRef]
- Guda, K.; Veigl, M.L.; Varadan, V.; Nosrati, A.; Ravi, L.; Lutterbaugh, J.; Beard, L.; Willson, J.K.V.; Sedwick, W.D.; Wang, Z.J.; et al. Novel recurrently mutated genes in African American colon cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 1149–1154. [Google Scholar] [CrossRef]
- Augustus, G.J.; Ellis, N.A. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. Am. J. Pathol. 2018, 188, 291–303. [Google Scholar] [CrossRef]
- Aoki, K.; Taketo, M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell Sci. 2007, 120, 3327–3335. [Google Scholar] [CrossRef]
- Schell, M.J.; Yang, M.; Teer, J.K.; Lo, F.Y.; Madan, A.; Coppola, D.; Monteiro, A.N.A.; Nebozhyn, M.V.; Yue, B.; Loboda, A.; et al. A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat. Commun. 2016, 7, 11743. [Google Scholar] [CrossRef]
- Zafra, M.P.; Parsons, M.J.; Kim, J.; Alonso-Curbelo, D.; Goswami, S.; Schatoff, E.M.; Han, T.; Katti, A.; Fernandez, M.T.C.; Wilkinson, J.E.; et al. An In Vivo Kras Allelic Series Reveals Distinct Phenotypes of Common Oncogenic Variants. Cancer Discov. 2020, 10, 1654–1671. [Google Scholar] [CrossRef]
- Cronjé, L.; Becker, P.J.; Paterson, A.C.; Ramsay, M. Hereditary non-polyposis colorectal cancer is predicted to contribute towards colorectal cancer in young South African blacks. S. Afr. J. Sci. 2009, 105, 68–72. [Google Scholar] [CrossRef]
- Wang, D.; Agrawal, R.; Zou, S.; Haseeb, M.A.; Gupta, R. Anatomic location of colorectal cancer presents a new paradigm for its prognosis in African American patients. PLoS ONE 2022, 17, e0271629. [Google Scholar] [CrossRef]
- Silva, M.G. The Role of HOXB13 Gene in Colorectal Cancer. Master’s Thesis, Universidade do Porto, Porto, Portugal, 2013. [Google Scholar]
- Xie, B.; Bai, B.; Xu, Y.; Liu, Y.; Lv, Y.; Gao, X.; Wu, F.; Fang, Z.; Lou, Y.; Pan, H.; et al. Tumor-suppressive function and mechanism of HOXB13 in right-sided colon cancer. Signal Transduct. Target. Ther. 2019, 4, 51:1–51:14. [Google Scholar] [CrossRef]
- Akbari, M.R.; Anderson, L.N.; Buchanan, D.D.; Clendenning, M.; Jenkins, M.A.; Win, A.K.; Hopper, J.L.; Giles, G.G.; Nam, R.; Narod, S.; et al. Germline HOXB13 p.Gly84Glu mutation and risk of colorectal cancer. Cancer Epidemiol. 2013, 37, 424–427. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, X.; Li, X.; Gong, R.; Guo, X.; Tang, Y.; Yang, K.; Niu, Y.; Zhao, Y. Germline HOXB13 p.Gly84Glu mutation and cancer susceptibility: A pooled analysis of 25 epidemiological studies with 145,257 participates. Oncotarget 2015, 6, 42312–42321. [Google Scholar] [CrossRef]
- Laitinen, V.H.; Wahlfors, T.; Saaristo, L.; Rantapero, T.; Pelttari, L.M.; Kilpivaara, O.; Laasanen, S.-L.; Kallioniemi, A.; Nevanlinna, H.; Aaltonen, L.; et al. HOXB13 G84E mutation in Finland: Population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2013, 22, 452–460. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. Diagnostic Histopathology of Tumors, 5th ed.; Elsevier: Philadelphia, Pennsylvania, 2019; Volume 1, p. 493. [Google Scholar]
- WHO Classification of Tumors Editorial Board. Digestive system tumors. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1, p. 182. [Google Scholar]
- Kohler, E.M.; Derungs, A.; Daum, G.; Behrens, J.; Schneikert, J. Functional definition of the mutation cluster region of adenomatous polyposis coli in colorectal tumours. Hum. Mol. Genet. 2008, 17, 1978–1987. [Google Scholar] [CrossRef]
- Sameer, A.S.; Shah, Z.A.; Abdullah, S.; Chowdri, N.A.; Siddiqi, M.A. Analysis of molecular aberrations of Wnt pathway gladiators in colorectal cancer in the Kashmiri population. Hum. Genom. 2011, 5, 441. [Google Scholar] [CrossRef]
- Cheadle, J.P.; Krawczak, M.; Thomas, M.W.; Hodges, A.K.; Al-Tassan, N.; Fleming, N.; Sampson, J.R. Different combinations of biallelic APC mutation confer different growth advantages in colorectal tumours. Cancer Res. 2002, 62, 363–366. [Google Scholar]
- Christie, M.; Jorissen, R.N.; Mouradov, D.; Sakthianandeswaren, A.; Li, S.; Day, F.; Tsui, C.; Lipton, L.; Desai, J.; Jones, I.T.; et al. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/β-catenin signalling thresholds for tumourigenesis. Oncogene 2013, 32, 4675–4682. [Google Scholar] [CrossRef]
- Brechka, H.; Bhanvadia, R.R.; VanOpstall, C.; Vander Griend, D.J. HOXB13 mutations and binding partners in prostate development and cancer: Function, clinical significance, and future directions. Genes Dis. 2017, 4, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ibarra, H.E.; Reyes-Cortes, L.M.; López-Tavera, E.; Luna-Aguirre, C.M.; Barrera-Saldaña, H.A. Complete Screening of Exons 2, 3, and 4 of KRAS and NRAS Genes Reveals a Higher Number of Clinically Relevant Mutations than Food and Drug Administration Quantitative Polymerase Chain Reaction-Based Commercial Kits. Rev. Investig. Clin. 2020, 72, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T. The current understanding of KRAS protein structure and dynamics. Comput. Struct. Biotechnol. J. 2020, 18, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Natsume, H.; Szczepaniak, K.; Yamada, H.; Iwashita, Y.; Gędek, M.; Šuto, J.; Ishino, K.; Kasajima, R.; Matsuda, T.; Manirakiza, F.; et al. Non-CpG sites preference in G:C > A:T transition of TP53 in gastric cancer of Eastern Europe (Poland, Romania and Hungary) compared to East Asian countries (China and Japan). Genes Environ. 2023, 45, 1. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE team Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.M. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef]
- Steinhaus, R.; Proft, S.; Schuelke, M.; Cooper, D.N.; Schwarz, J.M.; Seelow, D. MutationTaster2021. Nucleic Acids Res. 2021, 49, W446–W451. [Google Scholar] [CrossRef]
- Darst, B.F.; Hughley, R.; Pfennig, A.; Hazra, U.; Fan, C.; Wan, P.; Sheng, X.; Xia, L.; Andrews, C.; Chen, F.; et al. A Rare Germline HOXB13 Variant Contributes to Risk of Prostate Cancer in Men of African Ancestry. Eur. Urol. 2022, 81, 458–462. [Google Scholar] [CrossRef]
- Musabende, M.; Dusabejambo, V.; Kabakambira, J.D.; Ndayisenga, L.; Page, C. Assessment of the Outcome of Gastrointestinal Neoplasms Diagnosed at Kigali University Teaching Hospital. World Wide J. Multidiscip. Res. Dev. 2021, 7, 36–42. [Google Scholar]
- Kiringa, K.S.; Zalzal, M.; Bahizi, K.E.; Hangi, M.S.; Kihemba, K.; Bartels, S.A. Familial Adenomatous Polyposis (FAP): A case observed in eastern Democratic Republic of the Congo. Afr. J. Gastroenterol. Hepatol. 2022, 5, 32–39. [Google Scholar] [CrossRef]
- Bojuwoye, M.O.; Olokoba, A.B.; Ogunlaja, O.A.; Agodirin, S.O.; Ibrahim, O.K.; Okonkwo, K.C.; Aliyu, A.M. Familial adenomatous polyposis syndrome with colorectal cancer in two Nigerians: A report of two cases and review of literature. Pan Afr. Med. J. 2018, 30, 6:1–6:5. [Google Scholar] [CrossRef]
- McQuaide, J.R.; Stewart, A.W. Familial polyposis of the colon in the Bantu. S. Afr. Med. J. 1972, 46, 1241–1246. [Google Scholar]
- Sebbaale, A.K.; Obote, W.W.; Kituuka, O. Familial adenomatous polyposis: A case report. East Cent. Afr. J. Surg. 2004, 9, 54–55. [Google Scholar]
- Osuagwu, C.C.; Okafor, O.C.; Ezeome, E.R.; Uche, C.E.; Ememonu, C.; Kesieme, E. Familial adenomatous polyposis with synchronous invasive colonic carcinomas and metastatic jejunal adenocarcinoma in a Nigerian male. Rare Tumors 2010, 2, 189–191. [Google Scholar] [CrossRef]
- Alese, O.B.; Irabor, D.O. Adenomatous polyposis coli in an elderly female nigerian. Ghana Med. J. 2009, 43, 139–141. [Google Scholar] [CrossRef]
- Wismayer, R. Familial Adenomatous Polyposis Coli in East Africa: A Case Report and Review of the Literature. J. Adv. Med. Med. Res. 2020, 32, 74–80. [Google Scholar] [CrossRef]
- Kakembo, N.; Kisa, P.; Sekabira, J.; Ogdzediz, D. Colorectal Polyposis in a 15 Year Old Boy in Uganda-Case Report. East Cent. Afr. J. Surg. 2016, 21, 105–108. [Google Scholar] [CrossRef]
- Udofot, S.U.; Ekpo, M.D.; Khalil, M.I. Familial polyposis coli: An unusual case in West Africa. Cent. Afr. J. Med. 1992, 38, 44–48. [Google Scholar]
- Alfred, R.; Mills, M. Adenomatous polyposis in a young jamaican male of african descent. West Indian Med. J. 2014, 63, 186–188. [Google Scholar] [CrossRef]
- Joseph, R.; Little, P.; Hayes, D.N.; Lee, M.S. Characterization of the number and site of APC mutations in sporadic colorectal cancer. J. Clin. Oncol. 2017, 35, 630. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Nagase, H.; Ando, H.; Horii, A.; Ichii, S.; Nakatsuru, S.; Aoki, T.; Miki, Y.; Mori, T.; Nakamura, Y. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum. Mol. Genet. 1992, 1, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Lüchtenborg, M.; Weijenberg, M.P.; Roemen, G.M.J.M.; de Bruïne, A.P.; van den Brandt, P.A.; Lentjes, M.H.F.M.; Brink, M.; van Engeland, M.; Goldbohm, R.A.; de Goeij, A.F.P.M. APC mutations in sporadic colorectal carcinomas from The Netherlands Cohort Study. Carcinogenesis 2004, 25, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lange, E.M.; Lu, L.; Zheng, S.L.; Wang, Z.; Thibodeau, S.N.; Cannon-Albright, L.A.; Teerlink, C.C.; Camp, N.J.; Johnson, A.M.; et al. HOXB13 is a susceptibility gene for prostate cancer: Results from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum. Genet. 2013, 132, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Greenwood, C.; Isaacs, W.B.; Foulkes, W.D.; Sun, J.; Zheng, S.L.; Condreay, L.D.; Xu, J. The G84E mutation of HOXB13 is associated with increased risk for prostate cancer: Results from the REDUCE trial. Carcinogenesis 2013, 34, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Momozawa, Y.; Iwasaki, Y.; Hirata, M.; Liu, X.; Kamatani, Y.; Takahashi, A.; Sugano, K.; Yoshida, T.; Murakami, Y.; Matsuda, K.; et al. Germline Pathogenic Variants in 7636 Japanese Patients With Prostate Cancer and 12 366 Controls. J. Natl. Cancer Inst. 2020, 112, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Qu, L.; Chen, Z.; Xu, C.; Ye, D.; Shao, Q.; Wang, X.; Qi, J.; Chen, Z.; Zhou, F.; et al. A novel Germline mutation in HOXB13 is associated with prostate cancer risk in Chinese men. Prostate 2013, 73, 169–175. [Google Scholar] [CrossRef]
- Ben Salah, K.; Emaetig, F.; Jebriel, A.; Shagan, A.; El Mahjoubi, E.; Obaid, E. KRAS Testing for Colorectal Cancer Patients: Our Laboratory Experience in The Libyan National Cancer Institute. Libyan Int. J. Oncol. 2022, 1, 14–21. [Google Scholar]
- Marbun, V.M.G.; Erlina, L.; Lalisang, T.J.M. Genomic landscape of pathogenic mutation of APC, KRAS, TP53, PIK3CA, and MLH1 in Indonesian colorectal cancer. PLoS ONE 2022, 17, e0267090. [Google Scholar] [CrossRef]
- Koulouridi, A.; Karagianni, M.; Messaritakis, I.; Sfakianaki, M.; Voutsina, A.; Trypaki, M.; Bachlitzanaki, M.; Koustas, E.; Karamouzis, M.V.; Ntavatzikos, A.; et al. Prognostic Value of KRAS Mutations in Colorectal Cancer Patients. Cancers 2022, 14, 3320:1–3320:10. [Google Scholar] [CrossRef]
- Abudabous, A.; Drah, M.; Aldehmani, M.; Parker, I.; Alqawi, O. KRAS mutations in patients with colorectal cancer in Libya. Mol. Clin. Oncol. 2021, 15, 1–6. [Google Scholar] [CrossRef]
- Mounjid, C.; El Agouri, H.; Mahdi, Y.; Laraqui, A.; Chtati, E.; Ech-charif, S.; Khmou, M.; Bakri, Y.; Souadka, A.; El Khannoussi, B. Assessment of KRAS and NRAS status in metastatic colorectal cancer--Experience of the National Institute of Oncology in Rabat Morocco. Ann. Cancer Res. Ther. 2022, 30, 80–84. [Google Scholar] [CrossRef]
- Wismayer, R.; Kiwanuka, J.; Wabinga, H.; Odida, M. Risk Factors for Colorectal Adenocarcinoma in an Indigenous Population in East Africa. Cancer Manag. Res. 2022, 14, 2657–2669. [Google Scholar] [CrossRef]
- Wang, L.; Du, M.; Wang, K.; Khandpur, N.; Rossato, S.L.; Drouin-Chartier, J.-P.; Steele, E.M.; Giovannucci, E.; Song, M.; Zhang, F.F. Association of ultra-processed food consumption with colorectal cancer risk among men and women: Results from three prospective US cohort studies. BMJ 2022, 378, e068921:1–e068921:10. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Mashingaidze, N.; Ekesa, B.; Ndayisaba, C.P.; Njukwe, E.; Groot, J.C.J.; Gwazane, M.; Vanlauwe, B. Participatory Exploration of the Heterogeneity in Household Socioeconomic, Food, and Nutrition Security Status for the Identification of Nutrition-Sensitive Interventions in the Rwandan Highlands. Front. Sustain. Food Syst. 2020, 4, 47. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Sudo, N.; Amitani, Y.; Caballero, Y.; Sekiyama, M.; Mukamugema, C.; Matsuoka, T.; Imanishi, H.; Sasaki, T.; Matsuda, H. Development and Validation of a Data-Based Food Frequency Questionnaire for Adults in Eastern Rural Area of Rwanda. Nutr. Metab. Insights 2016, 9, 31–42. [Google Scholar] [CrossRef]
- Paridaens, A.-M. Rwanda 2021|Comprehensive Food Security and Vulnerability Analysis; National Institute of Statistics of Rwanda (NISR): Kigali, Rwanda, 2021; pp. 41–47.
| Case No | Age at Diagnosis | Sex | Site | Grade | Ever Smoker (Self-Reported) | Ever Alcohol Intake (Self-Reported) | Family History of Cancer (Self-Reported) | APC Mutation # | KRAS Mutation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | F | Rectum | Grade 2 | No | Yes | No | ||
| 2 | 59 | F | Rectum | Grade 2 | No | No | No | Yes | |
| 3 | 31 | M | Rectum | Grade 1 | No | Yes | No | Yes | Yes |
| 4 | 83 | F | Anorectum | Grade 2 | NA | NA | No information | Yes 2 | Yes |
| 5 | 47 | F | Rectum | Grade 3 | No | Yes | No | Yes | |
| 6 | 50 | M | Rectum | Grade 2 | No | Yes | No | ||
| 7 | 89 | F | Rectum | Grade 1 | Yes | Yes | No | ||
| 8 | 61 | F | Rectum | Grade 1 | NA | NA | No | Yes 3 | Yes |
| 9 | 66 | F | Colon | Grade 3 | No | No | No | Yes | |
| 10 | 32 | F | Rectum | Grade 3 | No | Yes | No | Yes | Yes |
| 11 | 76 | F | Rectum | Grade 3 | No | Yes | No information | ||
| 12 | 51 | F | Rectum | Grade 1 | No | No | No information | ||
| 13 | 81 | M | Rectum | Grade 2 | Yes | Yes | No | Yes | |
| 14 | 49 | F | Rectum | Grade 2 | No | No | No | Yes | |
| 15 | 72 | M | Rectum | Grade 1 | No | Yes | No | ||
| 16 | 63 | F | Rectum | Grade 2 | No | Yes | No | Yes | |
| 17 | 59 | F | Rectum | Grade 2 | No | Yes | No | Yes | Yes |
| 18 | 61 | F | Colon | Grade 1 | No | No | No | Yes 2 | Yes |
| 19 | 68 | M | Rectum | Grade 2 | No | Yes | No information | Yes | Yes |
| 20 | 59 | F | Rectum | Grade 1 | No | Yes | No information | Yes | |
| 21 | 53 | M | Colon | Grade 2 | No | Yes | No | Yes | Yes |
| 22 | 61 | M | Rectum | Grade 1 | Yes | Yes | No | Yes | Yes |
| 23 | 59 | M | Rectum | Grade 2 | No | Yes | No | Yes | |
| 24 | 64 | F | Rectum | Grade 1 | No | Yes | No information | Yes 2 | |
| 25 | 63 | M | Colon | Grade 1 | No | Yes | No | Yes | |
| 26 | 49 | M | Colon | Grade 1 | No | Yes | No information | Yes | Yes |
| 27 | 56 | M | Rectum | Grade 1 | Yes | Yes | No information | Yes | |
| 28 | 73 | F | Rectum | Grade 2 | No | Yes | No information | ||
| 29 | 77 | F | Rectum | Grade 2 | Yes | Yes | No information | Yes | |
| 30 | 73 | M | Rectum | Grade 2 | Yes | Yes | No | Yes | |
| 31 | 62 | M | Rectum | Grade 2 | NA | Yes | No | Yes | Yes |
| 32 | 79 | F | Rectum | Grade 2 | No | Yes | No information | ||
| 33 | 50 | F | Rectum | Grade 2 | No | No | No | ||
| 34 | 67 | F | Rectum | Grade 1 | No | No | No | Yes 2 | Yes |
| 35 | 65 | F | Rectum | Grade 2 | No | Yes | No | Yes | |
| 36 | 45 | M | Rectum | Grade 1 | No | Yes | No information | Yes | |
| 37 | 40 | M | Rectum | Grade 2 | NA | NA | NA | ||
| 38 | 51 | M | Rectum | Grade 2 | No | No | No | Yes | Yes |
| 39 | 57 | F | Rectum | Grade 1 | Yes | No | No information | ||
| 40 | 63 | F | Rectum | Grade 2 | Yes | Yes | No information | ||
| 41 | 78 | F | Rectum | Grade 2 | Yes | Yes | No information | Yes | |
| 42 | 38 | M | Rectum | Grade 1 | No | No | No | ||
| 43 | 45 | F | Rectum | Grade 1 | No | No | No | ||
| 44 | 61 | M | Rectum | Grade 2 | No | No | No | ||
| 45 | 63 | F | Colon | Grade 2 | No | Yes | No | Yes | |
| 46 | 75 | F | Rectum | Grade 1 | Yes | Yes | No information | Yes | |
| 47 | 54 | F | Rectum | Grade 2 | Yes | Yes | No information | Yes | |
| 48 | 36 | M | Rectum | Grade 2 | No | No | No information | Yes | |
| 49 | 65 | M | Rectum | Grade 1 | No | No | No | Yes | |
| 50 | 68 | F | Colon | Grade 2 | No | No | No | ||
| 51 | 52 | F | Rectum | Grade 2 | NA | NA | NA | ||
| 52 | 64 | F | Rectum | Grade 2 | No | Yes | No | ||
| 53 | 74 | F | Rectum | Grade 2 | No | Yes | No | Yes | |
| 54 | 59 | F | Colon | Grade 1 | No | NA | No information | Yes |
| No | dbSNP ID | GRCh38 Coordinate (NC-000005.10) | NM_000038.6 | Protein Change (NP_000029.2) | Codon Change | Clinical Significance $ | Number of Cases with Mutation (N = 54) | MAF ALFA/Global | MAF ALFA/ African | MAF 1000G/ Global | MAF 1000G/ African |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs121913331 | g.112838934C>T | c.3340C>T | p.Arg1114Ter | CGA>TGA | Pathogenic | 1 | NA | NA | NA | NA |
| 2 | rs386833391 | g.112839054-112839056delGAA | c.3460_3462delGAA | p.Glu1157del | NA* | Benign | 3 | 0.00007 | 0.0009 | 0.0048 | 0.0174 |
| 3 | rs138933660 | g.112839244A>C | c.3650A>C | p.Asn1217Thr | AAT>ACT | Likely benign/US | 1 | 0.000065 | 0.000014 | 0.001 | 0.0038 |
| 4 | rs74380081 | g.112839326A>G | c.3732A>G | p.Glu1244= | CAA>CAG | Benign | 4 | 0.00246 | 0.0282 | 0.0142 | 0.0537 |
| 5 | NA | g.112839451_112839453dupA | c.3859dupA | p.Ile1287Asnfs*4 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 6 | rs1064794229 | g.112839514_112839518delTAAAA | c.3920_3924delTAAAA | p.Ile1307Argfs*6 | NA* | Pathogenic | 1 | NA | NA | NA | NA |
| 7 | rs121913224 | g.112839515_112839525delAAAGA | c.3927_3931delAAAGA | p.Glu1309Aspfs*4 | NA* | Pathogenic | 2 | 0.0001 | Zero | NA | NA |
| 8 | NA | g.112839534delA | c.3940delA | p.Arg1314Glyfs*7 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 9 | rs1057517558 | g.112839549_112839550delC | c.3956delC | p.Pro1319Leufs*2 | NA* | Pathogenic | 1 | NA | NA | NA | NA |
| 10 | rs1554085480 | g.112839661C>A | c.4067C>A | p.Ser1356Ter | TCA>TAA | Pathogenic | 1 | NA | NA | NA | NA |
| 11 | rs121913328 | g.112839693C>T | c.4099C>T | p.Glu1367Ter | CAG>TAG | Pathogenic | 1 | NA | NA | NA | NA |
| 12 | NA | g.112839702A>T | c.4108A>T | p.Lys1370Ter | AAA>TAA | Pathogenic | 1 | NA | NA | NA | NA |
| 13 | rs1580645082 | g.112839783G>T | c.4189G>T | p.Glu1397Ter | GAG>TAG | Pathogenic | 1 | NA | NA | NA | NA |
| 14 | rs587782518 | g.112839810C>T | c.4216C>T | p.Gln1406Ter | CAG>TAG | Pathogenic | 1 | NA | NA | NA | NA |
| 15 | NA | g.112839904_112839913delAAACACCTCC | c.4310_4319delAAACACCTCC | p.Lys1437Asnfs*33 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 16 | rs111866410 | g.112839954A>G | c.4360A>G | p.Lys1454Glu | AAA>GAA | Benign | 1 | 0.000392 | 0.0099 | 0.0008 | 0.0023 |
| 17 | rs387906234 | g.112839979_112839988delAG | c.4393_4394delAG | p.Ser1465Trpfs*3 | NA* | Pathogenic | 1 | NA | NA | NA | NA |
| 18 | Rs387906234 | g.112839979_112839988dupAG | c.4393_4394dupAG | p.Ser1465Argfs*9 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 19 | NA | g.112840012A>G | c.4418A>G | p.Asn1473Ser | AAT>AGT | US | 1 | NA | NA | NA | NA |
| 20 | rs139387758 | g.112840014G>A | c.4420G>A | p.Ala1474Thr | GCT>ACT | Benign | 2 | 0.000326 | 0.0105 | 0.0026 | 0.0098 |
| 21 | NA | g.112840057_112840064delinsA | c.4463_4470delinsA | p.Leu1488Tyrfs*17 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 22 | rs41115 | g.112840073G>A | c.4479G>A | p.Thr1493= | ACG>ACA | Benign | 28 | 0.61 | 0.53 | 0.665 | 0.517 |
| 23 | NA | g.112840100_112840101delT | c.4506_4507delT | p.Ser1503Hisfs*4 | NA* | Deleterious (in silico) | 1 | NA | NA | NA | NA |
| 24 | rs587783031 | g.112840255_112840260delA | c.4666delA | p.Thr1556Leufs*9 | NA* | Pathogenic | 1 | NA | NA | NA | NA |
| 25 | rs587783031 | g.112840255_112840260dupA | c.4666dup | p.Thr1556Asnfs*3 | NA* | Pathogenic | 2 | NA | NA | NA | NA |
| 26 | rs35634377 | g.112840487T>C | c.4893T>C | p.Ser1631= | AGT>AGC | Benign/Likely benign | 1 | 0.00098 | 0.0104 | 0.0008 | 0.0023 |
| 27 | rs1554086285 | g.112840496_112840500dupG | c.4906dupG | p.Asp1636Glyfs*2 | NA* | Pathogenic | 1 | NA | NA | NA | NA |
| No | dbSNP_ID | GRCh38 Coordinate | NM_006361.6 | Protein Change NP_006352.2 | Codon Change | Clinical Significance $ | Number of Cases with Mutation (N = 54) | MAF ALFA/Global | MAF ALFA/ African |
|---|---|---|---|---|---|---|---|---|---|
| 1 | rs33993186 | g.48728264G>T | c.330C>A | p.Pro110= | CCC>CCA | benign | 6 | 0.00403 | 0.0220 |
| 2 | rs8556 | g.48728228G>A | c.366C>T | p.Ser122= | AGC>AGT | benign | 26 | 0.131 | 0.172 |
| 3 | rs9900627 | g.48728081A>G | c.513T>C | p.Ser171= | TCT>TCC | benign | 20 | 0.104 | 0.117 |
| 4 | rs138675188 | g.48726910C>T | c.735G>A | p.Lys245= | AAG>AAA | Likely benign | 1 | 0.00055 | 0.0024 |
| No | dbSNP_ID | GRCh38 Coordinate | NM_004985.5 | Protein Change (NP_004976.2) | Codon Change | Clinical Significance $ | Number of Cases with Mutation (N = 54) | MAF ALFA/ Global | MAF ALFA/ African |
|---|---|---|---|---|---|---|---|---|---|
| 1 | rs121913529 | g.25245350C>T | c.35G>A | p.Gln12Asp | GGT>GAT | Pathogenic | 2 | 0.00001 | Zero |
| 2 | rs121913529 | g.25245350C>G | c.35G>C | p.Gln12Ala | GGT>GCT | Pathogenic/ likely pathogenic | 1 | NA | NA |
| 3 | rs121913529 | g.25245350C>A | c.35G>T | p.Gln12Val | GGT>GTT | Pathogenic | 7 | NA | NA |
| 4 | rs112445441 | g.25245347C>T | c.38G>A | p.Gly13Asp | GGC>GAC | C.I | 11 | NA | NA |
| 5 | rs17851045 | g.25227341T>G | c.183A>C | p.Gln61His | CAA>CAC | Pathogenic/ likely pathogenic | 1 | NA | NA |
| 6 | rs1137282 | g.25209843A>G | c.519T>C | p.Asp173= | GAT>GAC | Benign | 19 | 0.213945 | 0.1763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manirakiza, F.; Rutaganda, E.; Yamada, H.; Iwashita, Y.; Rugwizangoga, B.; Seminega, B.; Dusabejambo, V.; Ntakirutimana, G.; Ruhangaza, D.; Uwineza, A.; et al. Clinicopathological Characteristics and Mutational Landscape of APC, HOXB13, and KRAS among Rwandan Patients with Colorectal Cancer. Curr. Issues Mol. Biol. 2023, 45, 4359-4374. https://doi.org/10.3390/cimb45050277
Manirakiza F, Rutaganda E, Yamada H, Iwashita Y, Rugwizangoga B, Seminega B, Dusabejambo V, Ntakirutimana G, Ruhangaza D, Uwineza A, et al. Clinicopathological Characteristics and Mutational Landscape of APC, HOXB13, and KRAS among Rwandan Patients with Colorectal Cancer. Current Issues in Molecular Biology. 2023; 45(5):4359-4374. https://doi.org/10.3390/cimb45050277
Chicago/Turabian StyleManirakiza, Felix, Eric Rutaganda, Hidetaka Yamada, Yuji Iwashita, Belson Rugwizangoga, Benoit Seminega, Vincent Dusabejambo, Gervais Ntakirutimana, Deogratias Ruhangaza, Annette Uwineza, and et al. 2023. "Clinicopathological Characteristics and Mutational Landscape of APC, HOXB13, and KRAS among Rwandan Patients with Colorectal Cancer" Current Issues in Molecular Biology 45, no. 5: 4359-4374. https://doi.org/10.3390/cimb45050277
APA StyleManirakiza, F., Rutaganda, E., Yamada, H., Iwashita, Y., Rugwizangoga, B., Seminega, B., Dusabejambo, V., Ntakirutimana, G., Ruhangaza, D., Uwineza, A., Shinmura, K., & Sugimura, H. (2023). Clinicopathological Characteristics and Mutational Landscape of APC, HOXB13, and KRAS among Rwandan Patients with Colorectal Cancer. Current Issues in Molecular Biology, 45(5), 4359-4374. https://doi.org/10.3390/cimb45050277







