AKR1B1 Represses Glioma Cell Proliferation through p38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Patient Samples
2.3. Cell Cultures
2.4. DNA Transfection and RNA Interference
2.5. Caspase3/7 Activity Assay
2.6. MTT Assay
2.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Western Blot Analysis
2.9. Annexin V-FITC/PI Double-Staining Assay
2.10. Statistical Analysis
3. Results
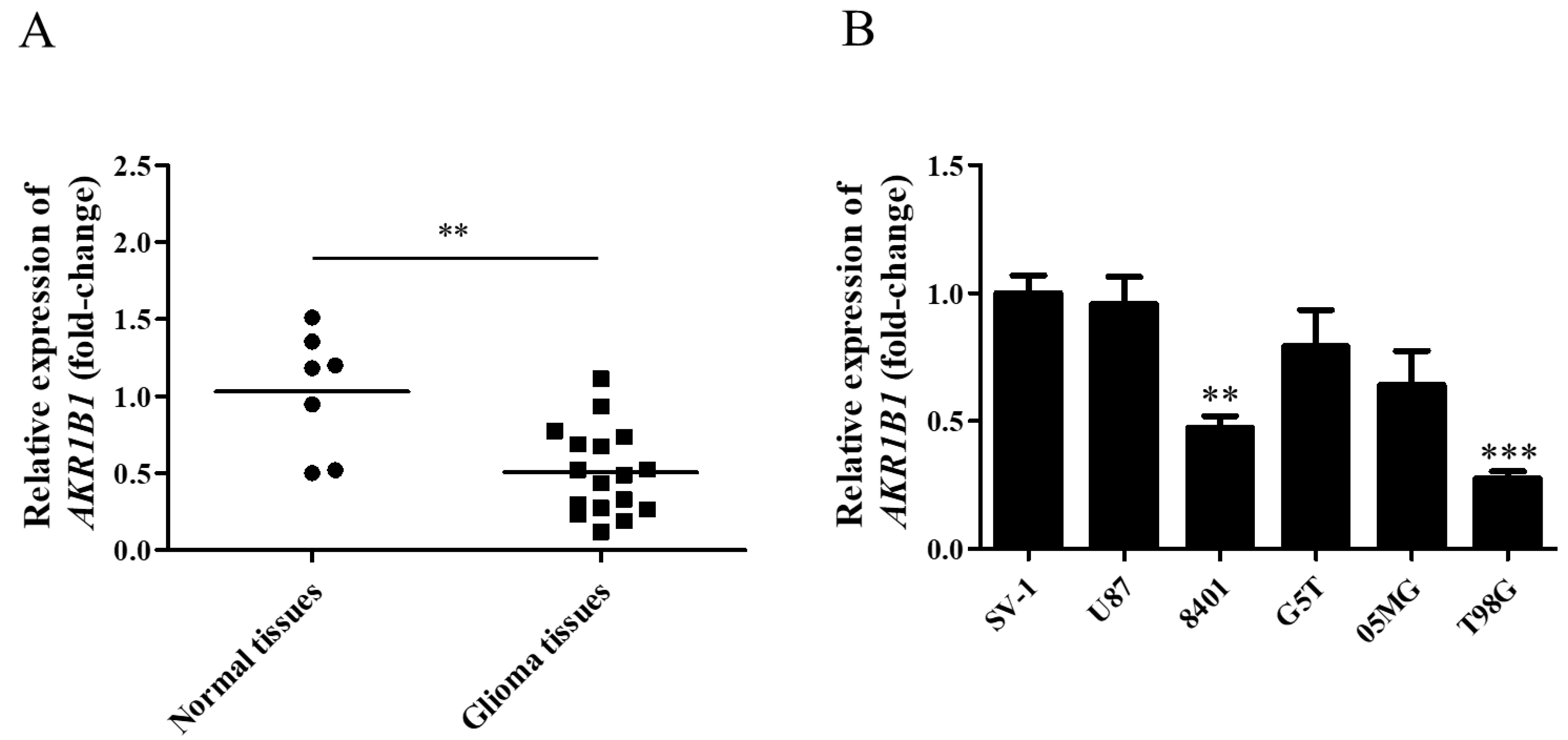
3.1. AKR1B1 Expression in Human Glioma Tissue and Various Glioma Cell Lines
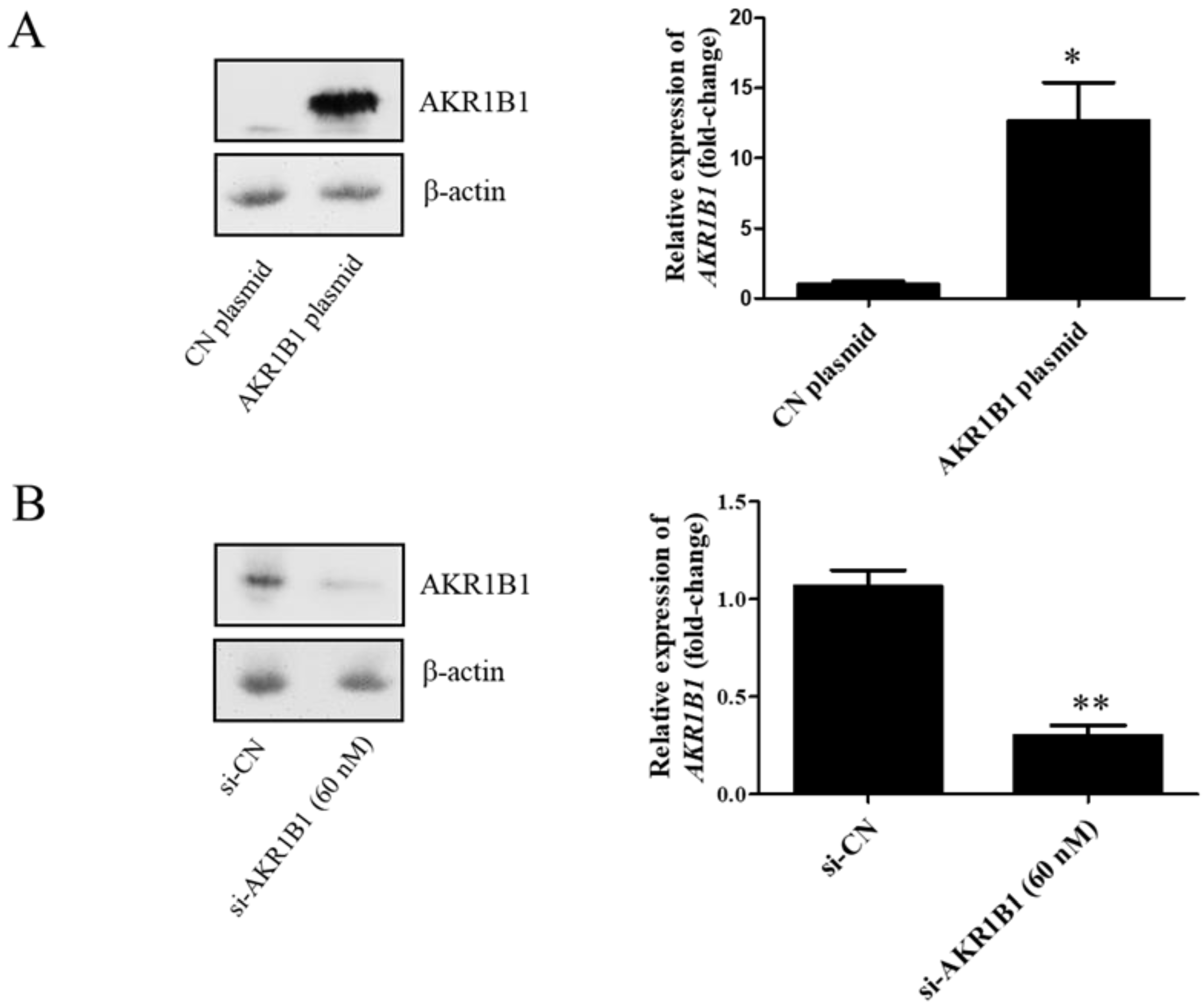
3.2. AKR1B1 Caused a Cytotoxic Effect on Human Glioblastoma Cell Lines
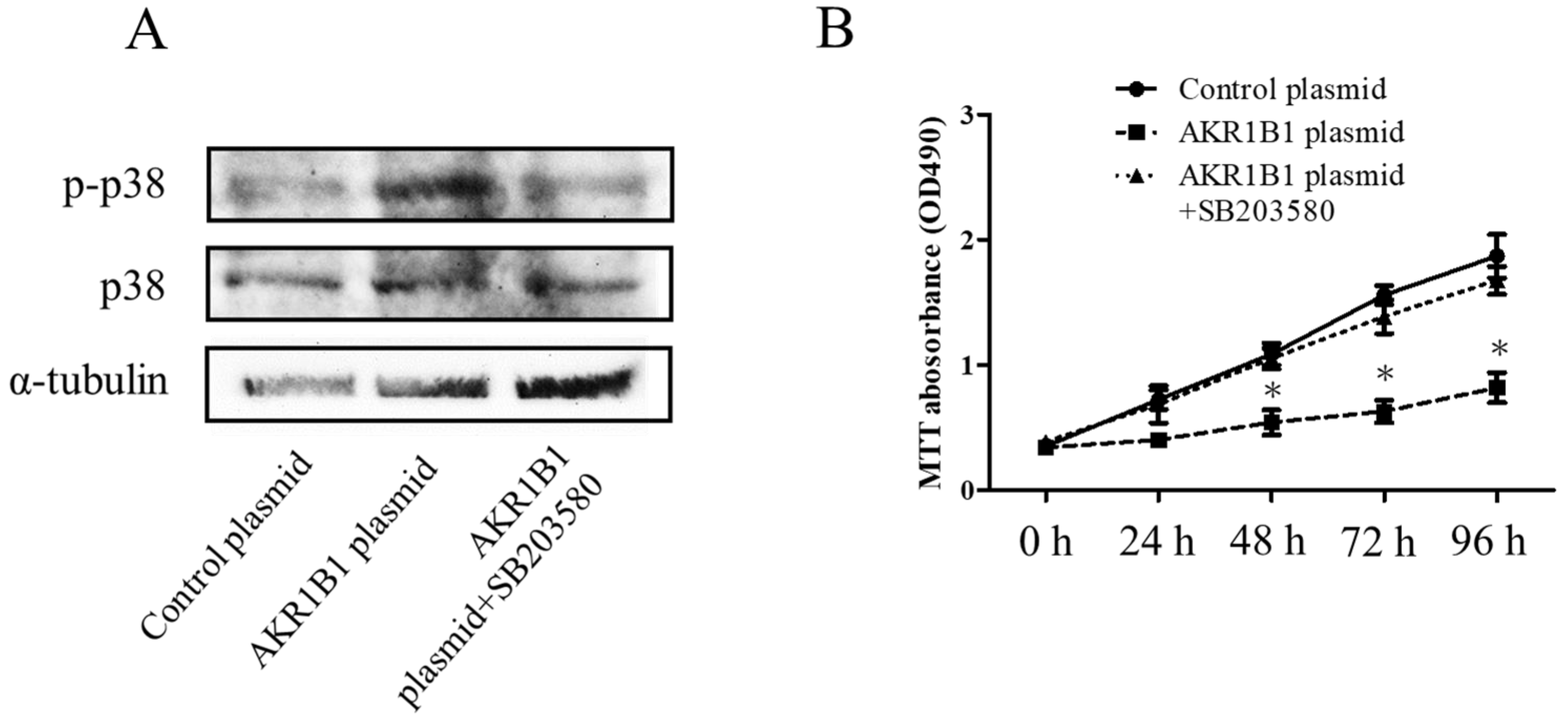
3.3. AKR1B1 Activated p38 Signaling in Glioma Cells
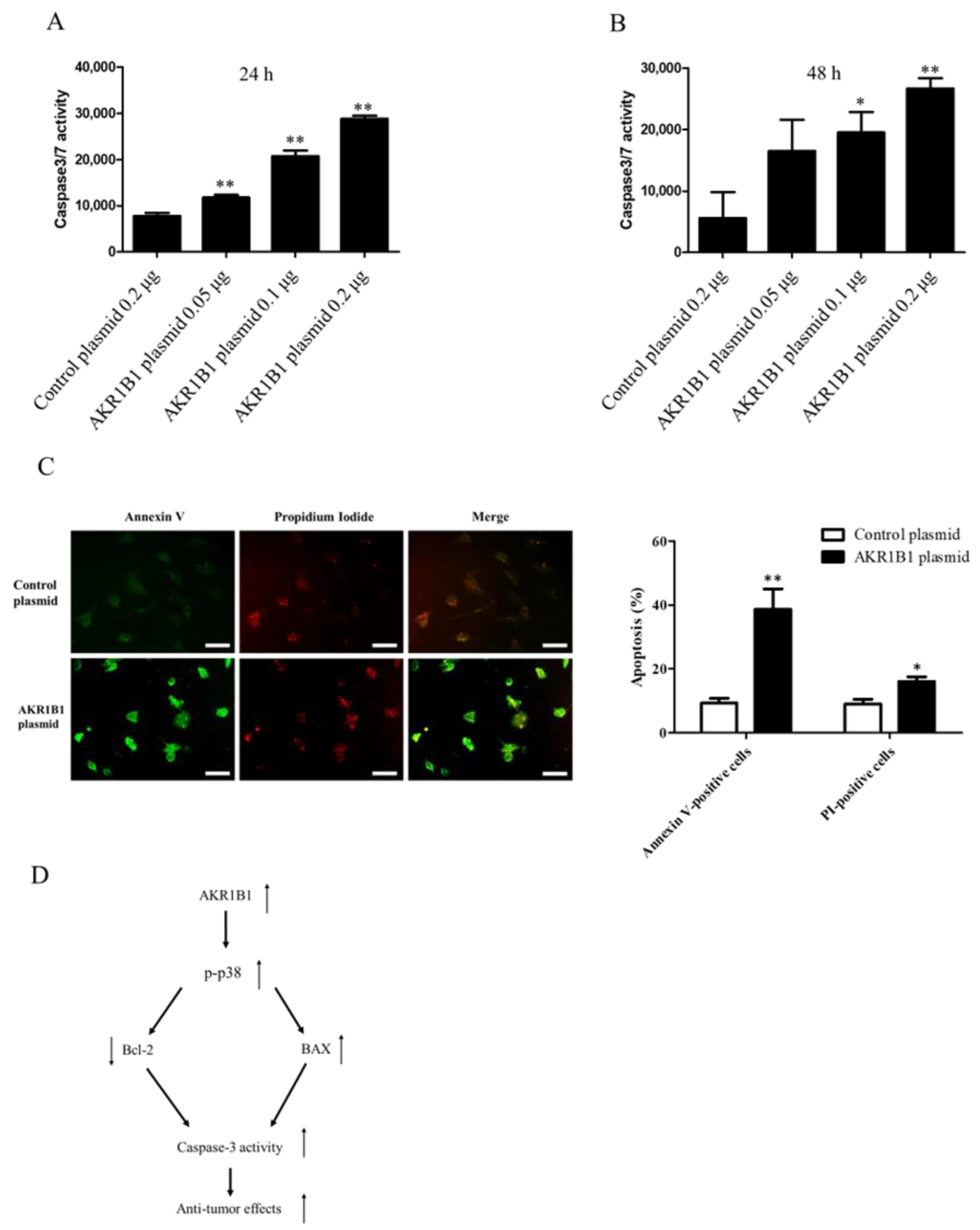
3.4. AKR1B1 Induced Apoptosis in Glioma Cells
3.5. AKR1B1 Activated Caspase-3/7 in Glioma Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Fahmideh, M.A.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk Factors for Childhood and Adult Primary Brain Tumors. Neuro-Oncology 2019, 21, 1357–1375. [Google Scholar] [CrossRef] [PubMed]
- Witthayanuwat, S.; Pesee, M.; Supaadirek, C.; Supakalin, N.; Thamronganantasakul, K.; Krusun, S. Survival Analysis of Glioblastoma Multiforme. Asian Pac. J. Cancer Prev. 2018, 19, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.F.; Chu, S.M.; Liao, C.C.; Wang, C.J.; Wang, Y.S.; Lai, M.Y.; Wang, H.C.; Huang, H.R.; Tsai, M.H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- Prajapati, H.P.; Kannaujia, S.K. A Simplified Overview of the World Health Organization Classification of Central Nervous System Tumors 2021. Surg. Neurol. Int. 2022, 13, 252. [Google Scholar] [CrossRef]
- Laffin, B.; Petrash, J.M. Expression of the Aldo-Ketoreductases AKR1B1 and AKR1B10 in Human Cancers. Front. Pharmacol. 2012, 3, 104. [Google Scholar] [CrossRef]
- Banerjee, S. Aldo Keto Reductases AKR1B1 and AKR1B10 in Cancer: Molecular Mechanisms and Signaling Networks. Cell Biol. Transl. Med. 2021, 14, 65–82. [Google Scholar]
- Shi, Z.; Kong, X.; Li, C.; Liu, H.; Aliagan, A.I.; Liu, L.; Shi, Y.; Shi, X.; Ma, B.; Jin, R.; et al. Bioinformatic Analysis of Differentially Expressed Genes as Prognostic Markers in Pheochromocytoma and Paraganglioma Tumors. Genes Genet. Syst. 2021, 96, 55–69. [Google Scholar] [CrossRef]
- Yamada, N.; Yasui, K.; Dohi, O.; Gen, Y.; Tomie, A.; Kitaichi, T.; Iwai, N.; Mitsuyoshi, H.; Sumida, Y.; Moriguchi, M.; et al. Genome-Wide DNA Methylation Analysis in Hepatocellular Carcinoma. Oncol. Rep. 2016, 35, 2228–2236. [Google Scholar] [CrossRef]
- Hevir, N.; Šinkovec, J.; Lanišnik Rižner, T. Decreased Levels of AKR1B1 and AKR1B10 in Cancerous Endometrium Compared to Adjacent Non-Cancerous Tissue. Chem. -Biol. Interact. 2013, 202, 226–233. [Google Scholar] [CrossRef]
- Lefrançois-Martinez, A.M.; Bertherat, J.; Val, P.; Tournaire, C.; Gallo-Payet, N.; Hyndman, D.; Veyssière, G.; Bertagna, X.; Jean, C.; Martinez, A. Decreased Expression of Cyclic Adenosine Monophosphate-Regulated Aldose Reductase (AKR1B1) Is Associated with Malignancy in Human Sporadic Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2004, 89, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xu, M.X.; Qian, T.Y.; Zhu, S.Z.; Jiang, F.; Liu, Z.X.; Xu, W.S.; Zhou, J.; Xiao, M.B. The AKR1B1 Inhibitor Epalrestat Suppresses the Progression of Cervical Cancer. Mol. Biol. Rep. 2020, 47, 6091–6103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.R.; Zhang, Y.F.; Lei, H.M.; Tang, Y.B.; Ma, C.S.; Lv, Q.M.; Wang, S.Y.; Lu, L.M.; Shen, Y.; Chen, H.Z.; et al. Targeting AKR1B1 Inhibits Glutathione de Novo Synthesis to Overcome Acquired Resistance to EGFR-Targeted Therapy in Lung Cancer. Sci. Transl. Med. 2021, 13, eabg6428. [Google Scholar] [CrossRef] [PubMed]
- Khayami, R.; Hashemi, S.R.; Kerachian, M.A. Role of Aldo-Keto Reductase Family 1 Member B1 (AKR1B1) in the Cancer Process and Its Therapeutic Potential. J. Cell Mol. Med. 2020, 24, 8890–8902. [Google Scholar] [CrossRef] [PubMed]
- Hojnik, M.; Šuster, N.K.; Smrkolj, Š.; Sisinger, D.; Grazio, S.F.; Verdenik, I.; Rižner, T.L. AKR1B1 as a Prognostic Biomarker of High-Grade Serous Ovarian Cancer. Cancers 2022, 14, 809. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. Killing Cancer Cells by Flipping the Bcl-2/Bax Switch. Cancer Cell 2005, 8, 5–6. [Google Scholar] [CrossRef]
- Dai, Y.; Grant, S. Targeting Multiple Arms of the Apoptotic Regulatory Machinery. Cancer Res. 2007, 67, 2908–2911. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014. [Google Scholar] [CrossRef]
- Kunac, N.; Filipović, N.; Kostić, S.; Vukojević, K. The Expression Pattern of Bcl-2 and Bax in the Tumor and Stromal Cells in Colorectal Carcinoma. Medicina 2022, 58, 1135. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.; Luedtke, D.; Edwards, H.; Taub, J.W.; Ge, Y. A Delicate Balance–The BCL-2 Family and Its Role in Apoptosis, Oncogenesis, and Cancer Therapeutics. Biochem. Pharmacol. 2019, 162, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Azmi, A.S.; Mohammad, R.M. Small Molecule Inhibitors of Bcl-2 Family Proteins for Pancreatic Cancer Therapy. Cancers 2011, 3, 1527–1549. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Park, J.H.; Kim, M.J.; Kim, W.J.; Ha, K.T.; Choi, B.T.; Lee, S.Y.; Shin, H.K. Isolinderalactone Regulates the BCL-2/Caspase-3/PARP Pathway and Suppresses Tumor Growth in a Human Glioblastoma Multiforme Xenograft Mouse Model. Cancer Lett. 2019, 443, 25–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Chen, L.; Yang, X.; Zhong, H.; Qi, X.; Bi, Y.; Xu, K. Low Frequency and Intensity Ultrasound Induces Apoptosis of Brain Glioma in Rats Mediated by Caspase-3, Bcl-2, and Survivin. Brain Res. 2012, 1473, 25–34. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Yang, J.; Pan, T.; Zhang, S.; Wang, Z. MiR-21 Protected Human Glioblastoma U87MG Cells from Chemotherapeutic Drug Temozolomide Induced Apoptosis by Decreasing Bax/Bcl-2 Ratio and Caspase-3 Activity. Brain Res. 2010, 1352, 255–264. [Google Scholar] [CrossRef]
- Skała, E.; Sitarek, P.; Toma, M.; Szemraj, J.; Radek, M.; Nieborowska-Skorska, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. Inhibition of Human Glioma Cell Proliferation by Altered Bax/Bcl-2-P53 Expression and Apoptosis Induction by Rhaponticum Carthamoides Extracts from Transformed and Normal Roots. J. Pharm. Pharmacol. 2016, 68, 1454–1464. [Google Scholar] [CrossRef]
- Zeng, Y.; Cai, Y.; Chai, P.; Mao, Y.; Chen, Y.; Wang, L.; Zeng, K.; Zhan, Z.; Xie, Y.; Li, C.; et al. Optimization of Cancer Immunotherapy through Pyroptosis: A Pyroptosis-Related Signature Predicts Survival Benefit and Potential Synergy for Immunotherapy in Glioma. Front. Immunol. 2022, 13, 961933. [Google Scholar] [CrossRef]
- Manero, F.; Gautier, F.; Gallenne, T.; Cauquil, N.; Grée, D.; Cartron, P.F.; Geneste, O.; Grée, R.; Vallette, F.M.; Juin, P. The Small Organic Compound HA14-1 Prevents Bcl-2 Interaction with Bax to Sensitize Malignant Glioma Cells to Induction of Cell Death. Cancer Res. 2006, 66, 2757–2764. [Google Scholar] [CrossRef]
- Huang, J.; Gao, J.; Lv, X.; Li, G.; Hao, D.; Yao, X.; Zhou, L.; Liu, D.; Wang, R. Target Gene Therapy of Glioma: Overexpression of BAX Gene under the Control of Both Tissue-Specific Promoter and Hypoxia-Inducible Element. Acta Biochim. Biophys. Sin. 2010, 42, 274–280. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T.; Tu, H. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Dhaheri, Y.A.; Iratni, R. Origanum Majorana Essential Oil Triggers P38 Mapk-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and Apoptotic Effects of Quercetin on Human Melanoma Cells Involving JNK/P38 MAPK Signaling Activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef]
- Taylor, C.A.; Zheng, Q.; Liu, Z.; Thompson, J.E. Role of P38 and JNK MAPK Signaling Pathways and Tumor Suppressor P53 on Induction of Apoptosis in Response to Ad-EIF5A1 in A549 Lung Cancer Cells. Mol. Cancer 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Lamy, V.; Bousserouel, S.; Gossé, F.; Minker, C.; Lobstein, A.; Raul, F. Lupulone Triggers P38 MAPK-Controlled Activation of P53 and of the TRAIL Receptor Apoptotic Pathway in Human Colon Cancer-Derived Metastatic Cells. Oncol. Rep. 2011, 26, 109–114. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Gu, J.H.; Ge, J.B.; Liang, Z.Q.; Qin, Z.H. P38MAPK/P53-Mediated Bax Induction Contributes to Neurons Degeneration in Rotenone-Induced Cellular and Rat Models of CParkinson’s Disease. Neurochem. Int. 2013, 63, 133–140. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, T.; Wang, Z.; Zhang, Y.; Wang, J.; Yang, B.; Zhao, Y.; Rao, Z.; Gao, J. P38MAPK Activation Mediates Tumor Necrosis Factor-α-Induced Apoptosis in Glioma Cells. Mol. Med. Rep. 2015, 11, 3101–3107. [Google Scholar] [CrossRef]
- de Chiara, G.; Marcocci, M.E.; Torcia, M.; Lucibello, M.; Rosini, P.; Bonini, P.; Higashimoto, Y.; Damonte, G.; Armirotti, A.; Amodei, S.; et al. Bcl-2 Phosphorylation by P38 MAPK: Identification of Target Sites and Biologic Consequences. J. Biol. Chem. 2006, 281, 21353–21361. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Q.; Yuan, F.; Dong, H.; Zhang, H.; Geng, R.; Qi, Y.; Xiong, X.; Chen, Q.; Liu, B. RND2 Attenuates Apoptosis and Autophagy in Glioblastoma Cells by Targeting the P38 MAPK Signalling Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-Induced Apoptosis of Glioma Cells Is Mediated by TRPV1 Vanilloid Receptor and Requires P38 MAPK Activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Oliveira Battastini, A.M.; Fonseca Moreira, J.C. Proteasome Inhibitor MG132 Induces Selective Apoptosis in Glioblastoma Cells through Inhibition of PI3K/Akt and NFkappaB Pathways, Mitochondrial Dysfunction, and Activation of P38-JNK1/2 Signaling. Investig. New Drugs 2012, 30, 2252–2262. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Yan, Q.; Lv, W.; Zhang, Y.; He, S. Exogenous Hydrogen Sulfide Exhibits Anti-Cancer Effects Though P38 MAPK Signaling Pathway in C6 Glioma Cells. Biol. Chem. 2015, 396, 1247–1253. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key Mediators of Mitochondrial Events of Apoptosis. Science (1979) 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; de Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Scabini, M.; Stellari, F.; Cappella, P.; Rizzitano, S.; Texido, G.; Pesenti, E. In Vivo Imaging of Early Stage Apoptosis by Measuring Real-Time Caspase-3/7 Activation. Apoptosis 2011, 16, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mccomb, S.; Chan, K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.-P.; Bornhauser, B.C. Efficient Apoptosis Requires Feedback Amplification of Upstream Apoptotic Signals by Effector Caspase-3 or-7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, B.; Xu, Y.; Li, Y.; Sun, Q.; Xu, P.; Geng, R.; Den, G.; Yang, J.; Zhang, S.; et al. TIPE3 Is a Regulator of Cell Apoptosis in Glioblastoma. Cancer Lett. 2019, 446, 1–14. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Anti-Tumor Effect of β-Elemene in Glioblastoma Cells Depends on P38 MAPK Activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Sadaf, N.; Kumar, N.; Ali, M.; Ali, V.; Bimal, S.; Haque, R. Arsenic Trioxide Induces Apoptosis and Inhibits the Growth of Human Liver Cancer Cells. Life Sci. 2018, 205, 9–17. [Google Scholar] [CrossRef]
- Penning, T.M. The Aldo-Keto Reductases (AKRs): Overview. Chem. Biol. Interact. 2015, 234, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Wang, Y.K.; Jiang, K.; Gai, X.D. Sorbitol Induces Apoptosis of Human Colorectal Cancer Cells via P38 MAPK Signal Transduction. Oncol Lett. 2014, 7, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Teramachi, K.; Izawa, M. Rapid Induction of Apoptosis in Human Gastric Cancer Cell Lines by Sorbitol. Apoptosis 2000, 5, 181–187. [Google Scholar] [CrossRef]
- Lou, X.; Zhou, Q.; Yin, Y.; Zhou, C.; Shen, Y. Inhibition of the Met Receptor Tyrosine Kinase Signaling Enhances the Chemosensitivity of Glioma Cell Lines to CDDP through Activation of P38 MAPK Pathway. Mol. Cancer Ther. 2009, 8, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Tsai, W.H.; Chuang, W.J.; Lin, Y.S.; Wu, J.J.; Liu, C.C.; Tsai, P.J.; Lin, M.T. Procaspase 8 and Bax Are Up-Regulated by Distinct Pathways in Streptococcal Pyrogenic Exotoxin B-Induced Apoptosis. J. Biol. Chem. 2009, 284, 33195–33205. [Google Scholar] [CrossRef]
- Hui, K.; Yang, Y.; Shi, K.; Luo, H.; Duan, J.; An, J.; Wu, P.; Ci, Y.; Shi, L.; Xu, C. The P38 MAPK-Regulated PKD1/CREB/Bcl-2 Pathway Contributes to Selenite-Induced Colorectal Cancer Cell Apoptosis in Vitro and in Vivo. Cancer Lett. 2014, 354, 189–199. [Google Scholar] [CrossRef]
- Milani, R.; Brognara, E.; Fabbri, E.; Manicardi, A.; Corradini, R.; Finotti, A.; Gasparello, J.; Borgatti, M.; Cosenza, L.C.; Lampronti, I.; et al. Targeting MiR-155-5p and MiR-221-3p by Peptide Nucleic Acids Induces Caspase-3 Activation and Apoptosis in Temozolomide-resistant T98G Glioma Cells. Int. J. Oncol. 2019, 55, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nasiraei-Moghadam, S.; Kazeminezhad, B.; Dargahi, L.; Ahmadiani, A. Maternal Oral Consumption of Morphine Increases Bax/Bcl-2 Ratio and Caspase 3 Activity during Early Neural System Development in Rat Embryos. J. Mol. Neurosci. 2010, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-K.; Chang, K.-C.; Li, C.-Y.; Lieu, A.-S.; Lin, C.-L. AKR1B1 Represses Glioma Cell Proliferation through p38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways. Curr. Issues Mol. Biol. 2023, 45, 3391-3405. https://doi.org/10.3390/cimb45040222
Huang Y-K, Chang K-C, Li C-Y, Lieu A-S, Lin C-L. AKR1B1 Represses Glioma Cell Proliferation through p38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways. Current Issues in Molecular Biology. 2023; 45(4):3391-3405. https://doi.org/10.3390/cimb45040222
Chicago/Turabian StyleHuang, Yu-Kai, Kun-Che Chang, Chia-Yang Li, Ann-Shung Lieu, and Chih-Lung Lin. 2023. "AKR1B1 Represses Glioma Cell Proliferation through p38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways" Current Issues in Molecular Biology 45, no. 4: 3391-3405. https://doi.org/10.3390/cimb45040222
APA StyleHuang, Y.-K., Chang, K.-C., Li, C.-Y., Lieu, A.-S., & Lin, C.-L. (2023). AKR1B1 Represses Glioma Cell Proliferation through p38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways. Current Issues in Molecular Biology, 45(4), 3391-3405. https://doi.org/10.3390/cimb45040222






