Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
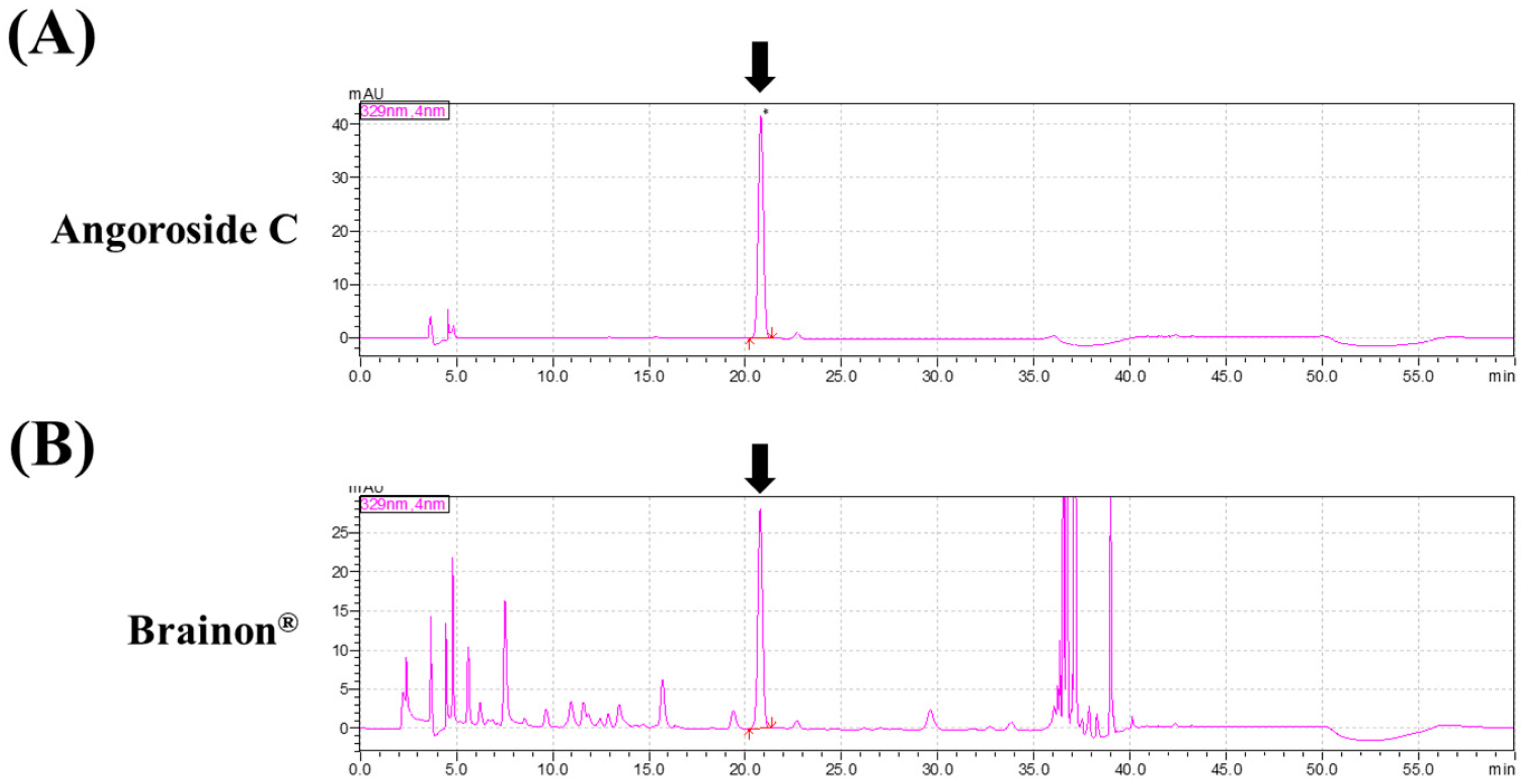
2.3. HPLC Analysis
2.4. Animals
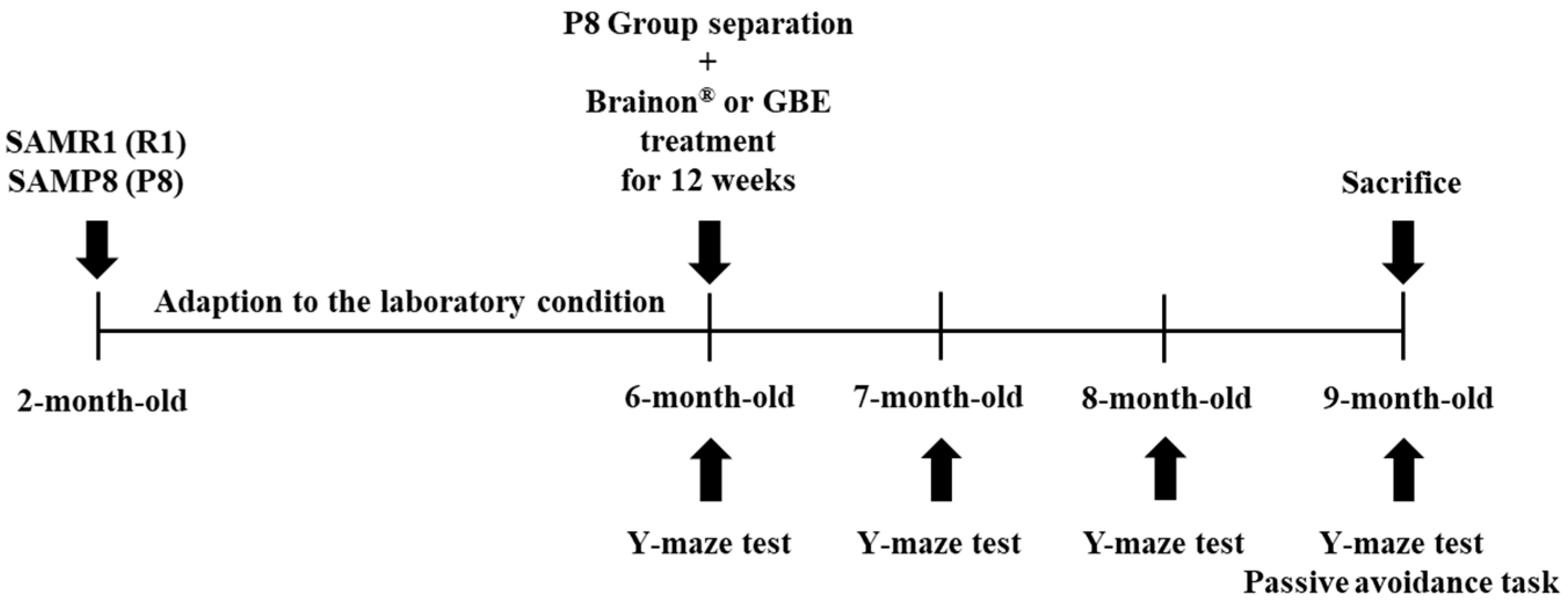
2.5. Design of Experiment
2.6. Y-Maze Test
2.7. Step-Through Passive Avoidance Task
2.8. Extraction of Protein and Western Blot Analysis
2.9. Immunofluorescence Staining
2.10. Statistical Analysis
3. Results
3.1. Composition of Brainon®
3.2. Brainon® Prevents Age-Dependent Cognitive Declines of SAMP8 Mice in the Y-Maze Test
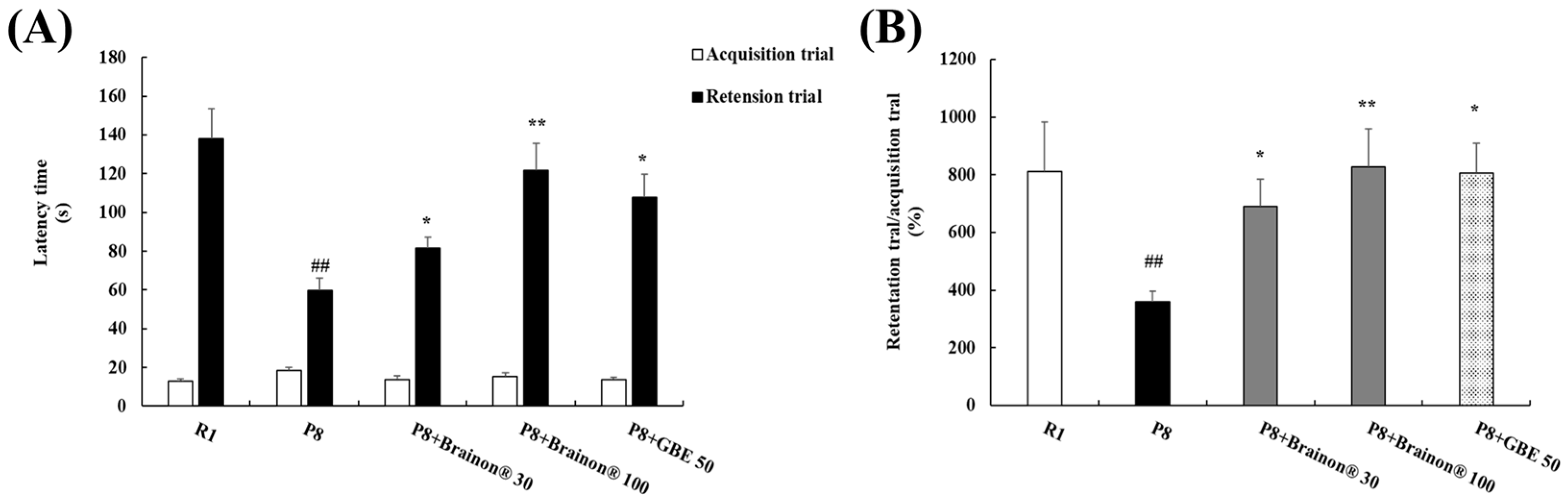
3.3. Brainon® Ameliorates Learning and Memory Impairments in the Passive Avoidance Task
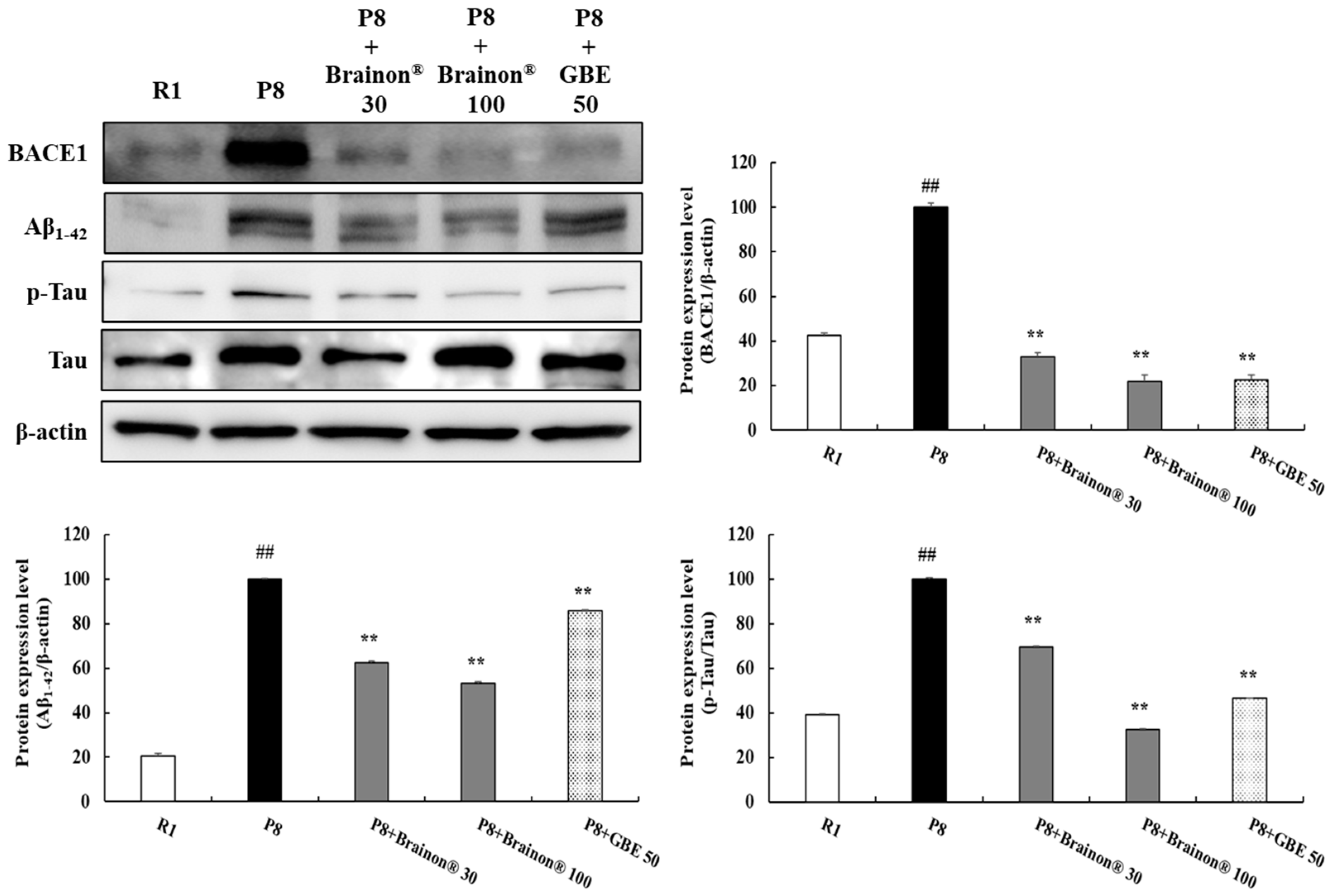
3.4. Effects of Brainon® on Inhibition of Aβ1–42 Production and Hyperphosphorylation of Tau in the Cerebral Cortex of SAMP8 Mice
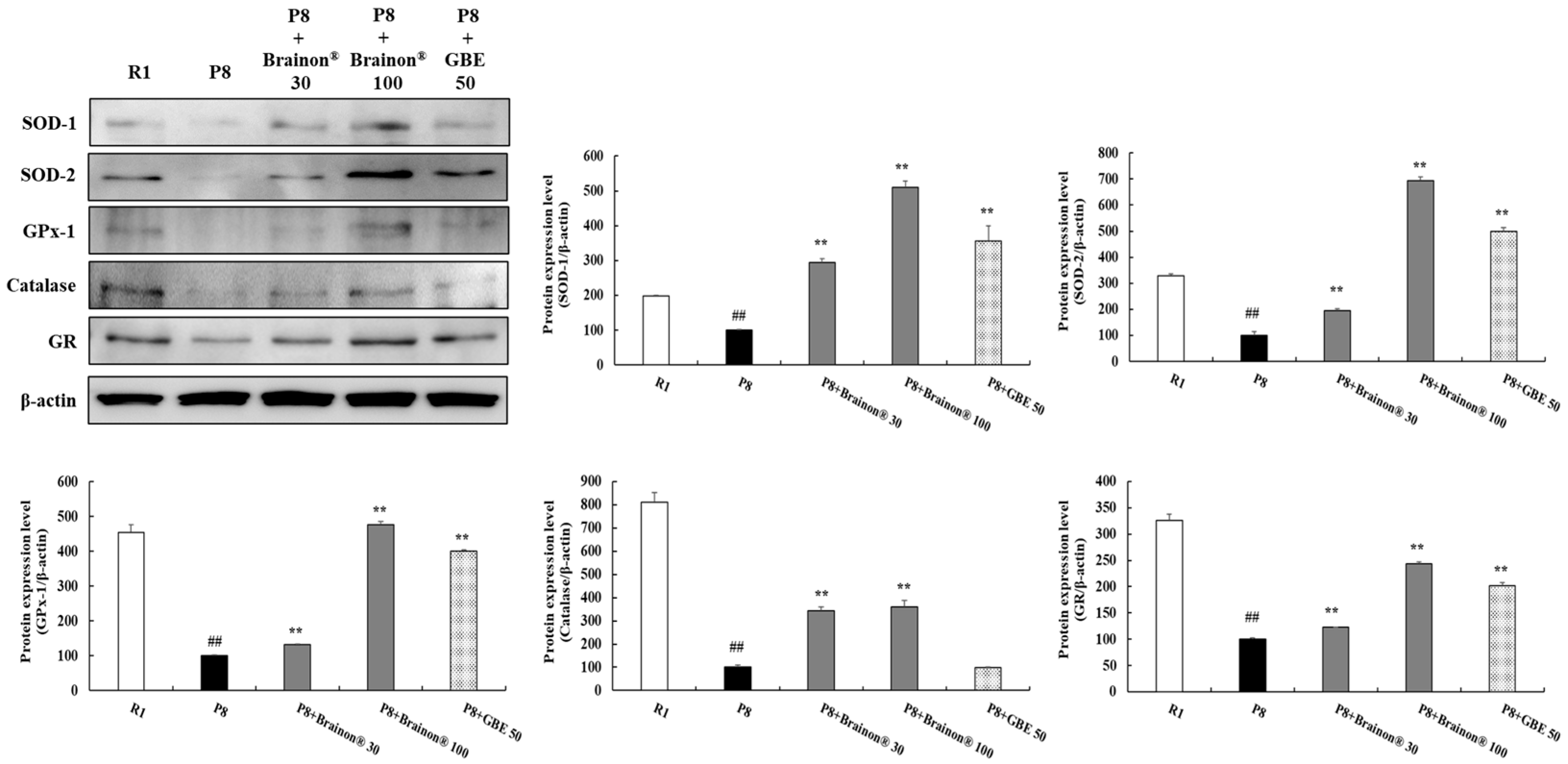
3.5. Brainon® Promotes Antioxidant Enzymes in the Cerebral Cortex of SAMP8 Mice
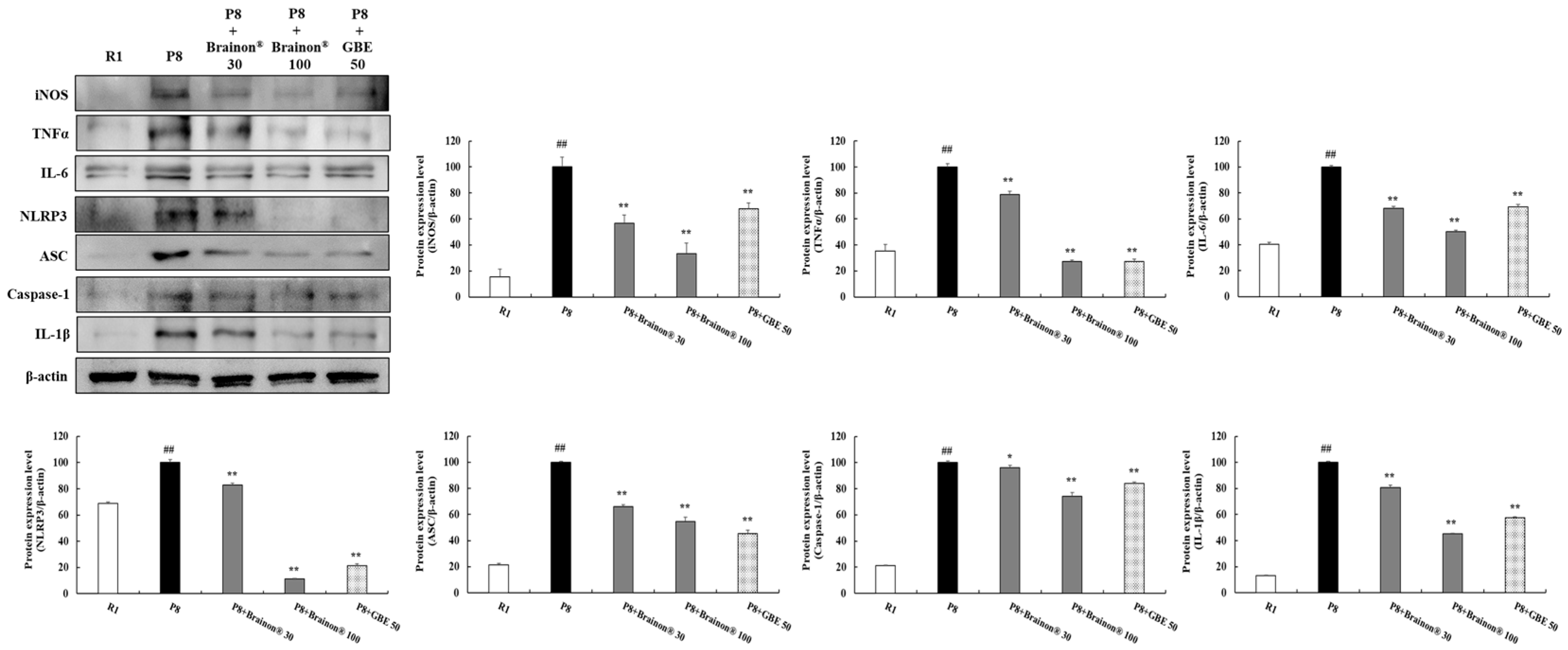
3.6. Brainon® Inhibits Expression of Inflammatory Cytokines in the Cerebral Cortex of SAMP8 Mice
3.7. Brainon® Inhibits Apoptosis-Related Proteins in the Cerebral Cortex of SAMP8 Mice
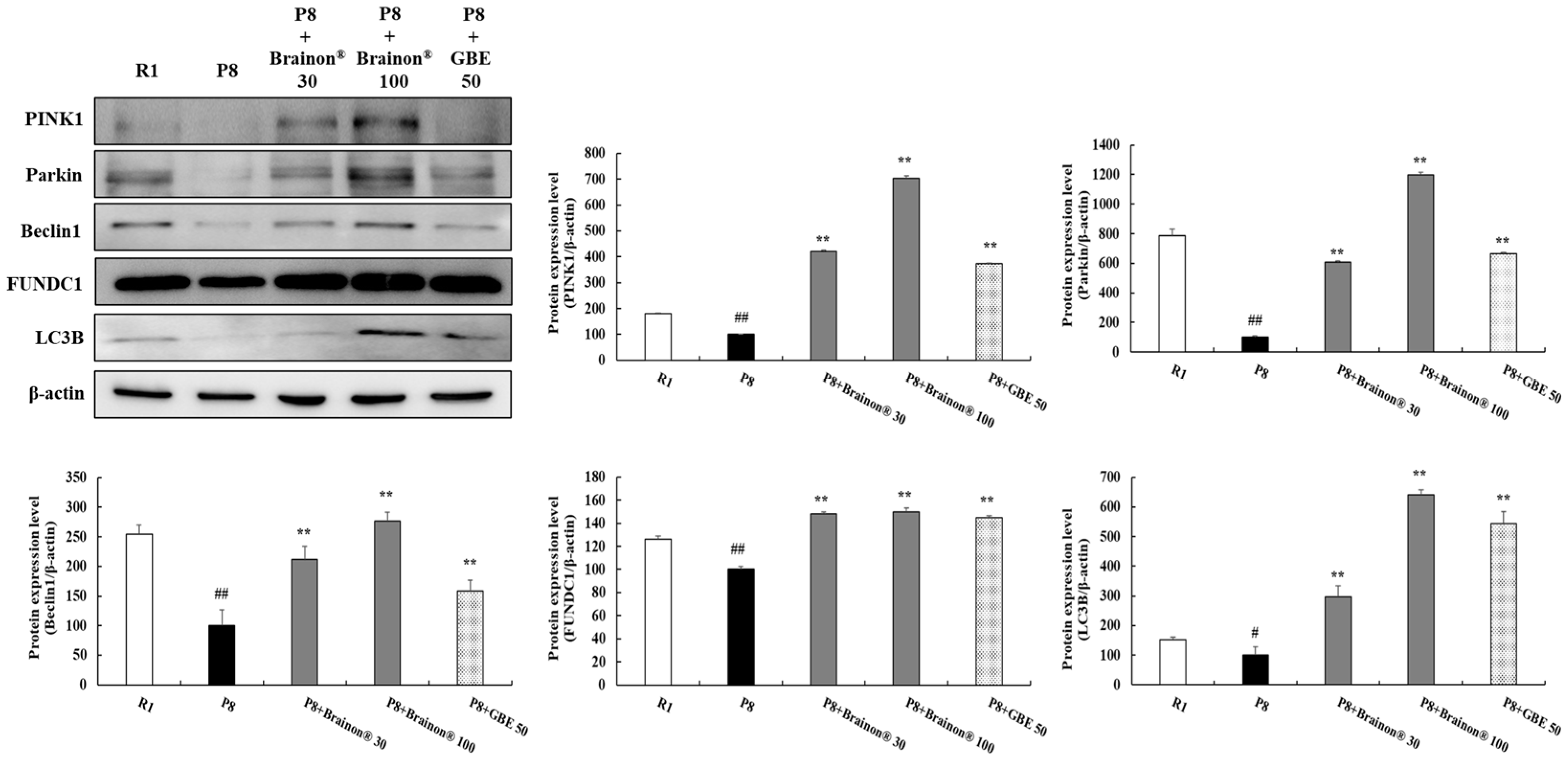
3.8. Brainon® Promotes Mitophagy/Autophagy in the Cerebral Cortex of SAMP8 Mice
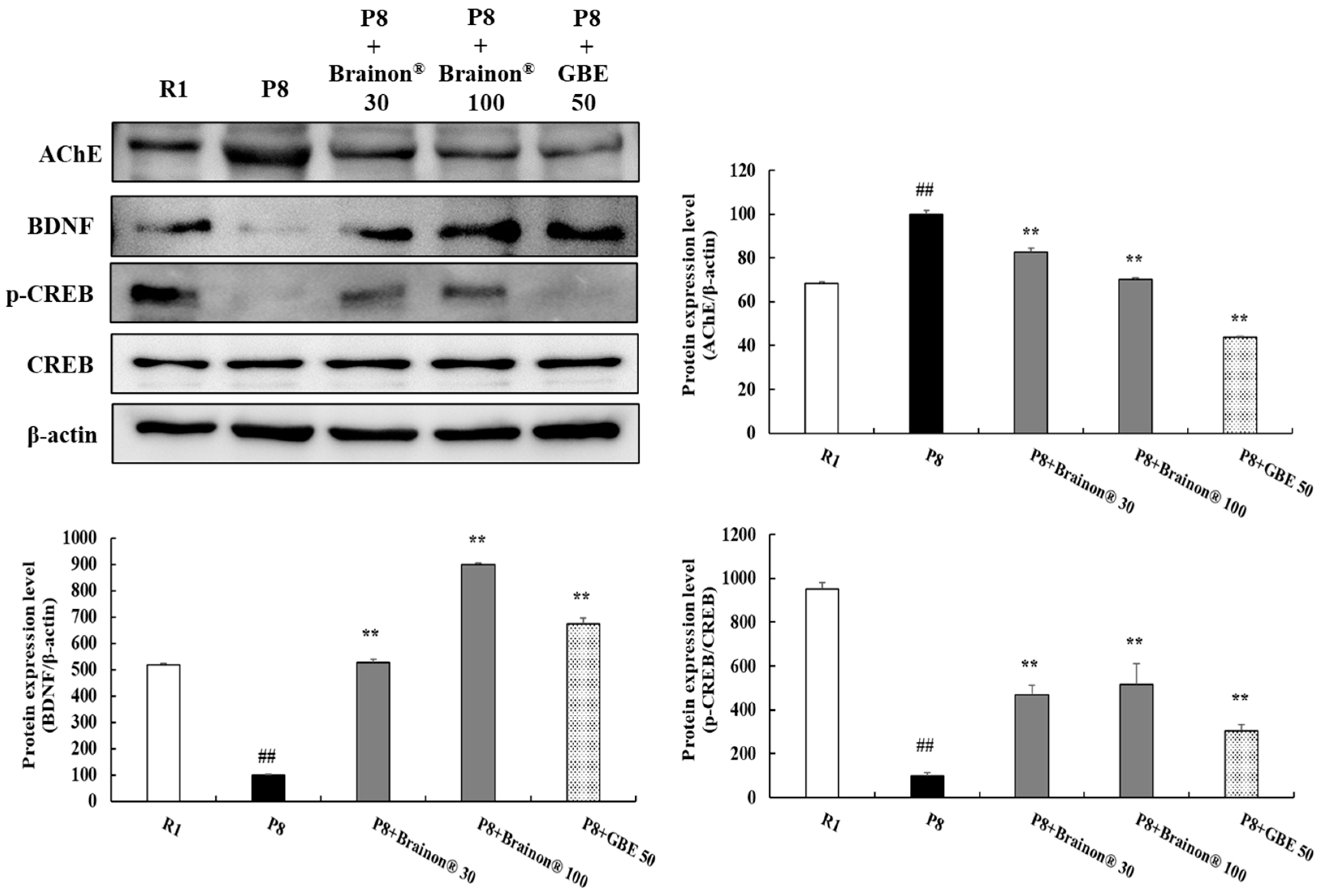
3.9. Brainon® Inhibits AChE Activity but Increases BDNF Expression and CREB Phosphorylation Levels in the Cerebral Cortex of SAMP8 Mice
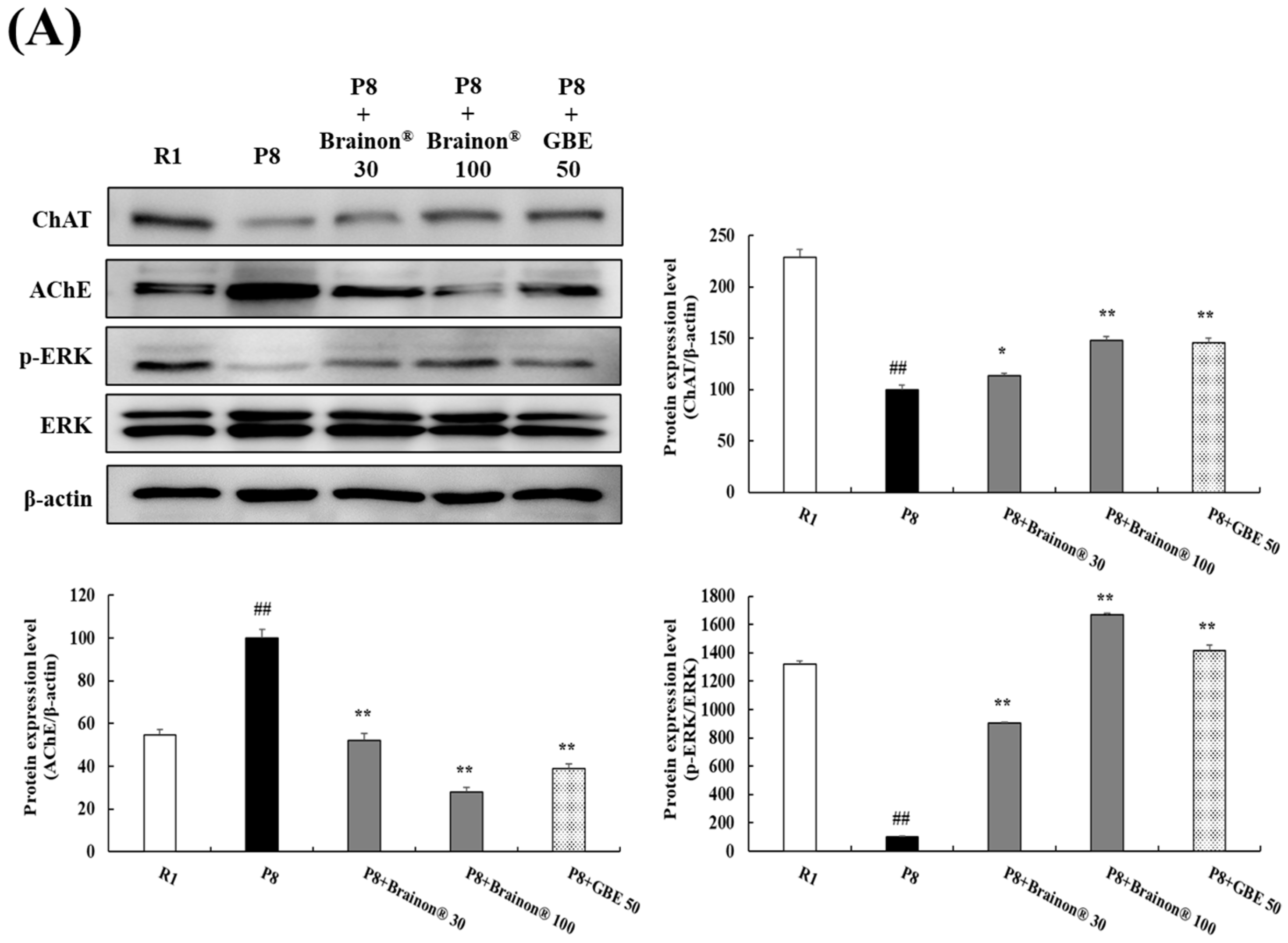
3.10. Brainon® Regulates the Expressions of AChE and ChAT and the Phosphorylation of ERK in the Hippocampus of SAMP8 Mice
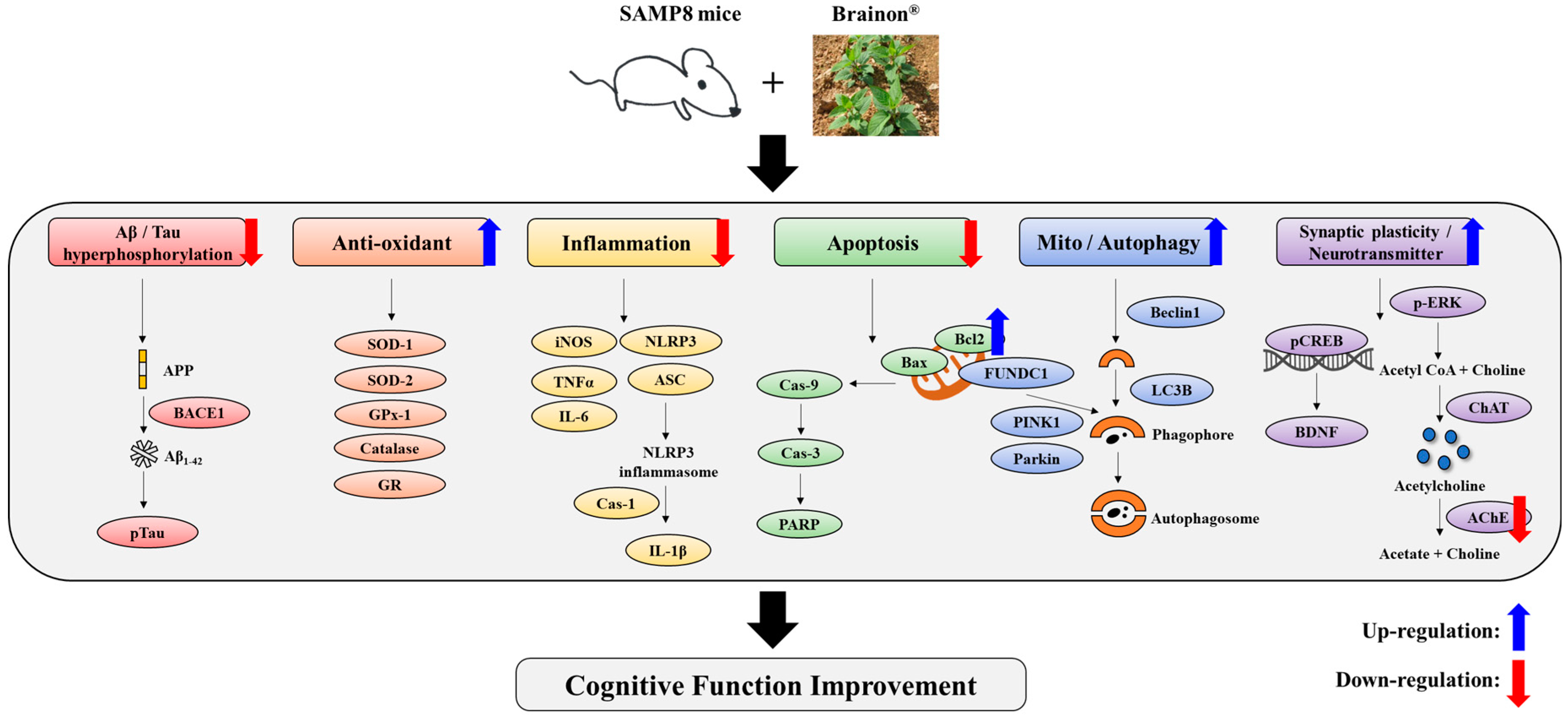
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Kanglan, L.; Shouchao, W.; Zhou, L.; Li, H.; Jiajing, L.I.N.; Shiting, T.A.N.; Yingern, M.A.I.; Wanjuan, P.E.N.G.; Hui, M.A.I.; Qi, H.O.U.; et al. The prevalence of Alzheimer’s disease in China: A systematic review and meta-analysis. Iran. J. Public Health 2018, 47, 1615–1626. [Google Scholar]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vicente, M. Neuronal mitophagy in neurodegenerative diseases. Front. Mol. Neurosci. 2017, 10, 64. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, H.; Huang, P.; Li, S.; Li, B.; Huo, L.; Zhong, J.; Pan, Z.; Li, Y.; Xia, X. Panax notoginseng saponins protect PC12 cells against Aβ induced injury via promoting parkin-mediated mitophagy. J. Ethnopharmacol. 2022, 285, 114859. [Google Scholar] [CrossRef] [PubMed]
- De Rechter, S.; Decuypere, J.P.; Ivanova, E.; van den Heuvel, L.P.; De Smedt, H.; Levtchenko, E.; Mekahli, D. Autophagy in renal diseases. Pediatr. Nephrol. 2016, 31, 737–752. [Google Scholar] [CrossRef]
- Kang, C.; Elledge, S.J. How autophagy both activates and inhibits cellular senescence. Autophagy 2016, 12, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Demirci, K.; Nazıroğlu, M.; Övey, İ.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Puri, A.; Srivastava, P.; Pandey, P.; Yadav, R.S.; Bhatt, P.C. Scopolamine induced behavioral and biochemical modifications and protective effect of Celastrus paniculatous and Angelica glauca in rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 158–169. [Google Scholar]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Manich, G.; Mercader, C.; Del Valle, J.; Duran-Vilaregut, J.; Camins, A.; Pallàs, M.; Vilaplana, J.; Pelegri, C. Characterization of amyloid-β granules in the hippocampus of SAMP8 mice. J. Alzheimer’s Dis. 2011, 25, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Akiguchi, I.; Pallàs, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathol 2017, 37, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Liu, B.; Wu, Q.; Liu, J.; Xu, Y.Y.; Zhou, S.Y.; Shi, J.S. Icariin delays brain aging in senescence-accelerated mouse prone 8 (SAMP8) model via inhibiting autophagy. J. Pharmacol. Exp. Ther. 2019, 369, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.H.; Lee, H.H.; Lim, S.S.; Park, K.W. Effect of Scrophularia buergeriana extract on the degranulation of mast cells and ear swelling induced by dinitrofluorobenzene in mice. Inflamm. 2012, 35, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Ma, C.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. KD-501, a standardized extract of Scrophularia buergeriana has both cognitive-enhancing and antioxidant activities in mice given scopolamine. J. Ethnopharmacol. 2009, 121, 98–105. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, D.Y.; Kim, H.L.; Yang, S.H. Scrophularia buergeriana extract improves memory impairment via inhibition of the apoptosis pathway in the mouse hippocampus. Appl. Sci. 2020, 10, 7987. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.L.; Lee, D.Y.; Lee, D.R.; Choi, B.K.; Yang, S.H. Scrophularia buergeriana extract (Brainon) improves scopolamine-induced neuronal impairment and cholinergic dysfunction in mice through CREB-BDNF signaling pathway. Appl. Sci. 2021, 11, 4286. [Google Scholar] [CrossRef]
- Dember, W.N.; Fowler, H. Spontaneous alternation behavior. Psychol. Bull. 1958, 55, 412–428. [Google Scholar] [CrossRef]
- Holcomb, L.; Gordon, M.N.; McGowan, E.; Yu, X.; Benkovic, S.; Jantzen, P.; Wright, K.; Saad, I.; Mueller, R.; Morgan, D.; et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998, 4, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Subbanna, S. CB1 receptor-mediated signaling underlies the hippocampal synaptic, learning, and memory deficits following treatment with JWH-081, a new component of spice/K2 preparations. Hippocampus 2014, 24, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 38, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Griñan-Ferré, C.; Palomera-Ávalos, V.; Puigoriol-Illamola, D.; Camins, A.; Porquet, D.; Plá, V.; Aguado, F.; Pallàs, M. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp. Gerontol. 2016, 80, 57–69. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, J.Q.; Zhou, Y.J.; Zhou, Y.J.; Li, Y.Y.; Huang, N.Q.; Deng, C.-M.; Luo, Y. Icariin improves cognitive deficits by reducing the deposition of β-amyloid peptide and inhibition of neurons apoptosis in SAMP8 mice. Neuroreport 2020, 31, 663–671. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Henrique, A.M.; Gianetti, N.G.; Ferrari, M.F. Parkin is downregulated among autophagy-related proteins prior to hyperphosphorylation of Tau in TS65DN mice. Biochem. Biophys. Res. Commun. 2021, 561, 59–64. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401. [Google Scholar] [CrossRef]
- Lei, L.; Yang, S.; Lu, X.; Zhang, Y.; Li, T. Research progress on the mechanism of mitochondrial autophagy in cerebral stroke. Front. Aging Neurosci. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; McClelland, J.L. Neural models of memory. Curr. Opin. Neurobiol. 1999, 9, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y. Choline acetyltransferase: The structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 1999, 49, 921–937. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in learning and memory: A review of recent research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef]
- Vasilopoulou, F.; Bellver-Sanchis, A.; Companys-Alemany, J.; Jarne-Ferrer, J.; Irisarri, A.; Palomera-Ávalos, V.; Gonzalez-Castillo, C.; Ortuño-Sahagún, D.; Sanfeliu, C.; Pallàs, M.; et al. Cognitive Decline and BPSD Are Concomitant with Autophagic and Synaptic Deficits Associated with G9a Alterations in Aged SAMP8 Mice. Cells 2022, 11, 2603. [Google Scholar] [CrossRef]
- Wood, H. Localized proteomics distinguishes AD subtypes. Nat. Rev. Neurol. 2017, 13, 260. [Google Scholar] [CrossRef]
- Sheng, C.; Xu, P.; Zhou, K.; Deng, D.; Zhang, C.; Wang, Z. Icariin attenuates synaptic and cognitive deficits in an Aβ1–42-induced rat model of Alzheimer’s disease. Biomed Res. Int. 2017, 2017, 7464872. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef]
- Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res. Rev. 2014, 13, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Busciglio, J.; Lorenzo, A.; Yeh, J.; Yankner, B.A. β-Amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 1995, 14, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Kowall, N.W.; Ferrante, R.J. Neurotoxicity and oxidative damage of beta amyloid 1-42 versus beta amyloid 1-40 in the mouse cerebral cortex. Ann. N. Y. Acad. Sci. 1999, 893, 314–320. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, W.Y.; Park, S.J.; Kim, J.M.; Lee, S.; Kim, Y.C.; Ryu, J.H. Anti-amnesic effect of ESP-102 on Aβ1–42-induced memory impairment in mice. Pharmacol. Biochem. Behav. 2010, 97, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Nomura, Y.; Wang, B.X.; Qi, S.B.; Namba, T.; Kaneko, S. Biochemical changes related to aging in the senescence-accelerated mouse. Exp. Gerontol. 1989, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Kurokawa, T.; Oda, N.; Ishibashi, S. Early appearance of abnormality of microperoxisomal enzymes in the cerebral cortex of senescence-accelerated mouse. Mech. Ageing Dev. 1996, 92, 175–184. [Google Scholar] [CrossRef]
- Kurokawa, T.; Asada, S.; Nishitani, S.; Hazeki, O. Age-related changes in manganese superoxide dismutase activity in the cerebral cortex of senescence-accelerated prone and resistant mouse. Neurosci. Lett. 2001, 298, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Oliver, J.; Roca, P. Role of uncoupling proteins in cancer. Cancers 2010, 2, 567–591. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984, 222, 649–655. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.I.; Escames, G.; López, L.C.; López, A.; García, J.A.; Ortiz, F.; Acuña-Castroviejo, D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007, 42, 272–279. [Google Scholar] [CrossRef]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017, 95, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S. ASC, Ipaf and Cryopyrin/Nalp3: Bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect. 2007, 9, 664–671. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Denes, A.; Lopez-Castejon, G.; Brough, D. Caspase-1: Is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012, 3, e338. [Google Scholar] [CrossRef]
- Gemma, C.; Bickford, P.C. Interleukin-1ß and Caspase-1: Players in the regulation of age-related cognitive dysfunction. Rev. Neurosci. 2007, 18, 137–148. [Google Scholar] [CrossRef]
- Zhu, W.L.; Zheng, J.Y.; Cai, W.W.; Dai, Z.; Li, B.Y.; Xu, T.T.; Liu, H.F.; Liu, X.Q.; Wei, S.F.; Luo, Y.; et al. Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Aging 2020, 12, 3175. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, H.; Wang, L.; Yi, H.; Li, Z.; Liu, R. Vitegnoside mitigates neuronal injury, mitochondrial apoptosis, and inflammation in an Alzheimer’s disease cell model via the p38 MAPK/JNK pathway. J. Alzheimer’s Dis. 2019, 72, 199–214. [Google Scholar] [CrossRef]
- Chien, C.Y.; Chien, C.T.; Wang, S.S. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J. Thorac. Cardiovasc. Surg. 2014, 148, 705–713. [Google Scholar] [CrossRef]
- Lian, W.; Fang, J.; Xu, L.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.L.; Du, G.H. DL0410 ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in mice. Molecules 2017, 22, 410. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Bramham, C.R.; Duarte, C.B. BDNF and hippocampal synaptic plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar]
- Kuczewski, N.; Porcher, C.; Lessmann, V.; Medina, I.; Gaiarsa, J.L. Activity-dependent dendritic release of BDNF and biological consequences. Mol. Neurobiol. 2009, 39, 37–49. [Google Scholar] [CrossRef]
- Fuster, J.M. Network memory. Trends Neurosci. 1997, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.L.; Lee, S.K.; Min, D.E.; Bastola, T.; Chang, B.Y.; Bae, J.H.; Lee, D.R. Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice. Curr. Issues Mol. Biol. 2023, 45, 1287-1305. https://doi.org/10.3390/cimb45020084
Kim HL, Lee SK, Min DE, Bastola T, Chang BY, Bae JH, Lee DR. Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice. Current Issues in Molecular Biology. 2023; 45(2):1287-1305. https://doi.org/10.3390/cimb45020084
Chicago/Turabian StyleKim, Hae Lim, Sung Kwon Lee, Da Eun Min, Tonking Bastola, Bo Yoon Chang, Jin Hye Bae, and Dong Ryung Lee. 2023. "Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice" Current Issues in Molecular Biology 45, no. 2: 1287-1305. https://doi.org/10.3390/cimb45020084
APA StyleKim, H. L., Lee, S. K., Min, D. E., Bastola, T., Chang, B. Y., Bae, J. H., & Lee, D. R. (2023). Effects of Scrophularia buergeriana Extract (Brainon®) on Aging-Induced Memory Impairment in SAMP8 Mice. Current Issues in Molecular Biology, 45(2), 1287-1305. https://doi.org/10.3390/cimb45020084






