Advances in Research on the Regulation of Floral Development by CYC-like Genes
Abstract
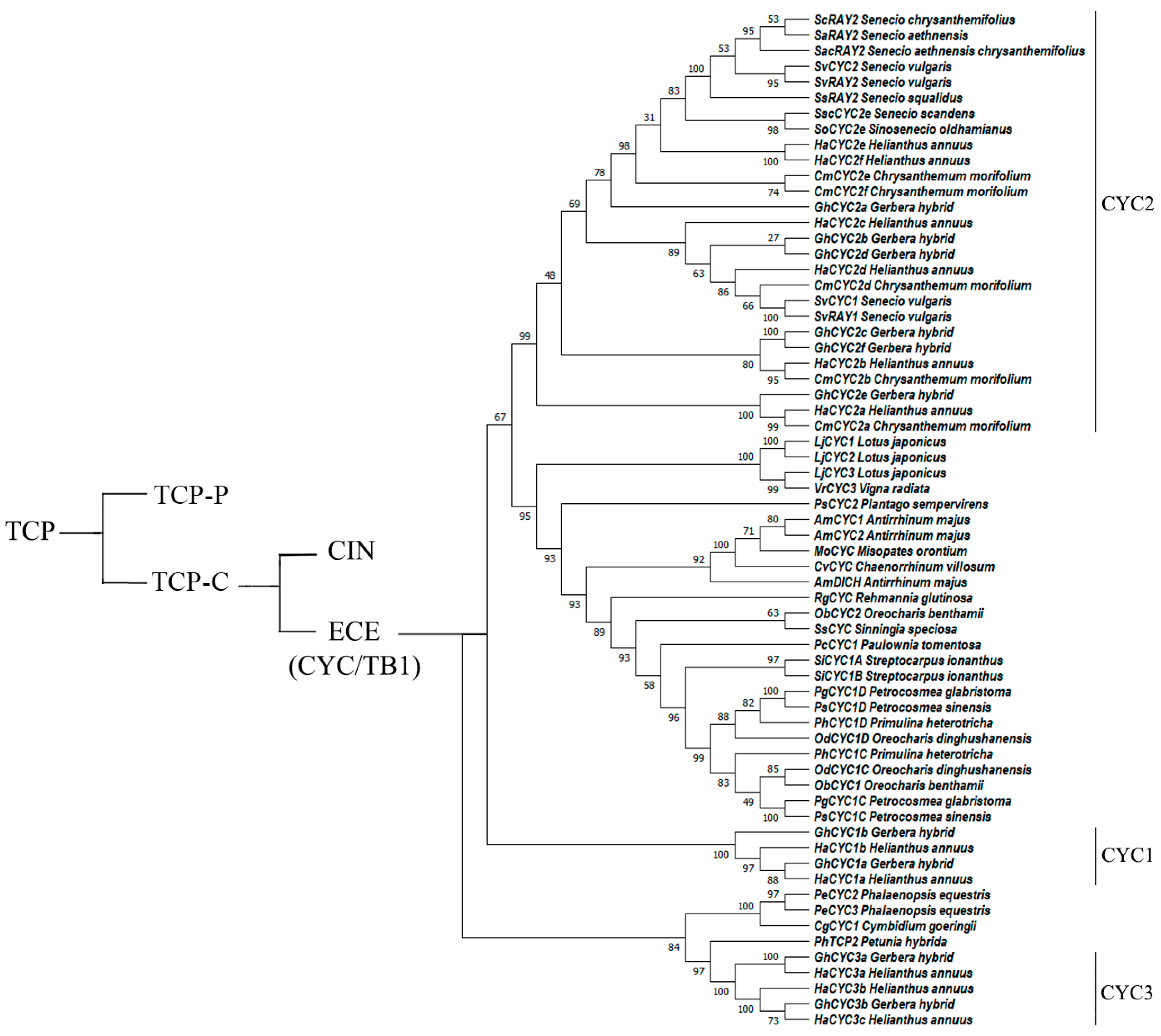
1. Introduction
2. Progress in Research on CYC-like Genes in Fabaceae
3. Progress in Research on CYC-like Genes in Asteraceae
3.1. CYC-like Genes of Helianthus
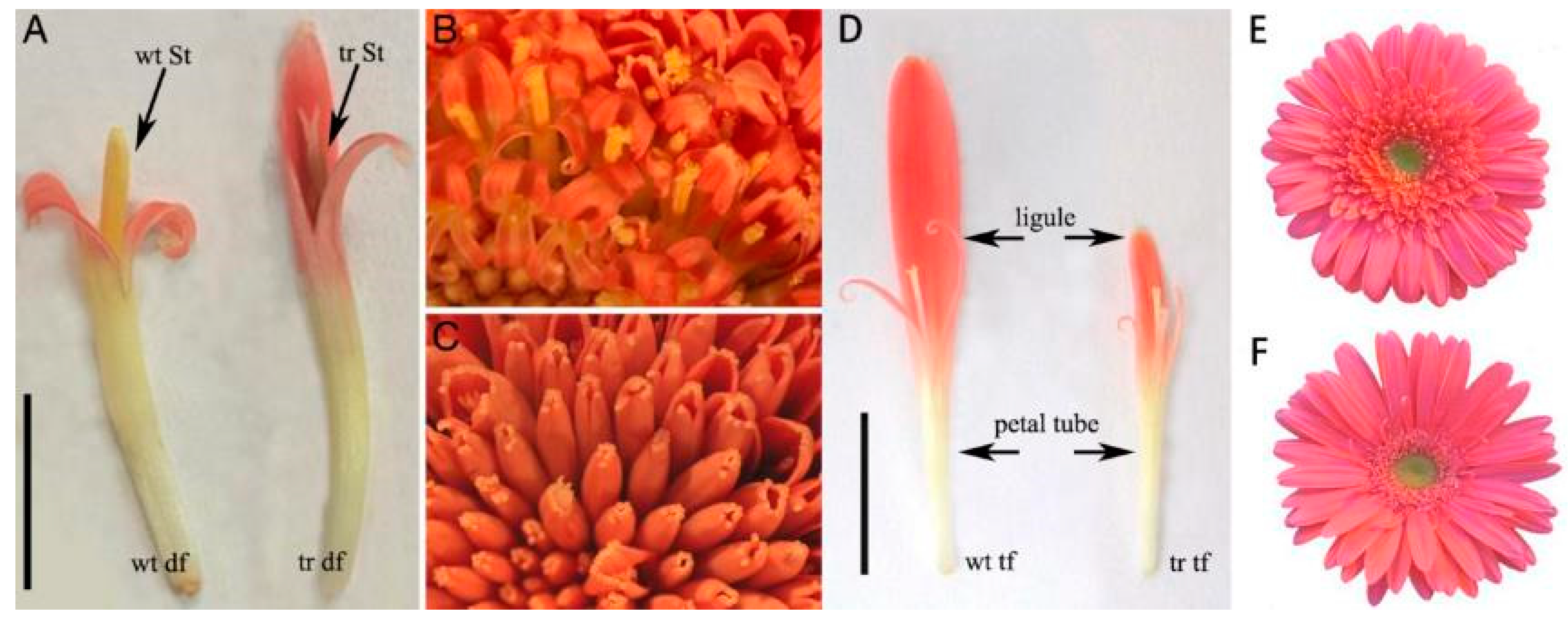
3.2. CYC-like Genes of Gerbera Hybrida
3.3. CYC-like Genes of Senecio
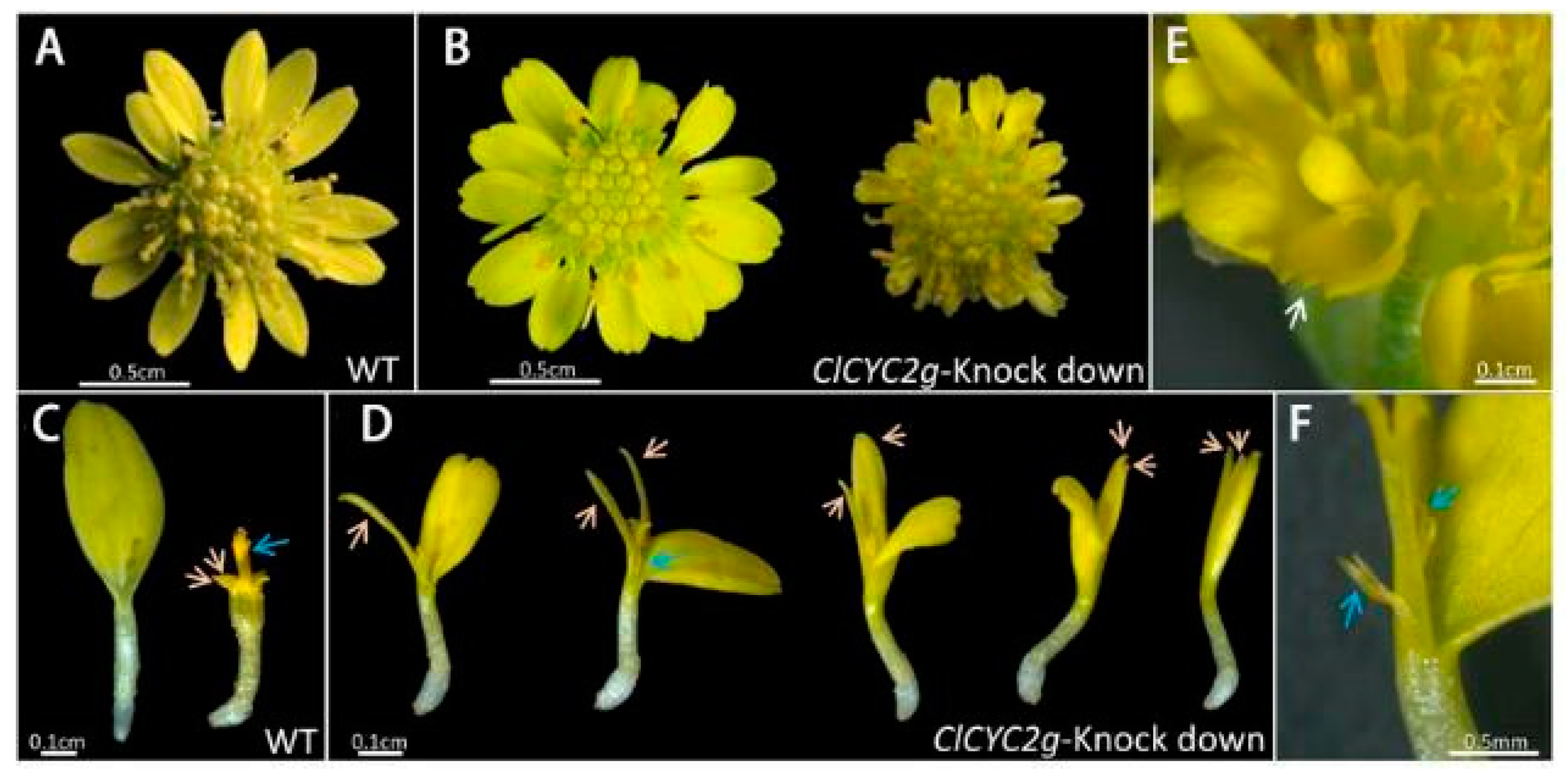
3.4. CYC-like Genes of Chrysanthemum
3.5. CYC-like Genes of Other Asteraceae Groups
4. Progress in Research on CYC-like Genes in Lamiales
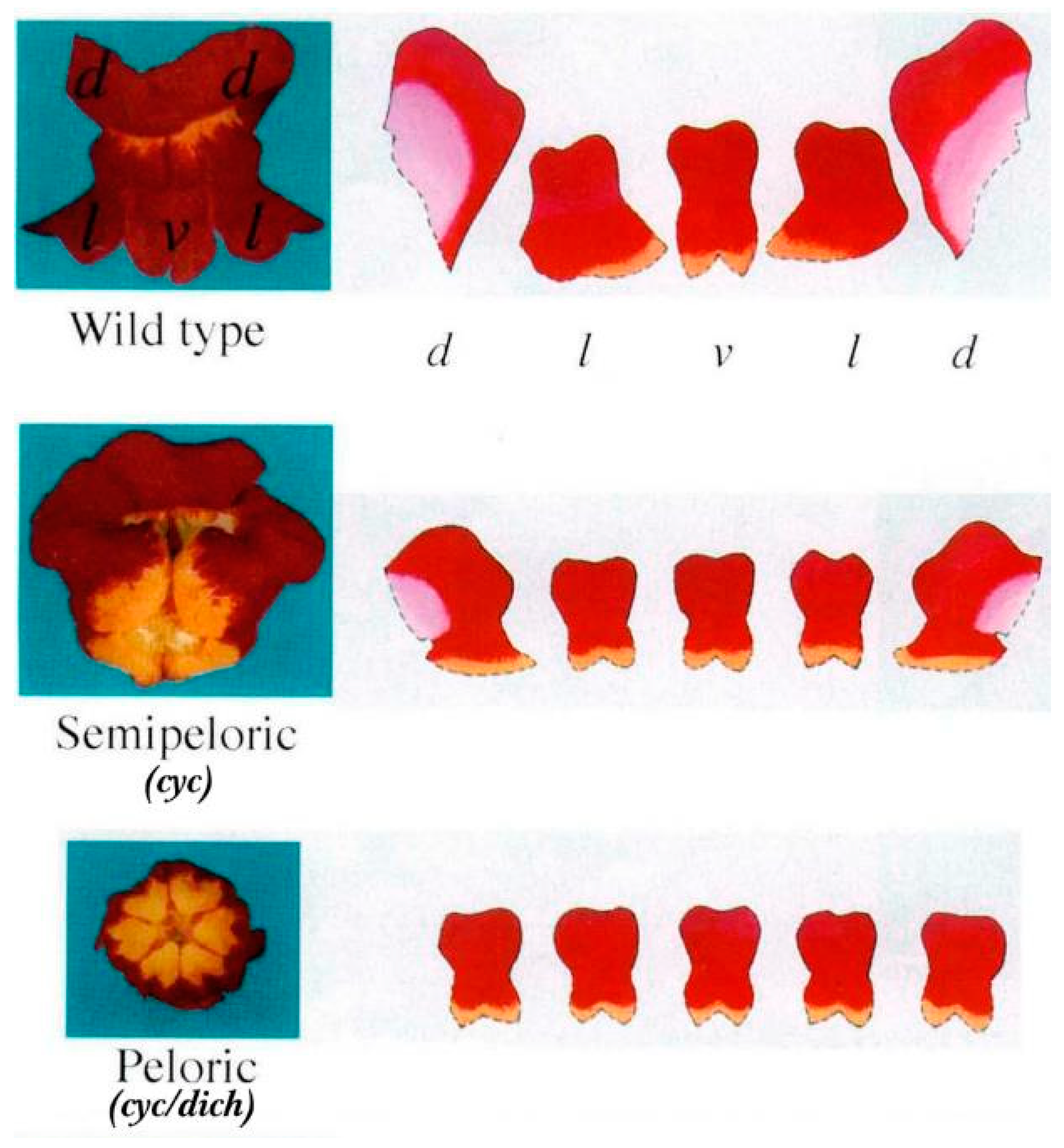
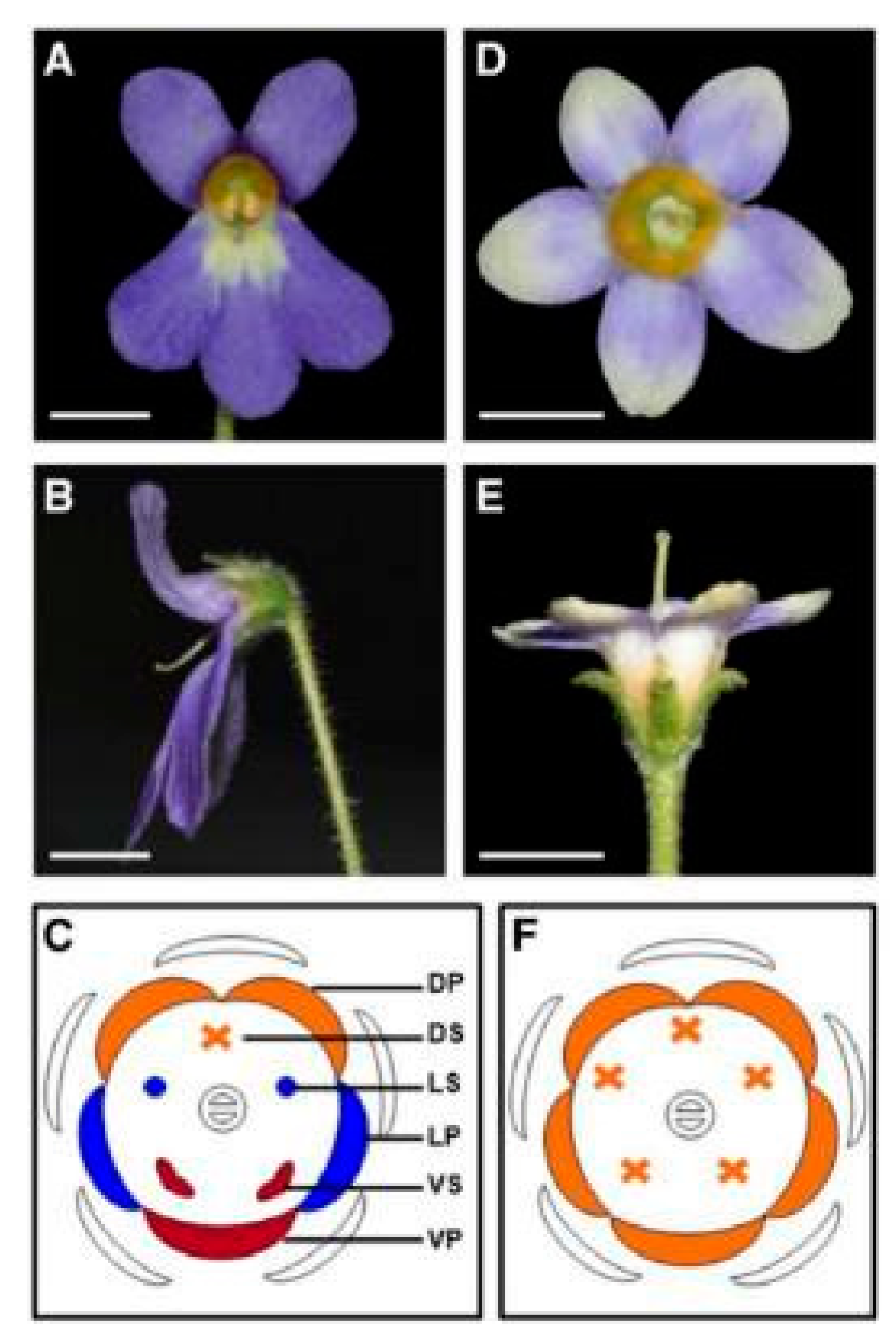
4.1. CYC-like Genes of Scrophulariaceae
4.2. CYC-like Genes of Gesneriaceae
4.3. CYC-like Genes of Phrymaceae
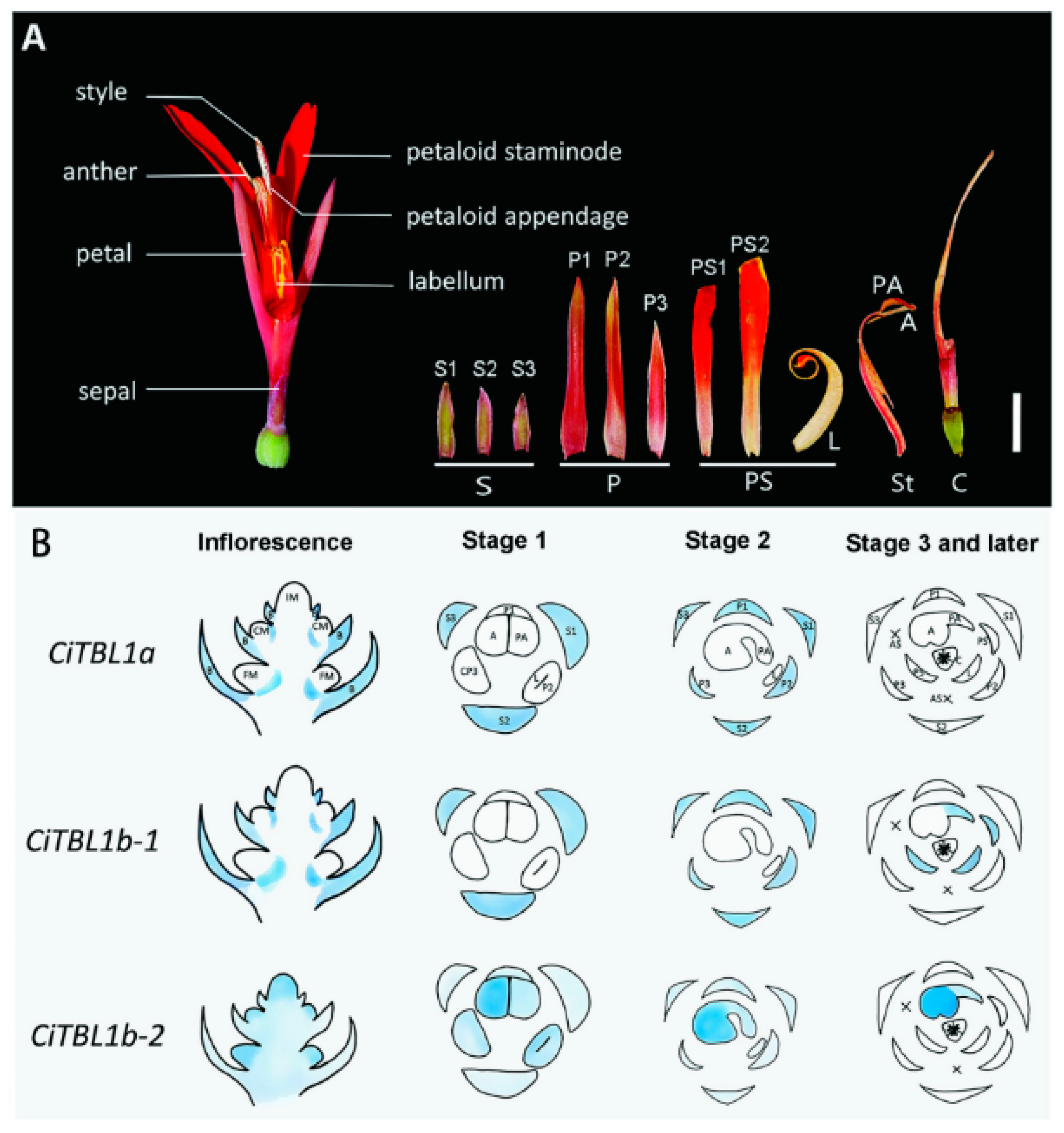
5. Progress in Research on CYC-like Genes in Orchidaceae
6. Progress in Research on CYC-like Genes in Solanaceae
7. Progress in Research on CYC-like Genes in Other Angiosperms
7.1. CYC-like Genes of Brassicaceae
7.2. CYC-like Genes of Dipsacales
7.3. CYC-like Genes of Zingiberales
7.4. CYC-like Genes of Ranunculales
7.5. CYC-like Genes of other Families
8. Outlook
8.1. Conduct Systematic Functional and Evolutionary Research, Especially Regarding CYC1 and CYC3 Clade Members
8.2. Functionally Characterize the CYC-like Genes in More Plant Groups
8.3. Investigate the Regulatory Elements Upstream of CYC-like Genes
8.4. Study the Phylogenetic Relationships and Expression of CYC-like Genes with New Techniques and Methods
9. Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of floral asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carpenter, R.; Copsey, L.; Vincent, C.; Clark, J.; Coen, E. Control of organ asymmetry in flowers of Antirrhinum. Cell 1999, 99, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Manassero, N.G.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Nicolas, M.; Torres-Perez, R.; Wahl, V.; Cruz-Oro, E.; Rodriguez-Buey, M.L.; Zamarreno, A.M.; Martin-Jouve, B.; Garcia-Mina, J.M.; Oliveros, J.C.; Prat, S.; et al. Spatial control of potato tuberization by the TCP transcription factor BRANCHED1b. Nat. Plants 2022, 8, 281–294. [Google Scholar] [CrossRef]
- Damerval, C.; Claudot, C.; Le Guilloux, M.; Conde, E.S.N.; Brunaud, V.; Soubigou-Taconnat, L.; Caius, J.; Delannoy, E.; Nadot, S.; Jabbour, F.; et al. Evolutionary analyses and expression patterns of TCP genes in Ranunculales. Front. Plant Sci. 2022, 13, 1055196. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Fri, N.; Ijast, I. Genome wide analysis and identification of TCP gene family in Wheat (Triticum aestivum L.). Int. J. Appl. Sci. Technol. 2022, 8, 19–35. [Google Scholar]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef]
- Baulies, J.L.; Bresso, E.G.; Goldy, C.; Palatnik, J.F.; Schommer, C. Potent inhibition of TCP transcription factors by miR319 ensures proper root growth in Arabidopsis. Plant Mol. Biol. 2022, 108, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Igartua, E.; Contreras-Moreira, B.; Casas, A.M. TB1: From domestication gene to tool for many trades. J. Exp. Bot. 2020, 71, 4621–4624. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.T.; Van Es, S.W.; Hernandez-Pinzon, I.; Kirschner, G.K.; Van Der Wal, F.; Da, S.S.; Busscher-Lange, J.; Angenent, G.C.; Moscou, M.; Immink, R.; et al. The TCP transcription factor HvTB2 heterodimerizes with VRS5 and controls spike architecture in barley. Plant Reprod. 2022, 35, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.E.; Pasquariello, M.; Boden, S.A. TEOSINTE BRANCHED1 regulates height and stem internode length in bread wheat. J. Exp. Bot. 2020, 71, 4742–4750. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, D.; Gao, Q.; Luo, Y.; Zhang, H.; Ma, B.; Chen, C.; Whibley, A.; Zhang, Y.; Cao, Y.; et al. Genome structure and evolution of Antirrhinum majus L. Nat. Plants 2019, 5, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Livne, S.; Kobinson-Katz, T.; Tal, L.; Pri-Tal, O.; Mosquna, A.; Tarkowska, D.; Mueller, B.; Tarkowski, P.; Weiss, D. The Putative O-Linked N-Acetylglucosamine Transferase SPINDLY Inhibits Class I TCP Proteolysis to Promote Sensitivity to Cytokinin. Plant Physiol. 2016, 171, 1485–1494. [Google Scholar] [CrossRef]
- Busch, A.; Deckena, M.; Almeida-Trapp, M.; Kopischke, S.; Kock, C.; Schussler, E.; Tsiantis, M.; Mithofer, A.; Zachgo, S. MpTCP1 controls cell proliferation and redox processes in Marchantia polymorpha. New Phytol. 2019, 224, 1627–1641. [Google Scholar] [CrossRef]
- Spears, B.J.; McInturf, S.A.; Collins, C.; Chlebowski, M.; Cseke, L.J.; Su, J.; Mendoza-Cozatl, D.G.; Gassmann, W. Class I TCP transcription factor AtTCP8 modulates key brassinosteroid-responsive genes. Plant Physiol. 2022, 190, 1457–1473. [Google Scholar] [CrossRef]
- Cubas, P. Floral zygomorphy, the recurring evolution of a successful trait. Bioessays 2004, 26, 1175–1184. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Bello, M.A.; Cubas, P.; Alvarez, I.; Sanjuanbenito, G.; Fuertes-Aguilar, J. Evolution and expression patterns of CYC/TB1 genes in Anacyclus: Phylogenetic insights for floral symmetry genes in Asteraceae. Front. Plant Sci. 2017, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Levin, K.A.; Boden, S.A. A new branch of understanding for barley inflorescence development. J. Exp. Bot. 2020, 71, 6869–6871. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P. Role of TCP genes in the evolution of morphological characters in angiosperms. In Role of TCP Genes in the Evolution of Key Morphological Characters in Angiosperms; Cronk, Q.C.B., Hawkins, J., Bateman, R.M., Eds.; Taylor & Francis: London, UK, 2002; pp. 247–266. [Google Scholar]
- Howarth, D.G.; Donoghue, M.J. Duplications in CYC-like genes from Dipsacales correlate with floral form. Int. J. Plant Sci. 2005, 166, 357–370. [Google Scholar] [CrossRef]
- Citerne, H.L.; Le Guilloux, M.; Sannier, J.; Nadot, S.; Damerval, C. Combining phylogenetic and syntenic analyses for understanding the evolution of TCP ECE genes in eudicots. PLoS ONE 2013, 8, e74803. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.G.; Donoghue, M.J. Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proc. Natl. Acad. Sci. USA 2006, 103, 9101–9106. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Knox, E.B.; Morden, C.W.; Cellinese, N.; Mossolem, F.; Zubair, A.S.; Howarth, D.G. Duplication and expression patterns of CYCLOIDEA-like genes in Campanulaceae. Evodevo 2022, 13, 5. [Google Scholar] [CrossRef]
- Ramage, E.; Soza, V.L.; Yi, J.; Deal, H.; Chudgar, V.; Hall, B.D.; Di Stilio, V.S. Gene Duplication and Differential Expression of Flower Symmetry Genes in Rhododendron (Ericaceae). Plants 2021, 10, 1994. [Google Scholar] [CrossRef]
- Carlson, S.E.; Howarth, D.G.; Donoghue, M.J. Diversification of CYCLOIDEA-like genes in Dipsacaceae (Dipsacales): Implications for the evolution of capitulum inflorescences. BMC Evol. Biol. 2011, 11, 325. [Google Scholar] [CrossRef]
- Busch, A.; Zachgo, S. Flower symmetry evolution: Towards understanding the abominable mystery of angiosperm radiation. Bioessays 2009, 31, 1181–1190. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Karaaslan, E.S.; Wang, N.; Faiss, N.; Liang, Y.; Montgomery, S.A.; Laubinger, S.; Berendzen, K.W.; Berger, F.; Breuninger, H.; Liu, C. Marchantia TCP transcription factor activity correlates with three-dimensional chromatin structure. Nat. Plants 2020, 6, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, L.V.; Gastaldi, V.; Ariel, F.D.; Viola, I.L.; Gonzalez, D.H. Class I TCP proteins TCP14 and TCP15 are required for elongation and gene expression responses to auxin. Plant Mol. Biol. 2021, 105, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Tabarelli, M.; Malnoy, M.; Janik, K. Chasing Consistency: An Update of the TCP Gene Family of Malus x Domestica. Genes 2022, 13, 1696. [Google Scholar] [CrossRef] [PubMed]
- Sinou, C.; Cardinal-McTeague, W.; Bruneau, A. Testing generic limits in Cercidoideae (Leguminosae): Insights from plastid and duplicated nuclear gene sequences: Phylogeny and generic limits in Cercidoideae. Taxon 2020, 69, 67–86. [Google Scholar] [CrossRef]
- Fambrini, M.; Pugliesi, C. CYCLOIDEA-2 Clade Genes: Key Players in the Control of Floral Symmetry, Inflorescence Architecture, and Reproductive Organ Development. Plant Mol. Biol. Rep. 2017, 35, 20–36. [Google Scholar] [CrossRef]
- Fambrini, M.; Pugliesi, C. Presence/absence of a CACTA transposon in the CYC2c gene of two genotypes of Helianthus × multiflorus cv. “Meteor” characterized by a radiate inflorescence with different shape of disk flower corollas. Biologia 2019, 74, 1675–1686. [Google Scholar] [CrossRef]
- Fambrini, M.; Bernardi, R.; Pugliesi, C. Ray flower initiation in the Helianthus radula inflorescence is influenced by a functional allele of the HrCYC2c gene. GENESIS 2020, 58, e23401. [Google Scholar] [CrossRef]
- Bukhari, G.; Zhang, J.; Stevens, P.F.; Zhang, W. Evolution of the process underlying floral zygomorphy development in pentapetalous angiosperms. Am. J. Bot. 2017, 104, 1846–1856. [Google Scholar] [CrossRef]
- Sengupta, A.; Hileman, L.C. Novel Traits, Flower Symmetry, and Transcriptional Autoregulation: New Hypotheses From Bioinformatic and Experimental Data. Front. Plant Sci. 2018, 9, 1561. [Google Scholar] [CrossRef]
- Kalisz, S.; Ree, R.H.; Sargent, R.D. Linking floral symmetry genes to breeding system evolution. Trends Plant Sci. 2006, 11, 568–573. [Google Scholar] [CrossRef]
- Endress, P.K. Evolution of floral symmetry. Curr. Opin. Plant Biol. 2010, 4, 86–91. [Google Scholar] [CrossRef]
- Coen, E.; Nugent, J.; Luo, D.; Bradley, D.; Cubas, P.; Chadwick, M.; Copsey, L.; Carpenter, R. Evolution of floral symmetry. Philos. Trans. R. Soc. B 1995, 350, 35–38. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, P.F.; Zhang, W. Evolution and development of inflorescences and floral symmetry in Solanaceae. Am. J. Bot. 2022, 109, 746–767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Moubayidin, L. Floral symmetry: The geometry of plant reproduction. Emerg. Top. Life Sci. 2022, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Rosin, F.M.; Kramer, E.M. Old dogs, new tricks: Regulatory evolution in conserved genetic modules leads to novel morphologies in plants. Dev. Biol. 2009, 332, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Fernandez-Mazuecos, M.; Vargas, P. Evolution in the Model Genus Antirrhinum Based on Phylogenomics of Topotypic Material. Front. Plant Sci. 2021, 12, 631178. [Google Scholar] [CrossRef]
- Nicolas, M.; Cubas, P. The Role of TCP Transcription Factors in Shaping Flower Structure, Leaf Morphology, and Plant Architecture. In Plant Transcription Factors; Gonzalez, D.H., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 249–267. [Google Scholar]
- Preston, J.C.; Hileman, L.C.; Cubas, P. Reduce, reuse, and recycle: Developmental evolution of trait diversification. Am. J. Bot. 2011, 98, 397–403. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Developmental genetics of floral symmetry evolution. Trends Plant Sci. 2009, 14, 147–154. [Google Scholar] [CrossRef]
- Hsin, K.T.; Lu, J.Y.; Moller, M.; Wang, C.N. Gene duplication and relaxation from selective constraints of GCYC genes correlated with various floral symmetry patterns in Asiatic Gesneriaceae tribe Trichosporeae. PLoS ONE 2019, 14, e210054. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Coen, E.; Zapater, J.M. Ancient asymmetries in the evolution of flowers. Curr. Biol. 2001, 11, 1050–1052. [Google Scholar] [CrossRef]
- Preston, J.C.; Martinez, C.C.; Hileman, L.C. Gradual disintegration of the floral symmetry gene network is implicated in the evolution of a wind-pollination syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 2343–2348. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.G.; Li, C.Q.; Liu, J.; Qiu, Z.J.; Dong, Y.; Wang, Y.Z. Distinct Regulatory Changes Underlying Differential Expression of TEOSINTE BRANCHED1-CYCLOIDEA-PROLIFERATING CELL FACTOR Genes Associated with Petal Variations in Zygomorphic Flowers of Petrocosmea spp. of the Family Gesneriaceae. Plant Physiol. 2015, 169, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.A.; Thompson, V.; Lim, A.; Ricigliano, V.; Howarth, D.G. Elaboration of bilateral symmetry across Knautia macedonica capitula related to changes in ventral petal expression of CYCLOIDEA-like genes. Evodevo 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kramer, E.M.; Davis, C.C. Differential Expression of CYC2 Genes and the Elaboration of Floral Morphologies in Hiptage, an Old World Genus of Malpighiaceae. Int. J. Plant Sci. 2016, 177, 551–558. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wang, C.N.; Liang, C.H.; Wang, C.C.; Kuo, Y.F. Association between Petal Form Variation and CYC2-like Genotype in a Hybrid Line of Sinningia speciosa. Front. Plant Sci. 2017, 8, 558. [Google Scholar] [CrossRef] [PubMed]
- Citerne, H.L.; Pennington, R.T.; Cronk, Q.C. An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proc. Natl. Acad. Sci. USA 2006, 103, 12017–12020. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Z.; Tian, Z.; Xu, S.; Luo, Y.; Cai, Z.; Wang, Y.; Yang, J.; Wang, Z.; Weng, L.; et al. Control of petal shape and floral zygomorphy in Lotus japonicus. Proc. Natl. Acad. Sci. USA 2006, 103, 4970–4975. [Google Scholar] [CrossRef]
- Citerne, H.L.; Luo, D.; Pennington, R.T.; Coen, E.; Cronk, Q.C. A phylogenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Plant Physiol. 2003, 131, 1042–1053. [Google Scholar] [CrossRef]
- Ree, R.H.; Citerne, H.L.; Lavin, M.; Cronk, Q.C. Heterogeneous selection on LEGCYC paralogs in relation to flower morphology and the phylogeny of Lupinus (Leguminosae). Mol. Biol. Evol. 2004, 21, 321–331. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Luo, D. LjCYC genes constitute floral dorsoventral asymmetry in Lotus japonicus. J. Integr. Plant Biol. 2010, 52, 959–970. [Google Scholar] [CrossRef]
- Woollacott, C.; Cronk, Q. The hooded mutant of Lathyrus odoratus (Fabaceae) is associated with a cycloidea gene mutation. Botany 2017, 96, 47–55. [Google Scholar] [CrossRef]
- Woollacott, C.; Wang, L.; Beyer, S.; Walus, K.; Cronk, Q. CYCLOIDEA gene activity, local growth and curvature in the dorsal petal of Lathyrus odoratus (Fabaceae). Bot. Lett. 2018, 166, 64–69. [Google Scholar] [CrossRef]
- Ojeda, D.I.; Jaen-Molina, R.; Santos-Guerra, A.; Caujape-Castells, J.; Cronk, Q. Temporal, but not spatial, changes in expression patterns of petal identity genes are associated with loss of papillate conical cells and the shift to bird pollination in Macaronesian Lotus (Leguminosae). Plant Biol. 2017, 19, 420–427. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, N.; Zhang, G.; Xu, S.; Gong, Y. Identification of Branch Related CYL Gene in Soybean and Analysis of Hormone Expression Pattern in Vegetable Soybean Species. Mol. Plant Breed. 2019, 17, 4865–4872. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, J.; Chen, S.; Luo, Z.; Luo, D.; Wen, J.; Tu, T.; Zhang, D. Evolution of CYCLOIDEA-like genes in Fabales: Insights into duplication patterns and the control of floral symmetry. Mol. Phylogenet. Evol. 2019, 132, 81–89. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Jia, Y.; Qiu, D.; Peng, Q.; Zhuang, L. Genetic control of the lateral petal shape and identity of asymmetric flowers in mungbean (Vigna radiata L.). Front. Plant Sci. 2022, 13, 996239. [Google Scholar] [CrossRef]
- Zoulias, N.; Duttke, S.; Garces, H.; Spencer, V.; Kim, M. The Role of Auxin in the Pattern Formation of the Asteraceae Flower Head (Capitulum). Plant Physiol. 2019, 179, 391–401. [Google Scholar] [CrossRef]
- Hernandez, L.F.; Palmer, J.H. Regeneration of the sunflower capitulum after cylindrical wounding of the receptacle. Am. J. Bot. 1988, 75, 1253–1261. [Google Scholar] [CrossRef]
- Broholm, S.K.; Tahtiharju, S.; Laitinen, R.A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 9117–9122. [Google Scholar] [CrossRef]
- Garces, H.M.; Spencer, V.M.; Kim, M. Control of Floret Symmetry by RAY3, SvDIV1B, and SvRAD in the Capitulum of Senecio vulgaris. Plant Physiol. 2016, 171, 2055–2068. [Google Scholar] [CrossRef]
- Sun, P.; Bao, Y.; Zhu, Y.; Huang, N.; Wang, X.; Wu, Z. Possible role of the CYC2c gene in the cornflower-like ray floret phenotype of Gaillardia cultivars. J. Plant Res. 2022, 135, 465–472. [Google Scholar] [CrossRef]
- Tahtiharju, S.; Rijpkema, A.S.; Vetterli, A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolution and diversification of the CYC/TB1 gene family in Asteraceae—A comparative study in Gerbera (Mutisieae) and sunflower (Heliantheae). Mol. Biol. Evol. 2012, 29, 1155–1166. [Google Scholar] [CrossRef]
- Juntheikki-Palovaara, I.; Tahtiharju, S.; Lan, T.; Broholm, S.K.; Rijpkema, A.S.; Ruonala, R.; Kale, L.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae). Plant J. 2014, 79, 783–796. [Google Scholar] [CrossRef]
- Spencer, V.; Kim, M. Re”CYC”ling molecular regulators in the evolution and development of flower symmetry. Semin. Cell Dev. Biol. 2018, 79, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Leebens-Mack, J.H.; Burke, J.M. Positive selection and expression divergence following gene duplication in the sunflower CYCLOIDEA gene family. Mol. Biol. Evol. 2008, 25, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Salvini, M.; Pugliesi, C. A transposon-mediate inactivation of a CYCLOIDEA-like gene originates polysymmetric and androgynous ray flowers in Helianthus annuus. Genetica 2011, 139, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Tang, S.; Draeger, D.; Nambeesan, S.; Shaffer, H.; Barb, J.G.; Knapp, S.J.; Burke, J.M. Genetic analysis of floral symmetry in Van Gogh’s sunflowers reveals independent recruitment of CYCLOIDEA genes in the Asteraceae. PLoS Genet. 2012, 8, e1002628. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Salvini, M.; Basile, A.; Pugliesi, C. Transposon-dependent induction of Vincent van Gogh’s sunflowers: Exceptions revealed. Genesis 2014, 52, 315–327. [Google Scholar] [CrossRef]
- Mizzotti, C.; Fambrini, M.; Caporali, E.; Masiero, S.; Pugliesi, C. A CYCLOIDEA -like gene mutation in sunflower determines an unusual floret type able to produce filled achenes at the periphery of the pseudanthium. Botany 2015, 93, 171–181. [Google Scholar] [CrossRef]
- He, Z.; Zeng, W.; Chen, W.; Wu, Y.; Wen, G.; Chen, X.; Wang, Q.; Zhou, J.; Li, Y.; Yang, Z.; et al. HaCYC2c regulating the heteromorphous development and functional differentiation of florets by recognizing HaNDUA2 in sunflower. Plant Cell Rep. 2022, 41, 1025–1041. [Google Scholar] [CrossRef]
- Fambrini, M.; Bellanca, M.; Costa, M.M.; Usai, G.; Cavallini, A.; Pugliesi, C. Ligulate inflorescence of Helianthus x multiflorus, cv. Soleil d’Or, correlates with a mis-regulation of a CYCLOIDEA gene characterised by insertion of a transposable element. Plant Biol. 2018, 20, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Broholm, S. The Role of MADS and TCP Transcription Factors in Gerbera Hybrida Flower Development. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2009. [Google Scholar]
- Zhao, Y.; Broholm, S.K.; Wang, F.; Rijpkema, A.S.; Lan, T.; Albert, V.A.; Teeri, T.H.; Elomaa, P. TCP and MADS-Box Transcription Factor Networks Regulate Heteromorphic Flower Type Identity in Gerbera hybrida. Plant Physiol. 2020, 184, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cui, M.L.; Cubas, P.; Gillies, A.; Lee, K.; Chapman, M.A.; Abbott, R.J.; Coen, E. Regulatory genes control a key morphological and ecological trait transferred between species. Science 2008, 322, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Po, C.; Hao, Y.; Feng, L.; Zhou, J.; Luan, S.; Liu, L.; Li, F.; Yuan, S.; Cui, M. Cloning and functional analysis of CYCLOIDEA(CYC)-like SvRAY1 gene from Senecio vulgaris. J. Zhejiang AF Univ. 2021, 38, 1153–1160. [Google Scholar] [CrossRef]
- Huang, D.; Sun, M.; Yuan, C.; Cheng, T.; Wang, J.; Zhang, Q. Isolation and functional analysis of CYC2d orthologous genes from several plants of the tribe Anthemideae. J. Beijing For. Univ. 2017, 39, 63–71. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Yuan, C.; Han, Y.; Zheng, T.; Cheng, T.; Wang, J.; Zhang, Q. Interactions between WUSCHEL and CYC2-like Transcription Factors in Regulating the Development of Reproductive Organs in Chrysanthemum morifolium. Int. J. Mol. Sci. 2019, 20, 1276. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, H.; Wen, X.; Lu, C.; Zhang, B.; Gu, X.; Qi, S.; Fan, G.; Wang, W.; Dai, S. Comprehensive transcriptomic analysis provides new insights into the mechanism of ray floret morphogenesis in chrysanthemum. BMC Genom. 2020, 21, 728. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, D.; Yang, Y.; Sun, M.; Cheng, T.; Wang, J.; Pan, H.; Zhang, Q. CmCYC2-like transcription factors may interact with each other or bind to the promoter to regulate floral symmetry development in Chrysanthemum morifolium. Plant Mol. Biol. 2020, 103, 159–171. [Google Scholar] [CrossRef]
- Liu, H.; Sun, M.; Pan, H.; Cheng, T.; Wang, J.; Zhang, Q. Two Cyc2CL transcripts (Cyc2CL-1 and Cyc2CL-2) may play key roles in the petal and stamen development of ray florets in chrysanthemum. BMC Plant Biol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.Z.; Guo, Y.P.; Rao, G.Y. Patterning the Asteraceae Capitulum: Duplications and Differential Expression of the Flower Symmetry CYC2-Like Genes. Front. Plant Sci. 2018, 9, 551. [Google Scholar] [CrossRef]
- Wen, X.; Qi, S.; Huang, H.; Wu, X.; Zhang, B.; Fan, G.; Yang, L.; Hong, Y.; Dai, S. The expression and interactions of ABCE-class and CYC2-like genes in the capitulum development of Chrysanthemum lavandulifolium and C. × morifolium. Plant Growth Regul. 2019, 88, 205–214. [Google Scholar] [CrossRef]
- Kironji, G.S. Patterning of the Chrysanthemum Inflorescence Roles of B-Class and CYC2 Subclade Genes. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2019. [Google Scholar]
- Yang, Y.; Yang, Y.J.; Yuan, C.Q.; Zhang, Q.X. Cloning and Expression Analysis of CmTCP7 in Chrysanthemum morifolium. Acta Bot. Boreali-Occident. Sin. 2019, 39, 595–602. [Google Scholar] [CrossRef]
- Shen, C.Z.; Chen, J.; Zhang, C.J.; Rao, G.Y.; Guo, Y.P. Dysfunction of CYC2g is responsible for the evolutionary shift from radiate to disciform flowerheads in the Chrysanthemum group (Asteraceae: Anthemideae). Plant J. 2021, 106, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, C.; Qi, S.; Dai, S. Difference Analysis of ClCYC2-Like Genes Expression and DNA Methylation between the Two Types of Florets in Chrysanthemum lavandulifolium. J. Plant Growth Regul. 2022, 41, 1316–1330. [Google Scholar] [CrossRef]
- Elomaa, P.; Zhao, Y.; Zhang, T. Flower heads in Asteraceae-recruitment of conserved developmental regulators to control the flower-like inflorescence architecture. Hortic. Res. 2018, 5, 36. [Google Scholar] [CrossRef]
- Zhong, J.; Kellogg, E.A. Duplication and expression of CYC2-like genes in the origin and maintenance of corolla zygomorphy in Lamiales. New Phytol. 2014, 205, 852–868. [Google Scholar] [CrossRef]
- Ha, Y.H.; Kim, C.; Choi, K.; Kim, J.H. Molecular Phylogeny and Dating of Forsythieae (Oleaceae) Provide Insight into the Miocene History of Eurasian Temperate Shrubs. Front. Plant Sci. 2018, 9, 99. [Google Scholar] [CrossRef]
- Smyth, D.R. Evolution and genetic control of the floral ground plan. New Phytol. 2018, 220, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Ajani, Y.; Jamzad, Z.; Claßen-Bockhoff, R. Floral biology in the endemic Iranian Salvia majdae—Implications for taxonomy, character evolution and conservation. Flora 2021, 287, 151986. [Google Scholar] [CrossRef]
- Wessinger, C.; Hileman, L. Parallelism in Flower Evolution and Development. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 387–408. [Google Scholar] [CrossRef]
- Zhong, J.; Kellogg, E.A. Stepwise evolution of corolla symmetry in CYCLOIDEA2-like and RADIALIS-like gene expression patterns in Lamiales. Am. J. Bot. 2015, 102, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Poulin, V.; Amesefe, D.; Gonzalez, E.; Alexandre, H.; Joly, S. Testing candidate genes linked to corolla shape variation of a pollinator shift in Rhytidophyllum (Gesneriaceae). PLoS ONE 2022, 17, e267540. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Preston, J.C.; Hileman, L.C.; Kellogg, E.A. Repeated and diverse losses of corolla bilateral symmetry in the Lamiaceae. Ann. Bot. 2017, 119, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Hileman, L.C.; Cubas, P. An expanded evolutionary role for flower symmetry genes. J. Biol. 2009, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Neel, M.C. Conservation implications of the reproductive ecology of Agalinis acuta (Scrophulariaceae). Am. J. Bot. 2002, 89, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hileman, L.C.; Kramer, E.M.; Baum, D.A. Differential regulation of symmetry genes and the evolution of floral morphologies. Proc. Natl. Acad. Sci. USA 2003, 100, 12814–12819. [Google Scholar] [CrossRef]
- Su, S.; Xiao, W.; Guo, W.; Yao, X.; Xiao, J.; Ye, Z.; Wang, N.; Jiao, K.; Lei, M.; Peng, Q.; et al. The CYCLOIDEA-RADIALIS module regulates petal shape and pigmentation, leading to bilateral corolla symmetry in Torenia fournieri (Linderniaceae). New Phytol. 2017, 215, 1582–1593. [Google Scholar] [CrossRef]
- Clark, J.I.; Coen, E.S. The cycloidea gene can respond to a common dorsoventral prepattern in Antirrhinum. Plant J. 2002, 30, 639–648. [Google Scholar] [CrossRef]
- Preston, J.C.; Kost, M.A.; Hileman, L.C. Conservation and diversification of the symmetry developmental program among close relatives of snapdragon with divergent floral morphologies. New Phytol. 2009, 182, 751–762. [Google Scholar] [CrossRef]
- Gubitz, T.; Caldwell, A.; Hudson, A. Rapid molecular evolution of CYCLOIDEA-like genes in Antirrhinum and its relatives. Mol. Biol. Evol. 2003, 20, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Corley, S.B.; Carpenter, R.; Copsey, L.; Coen, E. Floral asymmetry involves an interplay between TCP and MYB transcription factors in Antirrhinum. Proc. Natl. Acad. Sci. USA 2005, 102, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Theissen, G. Evolutionary developmental genetics of floral symmetry: The revealing power of Linnaeus’ monstrous flower. Bioessays 2000, 22, 209–213. [Google Scholar] [CrossRef]
- Kondo, M.; Tanikawa, N.; Nishijima, T. Mutation of CYCLOIDEA expands variation of dorsal-ventral flower asymmetry expressed as a pigmentation pattern in Torenia fournieri cultivars. Hortic. J. 2020, 89, 481–487. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Yuan, Z.; Wang, Y. Significance of consensus CYC-binding sites found in the promoters of both ChCYC and ChRAD genes in Chirita heterotricha (Gesneriaceae). J. Syst. Evol. 2010, 48, 249–256. [Google Scholar] [CrossRef]
- Yang, X.; Pang, H.B.; Liu, B.L.; Qiu, Z.J.; Gao, Q.; Wei, L.; Dong, Y.; Wang, Y.Z. Evolution of double positive autoregulatory feedback loops in CYCLOIDEA2 clade genes is associated with the origin of floral zygomorphy. Plant Cell 2012, 24, 1834–1847. [Google Scholar] [CrossRef]
- Hsu, H.J.; He, C.W.; Kuo, W.H.; Hsin, K.T.; Lu, J.Y.; Pan, Z.J.; Wang, C.N. Genetic analysis of floral symmetry transition in African violet suggests the involvement of trans-acting factor for CYCLOIDEA expression shifts. Front. Plant Sci. 2018, 9, 1008. [Google Scholar] [CrossRef]
- Liu, J.; Jie, W.; Yang, X.; Wang, Y. Regulatory pathways of CYC-like genes in patterning floral zygomorphy exemplified in Chirita pumila. J. Syst. Evol. 2020, 59, 567–580. [Google Scholar] [CrossRef]
- Yang, M.; Xu, J.; Pang, J. Flower Shape Changes of African Violets Caused by LjCYC1 Gene in Lotus japonicus. Acta Horticulturae Sinica 2020, 47, 708–716. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.J.; Wu, J.; Wang, Y.; Liu, Q.; Liu, F.P.; Yang, X.; Wang, Y.Z. An Optimized Transformation System and Functional Test of CYC-Like TCP Gene CpCYC in Chirita pumila (Gesneriaceae). Int. J. Mol. Sci. 2021, 22, 4544. [Google Scholar] [CrossRef]
- Ferraro, B.J. Examining the roles of CYCLOIDEA, RADIALIS and DIVARICATA in driving the evolution of flower shape Californian Diplacus pictus (Curran ex Greene) Nesom (Phrymaceae). Master’s Thesis, California State University, Long Beach, CA, USA, 2014. [Google Scholar]
- Lucibelli, F.; Valoroso, M.C.; Aceto, S. Radial or bilateral? The molecular basis of floral symmetry. Genes 2020, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Lucibelli, F.; Valoroso, M.; Theißen, G.; Nolden, S.; Mondragon Palomino, M.; Aceto, S. Extending the toolkit for beauty: Differential co-expression of DROOPING LEAF-like and class B MADS-Box genes during Phalaenopsis flower development. Int. J. Mol. Sci. 2021, 22, 7025. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, Y.; Pabon-Mora, N.; Alzate, J. Unraveling the genetic basis of floral symmetry variation in monocots with an emphasis on Asparagales. In Proceedings of the World Orchid Conference 2017, Guayaquil, Ecuador, 8–12 November 2017. [Google Scholar]
- Klepikova, A.V.; Kasianov, A.S.; Ezhova, M.A.; Penin, A.A.; Logacheva, M.D. Transcriptome atlas of Phalaenopsis equestris. PeerJ 2021, 9, e12600. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, Y.Y.; Hsiao, Y.Y.; Shen, C.Y.; Hsu, J.L.; Yeh, C.M.; Mitsuda, N.; Ohme-Takagi, M.; Liu, Z.J.; Tsai, W.C. Genome-wide identification and characterization of TCP genes involved in ovule development of Phalaenopsis equestris. J. Exp. Bot. 2016, 67, 5051–5066. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.K.; Zhang, C.; Zhao, X.; Ke, S.; Li, Y.; Zhang, D.; Zheng, Q.; Li, M.H.; Lan, S.; Liu, Z.J. Genome-wide analysis of the TCP gene family and their expression pattern in Cymbidium goeringii. Front. Plant Sci. 2022, 13, 1068969. [Google Scholar] [CrossRef]
- Madrigal, Y.; Alzate, J.F.; Pabon-Mora, N. Evolution and expression patterns of TCP genes in Asparagales. Front. Plant Sci. 2017, 8, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, P.; Zhang, W. Evolution of floral zygomorphy in androecium and corolla in Solanaceae. J. Syst. Evol. 2017, 55, 581–590. [Google Scholar] [CrossRef]
- Shimada, A.; Kimura, Y. Influence of glyphosate on flower morphogenesis and pigmentation in Petunia hybrida. Z. Naturforsch. C 2006, 61, 578–582. [Google Scholar] [CrossRef]
- Zou, S.; Wang, H.; Yu, Y.; Ma, J.; Guo, Y.; Li, M. Studies on molecular cloning, expression pattern of ECE Clade TCP genes in Petunia. Acta Hortic. Sin. 2013, 40, 307–316. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Chen, F.; Wu, L.; Liu, B.; Li, F.; Zhang, J.; Bao, M.; Liu, G. Genome-Wide Identification, Characterization and Expression Analysis of TCP Transcription Factors in Petunia. Int. J. Mol. Sci. 2020, 21, 6594. [Google Scholar] [CrossRef]
- Sengupta, A.; Hileman, L.C. A CYC-RAD-DIV-DRIF interaction likely pre-dates the origin of floral monosymmetry in Lamiales. Evodevo 2022, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Hileman, L.C. Parallel evolution of TCP and B-class genes in Commelinaceae flower bilateral symmetry. Evodevo 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Classen-Bockhoff, R.; Ruonala, R.; Bull-Herenu, K.; Marchant, N.; Albert, V.A. The unique pseudanthium of Actinodium (Myrtaceae)—Morphological reinvestigation and possible regulation by CYCLOIDEA-like genes. Evodevo 2013, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Pabon-Mora, N.; Theuss, V.S.; Busch, A.; Zachgo, S. Analysis of the CYC/TB1 class of TCP transcription factors in basal angiosperms and magnoliids. Plant J. 2015, 81, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.E.; Specht, C.D. Changes in expression pattern of the teosinte branched1-like genes in the Zingiberales provide a mechanism for evolutionary shifts in symmetry across the order. Am. J. Bot. 2011, 98, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tian, X.; Lin, C.; Specht, C.D.; Liao, J. Expression and function studies of CYC/TB1-like genes in the asymmetric flower Canna (Cannaceae, Zingiberales). Front. Plant Sci. 2020, 11, 580576. [Google Scholar] [CrossRef] [PubMed]
- Iljinska, A.P. The range of the morphological features of Brassicaceae s. l.: Inflorescence, flower. Ukr. Bot. J. 2015, 72, 122–134. [Google Scholar] [CrossRef]
- Nikolov, L.A. Brassicaceae flowers: Diversity amid uniformity. J. Exp. Bot. 2019, 70, 2623–2635. [Google Scholar] [CrossRef]
- Reichling, J.; Saller, R. Iberis amara L. (Bittere Schleifenblume)—Profil einer Heilpflanze. Complement. Med. Res. 2002, 9, 21–33. [Google Scholar] [CrossRef]
- Busch, A.; Zachgo, S. Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proc. Natl. Acad. Sci. USA 2007, 104, 16714–16719. [Google Scholar] [CrossRef]
- Busch, A.; Horn, S.; Zachgo, S. Differential transcriptome analysis reveals insight into monosymmetric corolla development of the crucifer Iberis amara. BMC Plant Biol. 2014, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Horn, S.; Muhlhausen, A.; Mummenhoff, K.; Zachgo, S. Corolla monosymmetry: Evolution of a morphological novelty in the Brassicaceae family. Mol. Biol. Evol. 2012, 29, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, F.; Cossard, G.; Le Guilloux, M.; Sannier, J.; Nadot, S.; Damerval, C. Specific duplication and dorsoventrally asymmetric expression patterns of Cycloidea-like genes in zygomorphic species of Ranunculaceae. PLoS ONE 2014, 9, e95727. [Google Scholar] [CrossRef] [PubMed]
- Zalko, J.; Frachon, S.; Morel, A.; Deroin, T.; Espinosa Moreno, F.; Xiang, K.; Wang, W.; Zhang, W.; Lang, S.; Dixon, L.; et al. Floral organogenesis and morphogenesis of Staphisagria (Ranunculaceae): Implications for the evolution of synorganized floral structures in Delphinieae. Int. J. Plant Sci. 2020, 182, 59–70. [Google Scholar] [CrossRef]
- Kolsch, A.; Gleissberg, S. Diversification of CYCLOIDEA-like TCP genes in the basal eudicot families Fumariaceae and Papaveraceae s.str. Plant Biol. 2006, 8, 680–687. [Google Scholar] [CrossRef]
- Damerval, C.; Le Guilloux, M.; Jager, M.; Charon, C. Diversity and evolution of CYCLOIDEA-like TCP genes in relation to flower development in Papaveraceae. Plant Physiol. 2007, 143, 759–772. [Google Scholar] [CrossRef]
- Zhao, Y.; Pfannebecker, K.; Dommes, A.B.; Hidalgo, O.; Becker, A.; Elomaa, P. Evolutionary diversification of CYC/TB1-like TCP homologs and their recruitment for the control of branching and floral morphology in Papaveraceae (basal eudicots). New Phytol. 2018, 220, 317–331. [Google Scholar] [CrossRef]
- Pabon-Mora, N.; Madrigal, Y.; Alzate, J.F.; Ambrose, B.A.; Ferrandiz, C.; Wanke, S.; Neinhuis, C.; Gonzalez, F. Evolution of Class II TCP genes in perianth bearing Piperales and their contribution to the bilateral calyx in Aristolochia. New Phytol. 2020, 228, 752–769. [Google Scholar] [CrossRef]
- Zhang, W.; Steinmann, V.W.; Nikolov, L.; Kramer, E.M.; Davis, C.C. Divergent genetic mechanisms underlie reversals to radial floral symmetry from diverse zygomorphic flowered ancestors. Front. Plant Sci. 2013, 4, 302. [Google Scholar] [CrossRef]
- Berger, B.A.; Ricigliano, V.A.; Savriama, Y.; Lim, A.; Thompson, V.; Howarth, D.G. Geometric morphometrics reveals shifts in flower shape symmetry and size following gene knockdown of CYCLOIDEA and ANTHOCYANIDIN SYNTHASE. BMC Plant Biol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Hileman, L.C. Trends in flower symmetry evolution revealed through phylogenetic and developmental genetic advances. Philos. Trans. R. Soc. B 2014, 369, 20130348. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Igarashi, T.; Ohshima, M.; Shinoda, K.; Murata, N.; Kanno, A.; Nakano, M. Characterization of CYCLOIDEA-like genes in controlling floral zygomorphy in the monocotyledon Alstroemeria. Sci. Hortic. 2014, 169, 6–13. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.; Liu, H.; Chen, W.; Guo, C.; Chen, H.; Cheng, X.; Chen, D.; Luo, C.; Zhou, X.; Huang, C. Advances in Research on the Regulation of Floral Development by CYC-like Genes. Curr. Issues Mol. Biol. 2023, 45, 2035-2059. https://doi.org/10.3390/cimb45030131
Chai Y, Liu H, Chen W, Guo C, Chen H, Cheng X, Chen D, Luo C, Zhou X, Huang C. Advances in Research on the Regulation of Floral Development by CYC-like Genes. Current Issues in Molecular Biology. 2023; 45(3):2035-2059. https://doi.org/10.3390/cimb45030131
Chicago/Turabian StyleChai, Yuhong, Hua Liu, Wendan Chen, Chenghu Guo, Haixia Chen, Xi Cheng, Dongliang Chen, Chang Luo, Xiumei Zhou, and Conglin Huang. 2023. "Advances in Research on the Regulation of Floral Development by CYC-like Genes" Current Issues in Molecular Biology 45, no. 3: 2035-2059. https://doi.org/10.3390/cimb45030131
APA StyleChai, Y., Liu, H., Chen, W., Guo, C., Chen, H., Cheng, X., Chen, D., Luo, C., Zhou, X., & Huang, C. (2023). Advances in Research on the Regulation of Floral Development by CYC-like Genes. Current Issues in Molecular Biology, 45(3), 2035-2059. https://doi.org/10.3390/cimb45030131




