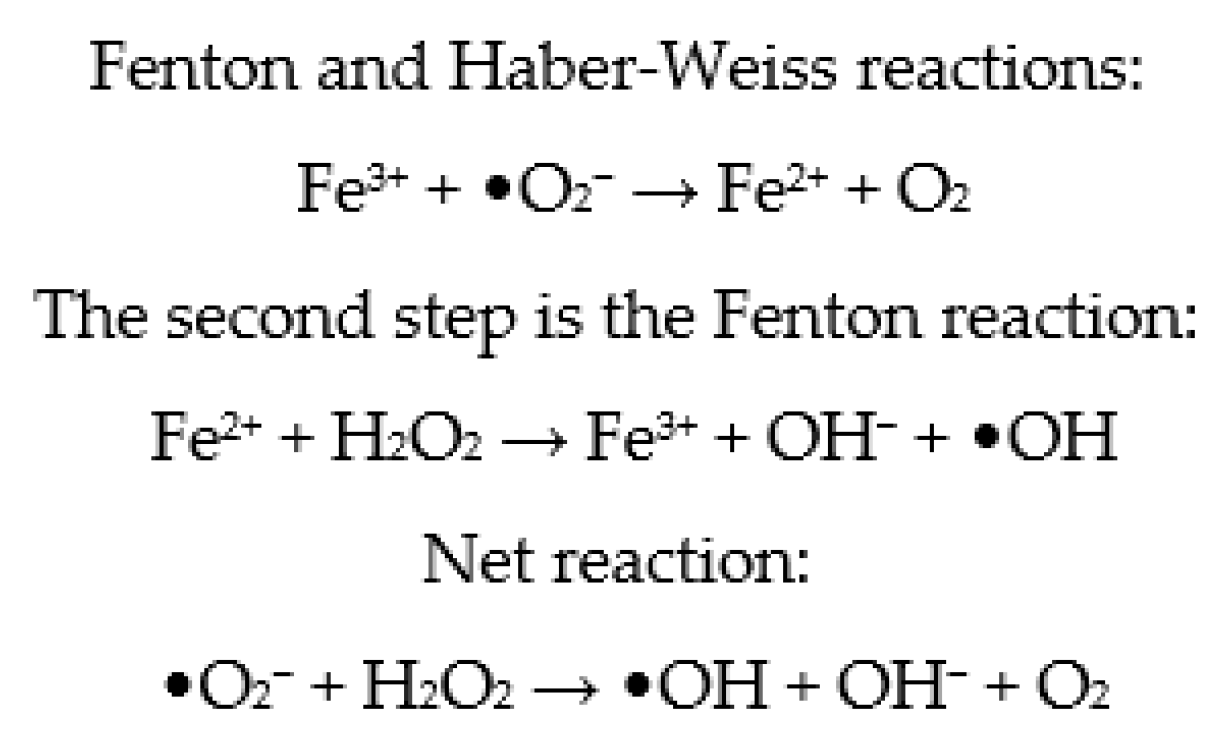Abstract
Based on current findings, the presence of oxidative stress has a significant impact on the quality of gametes and embryos when performing assisted reproductive techniques (ART). Unfortunately, in vitro manipulation of these cells exposes them to a higher level of reactive oxygen species (ROS). The primary goal of this review is to provide a comprehensive overview of the development of oxidative stress in female and male reproductive systems, as well as in the case of the pre-implantation embryo and its environment. This review also focuses on the origins of ROS and the mechanisms of oxidative stress-induced damage during ART procedures. A well-known but underestimated hazard, light exposure-related photo-oxidation, is particularly concerning. The effect of oxidative stress on ART outcomes, as well as the various strategies for preventing it, are also discussed. We emphasize the role and significance of antioxidants and light protection including forms, functions, and mechanisms in the development of gametes and embryos in vivo and in vitro.
1. Introduction
In the past few decades, the study of the role of oxidative stress (OS) in reproductive health has become more and more popular. Oxygen is a key element of aerobic life, and oxidative metabolism represents an essential supply of energy. All multicellular aerobic organisms require molecular oxygen for their survival. The electron configuration of oxygen is special, as it has two unpaired electrons in different orbits in its outer shell, which makes it prone to forming radicals. The reduction of molecular oxygen (O2) yields superoxide (•O2−), which is the precursor of most other reactive oxygen species (ROS) [1,2]. ROS originate from the mitochondria along with other superoxides, and have a complex role in numerous cell signaling pathways that control cell proliferation rates and other cellular activities, such as molecular responses to hypoxia [3,4,5].
Moreover, ROS have a significant effect on the oxidative modification of many macromolecules such as proteins, receptors, ion channels, or transcription factors [6,7]. Consequently, a small amount of ROS are essential for the natural cell functions [8]. There are two types of oxidants that can produce free radicals: endogenous and exogenous oxidants. ROS are highly reactive and thus unstable, but they can be stabilized by acquiring electrons from nearby molecules (e.g., lipids, proteins, nucleic acids), resulting in cell damage and pathology [9,10,11]. Therefore, OS can cause lipid peroxidation and DNA and protein damage. In a healthy environment, every aerobic cell has a defense system against ROS, there is a precisely adjusted balance (homeostasis) between prooxidants and antioxidants (AOX). Superoxide anions (O2−), hydroxyl radicals (OH−), peroxyls (ROO), alkoxyls (RO), and hydroperoxyls (HO2) have the biggest biological importance among ROS. Enzymatic and non-enzymatic antioxidants are the two types of antioxidants that can be found in the body under normal conditions. The most prominent enzymatic antioxidants are catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GSH-R), and superoxide dismutase (SOD), which can cause the reduction of hydrogen-peroxide (H2O2) to alcohol and water. For example, the non-enzymatic antioxidants are vitamins such as vitamin A, C, E, plant polyphenols, carotenoids, and glutathione or zinc [12]. These antioxidants are also treated as dietary supplements and synthetic antioxidants. By attaching to these harmful molecules, antioxidants reduce the effects of oxidants. Antioxidants, on the other hand, are beneficial at low concentrations and can function as oxidants at higher amounts. [13]. Therefore, the role of OS in male and female fertility is of particular importance. Therefore, in this report it will be briefly outlined.
2. Effect of Oxidative Stress on the Reproductive Tract of Males
2.1. Sources of ROS in Sperm
Spermatozoa obtain energy from two major metabolic pathways: glycolysis, which occurs in the main part of the flagellum, and oxidative phosphorylation, which occurs in mitochondria located in the flagellum’s midpiece [14]. There is no evidence that the process of the tricarboxylic acid cycle plays a role in adenosine triphosphate (ATP) production. By obtaining approximately 30 molecules of ATP by oxidizing one molecule of glucose, oxidative phosphorylation is the more efficient pathway. During glycolysis only two molecules of ATP are gained from each molecule of glucose.
2.2. Physiological Role of ROS in Sperm
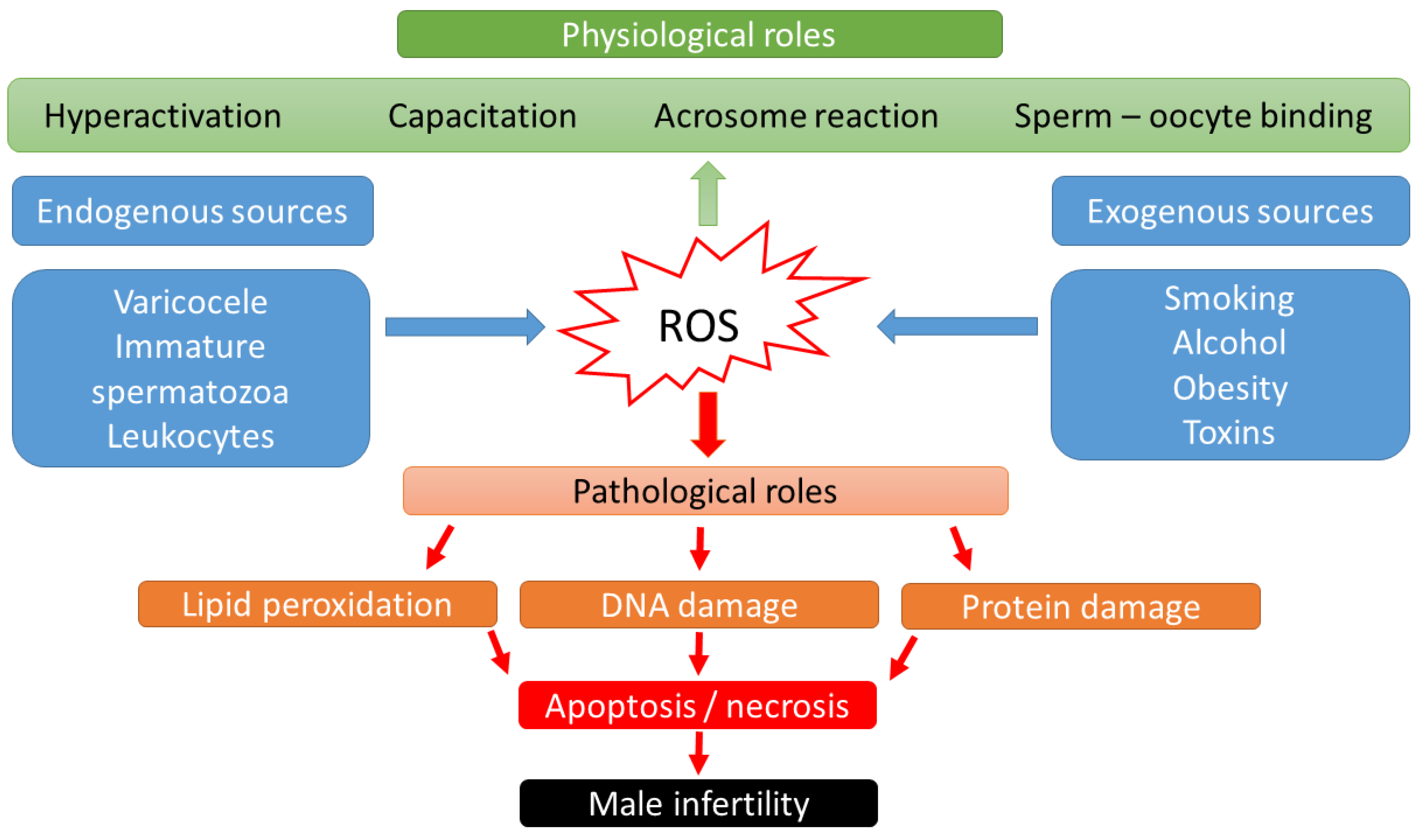
Over the last two decades, it has become known that ROS may have a dual role in sperm function [15]: low ROS levels promote numerous intracellular processes leading to oocyte fertilization, whereas higher ROS levels may lead to DNA damage and embryo loss [16,17] (Figure 1). Physiological levels of ROS have an impact on diverse signaling pathways that regulate physiological redox-sensitive activities, since ROS generally mediates cell proliferation, apoptotic pathways that regulate the cell cycle and programmed cell death [18]. In order to fertilize the oocyte, spermatozoa must undergo various processes in the epididymis, such as sperm maturation, and in the female reproductive tract after ejaculation, such as hyperactivation, capacitation, and the acrosome reaction. During sperm maturation, ROS levels in seminal fluid have been shown to be critical for membrane protein rearrangements, enzymatic modulations, and nuclear remodeling [19]. In the case of nuclear remodeling, in addition to the inevitable replacement of histone proteins with smaller protamines [20], ROS also play a non-negligible role in stabilizing disulfide bonds to maintain chromatin stability [19]. During the ROS-mediated process of hyperactivation, the motility pattern of sperm changes significantly [21]. The hyperactivated sperm is characterized by a high-amplitude, asymmetric beating pattern of the sperm tail (flagellum). The biochemical background was recently described by Dutta et al., 2020 [22]: Calcium ions (Ca2+) and ROS (superoxide, O2−) mediate activation of adenylate cyclase (AC) and increased production of intracellular cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) [23]. Increased levels of PKA lead to activation of protein tyrosine kinase (PTK), resulting in phosphorylation of serine (Ser) and tyrosine (Tyr) residues. These steps lead to the essential changes in the cytoskeleton of the flagellum and the fibrous sheath of the axoneme. In parallel with hyperactivation, the increased level of phosphorylated tyrosine residues (P-Tyr) also leads to the process of capacitation, when the sperm cell prepares for the acrosome reaction. The biochemical features of the acrosome reaction overlap with those of capacitation. Both processes involve the influx of Ca2+ and increased levels of cAMP, PKA, and PKC. However, molecules such as phospholipase A2 (PLA2) are also involved in the acrosome reaction. PLA2 is activated in spermatozoa by progesterone secreted from the cumulus cell and cleaves intact phosphoglycerolipids into free fatty acids and lysophospholipids, increasing the fluidity of the sperm plasma membrane in preparation for sperm–oocyte fusion [24]. Then, the capacitated sperm binds to a glycoprotein of the zona pellucida, the process of which leads to oocyte penetration, and sperm head decondensation [24,25]. ROS have a low-level role as a second messenger in these fertilization processes.
Figure 1.
Scheme of physiological and pathological effects of reactive oxygen species (ROS) on male fertility.
2.3. Pathological Role of ROS in Sperm
High levels of ROS biological markers were identified in semen samples from 25–40% of infertile men [26]. Thus, male sub- and infertility are frequently related to OS. The source of the OS could be categorized into endogenous and exogenous factors. Lifestyle habits, such as alcohol intake, smoking, contact with toxic materials (radiation or environmental pollutants), or pathological abnormalities such as obesity, varicocele, stress, and aging have been connected with elevated production of adipokines, cytokines, and high levels of ROS in seminal plasma [27]. Supraphysiologic ROS levels have been correlated with the presence of leukocytes in seminal fluid, as well as a high percentage of morphologically abnormal spermatozoa [28] or immature spermatozoa with cytoplasmatic droplets containing a high number of enzymes [24,29]. In cases of leukocytospermia (no. of leukocytes ≥ 1 × 106/mL), an increase in extracellular ROS generation is particularly evident, as the antioxidant protection of the seminal plasma becomes insufficient. Activated leukocytes can produce 100 times more ROS than non-activated leukocytes during inflammation or infection [30].
In the context of ART, gametes are exposed to in vitro modification, which regularly exposes these cells to OS [31]. However, leukocytes can be removed from sperm suspensions using procedures such as density gradient centrifugation (DGC) or swim-up; however, using these techniques without serum albumin has been related to sperm damage in several studies. In the absence of albumin, free radicals created by mitochondria during centrifugation trigger membrane lipid peroxidation and DNA damage [32,33]. This damage could be caused by peroxide produced by manganese superoxide dismutase (MnSOD) from superoxide radicals in tightly packed sperm pellets. It is released from damaged mitochondria and causes lipid peroxidation of the cell membrane, depolarization of mitochondria, decreased ATP synthesis, and sperm motility [34,35]. Advanced selection approaches such as microelectrophoresis, Zeta potential, and microfluidic technologies could be used to reduce the induction of OS and the resulting increase in DNA damage. However, such technologies are still used infrequently in clinics [36].
2.4. Effects of OS on Sperm Functions
Lipid peroxidation of the sperm membrane is the major mechanism of ROS-induced sperm destruction, which leads to infertility. Because their cell membrane and cytoplasm contain large quantities of polyunsaturated fatty acids, spermatozoa are sensitive to ROS [37]. This lipid peroxidation reduces sperm motility, likely due to a rapid loss of intracellular ATP, leading to a reduction in axonemal protein phosphorylation, which might result in decrease in motility and, subsequently, sperm immobility [38].
Furthermore, ROS may decrease sperm viability and enhance morphological defects in the mid-piece [39]. In case of DNA damage, the production of basis-free sites, deletions, frameshifts, DNA cross-links, chromosomal rearrangements, and DNA strand breaks could occur [40,41,42]. One study revealed that a 25% increase in ROS level in seminal plasma led to a 10% increase in DNA fragmentation [43]. These changes can cause the start or stop of gene transcription, accelerated degradation of telomeric DNA, epigenetic changes, replication mistakes, and GC-to-TA transversions [44]. In the case of the spermatozoa, only one base excision repair (BER) enzyme has been described, which is the 8-oxoguanine DNA glycosylase 1 (OGG1). Therefore, the DNA repair potential of spermatozoa is strongly limited, and much more exposed to ROS than other gametes [45]. However, cells normally rely on a variety of intrinsic and extrinsic antioxidant systems to neutralize high amounts of ROS. Enzymatic antioxidants such as SOD, catalase, and thiol peroxidases, as well as nonenzymatic antioxidants such as glutathione, are forms of endogenous antioxidants. Extrinsic antioxidants, on the other hand, are micronutrients such as vitamin C, vitamin E, L-carnitine, N-acetyl cysteine, and trace elements such as selenium or zinc [2] that must be provided from external sources in order to maintain a balance between oxidation and reduction (antioxidation) in any living cell of the body [1].
2.5. Methods Used to Counteract OS Effects
Although there are some contradictory reports that oral consumption of antioxidant-rich medication seems to improve sperm functional parameters such as motility and concentration, as well as decrease DNA damage, there is insufficient evidence that antioxidant consumption has a significant effect on the improving of fertility rates and live birth rates [46]. Furthermore, it is dependent on the type of antioxidants, the duration of treatment, and even the diagnosis of the man’s fertility, among further aspects [46]. A recent study discovered a significant effect of three-month lifestyle changes combined with oral antioxidant intake on DNA fragmentation index (DFI), but no effect on sperm concentration or total motile sperm count [47]. ‘Reductive stress’ refers to a change in the redox levels of the body to a more reduced state. According to reports, reductive stress is just as harmful as oxidative stress [48].
3. Effect of Oxidative Stress on the Reproductive Tract of Females
3.1. Physiological Roles of OS in Females Reproductive Tract
OS is the result of enormous ROS contributing to oocyte aging and several disorders affecting female reproduction. OS is considered to have cytotoxic effects by initiating the peroxidation of membrane phospholipids and altering nucleic acids, lipids, and proteins. These processes result in changes in the cellular physiology, including apoptosis, increased membrane permeability, even the total loss of membrane integrity, decreased enzyme activity, structural DNA damage, mitochondrial alterations, and ATP depletion [38,49,50]. The evolved free radicals may alter the oocyte, sperm, and embryos in their follicular and tubal fluids and peritoneal fluid microenvironments, and through these changes influence reproductive outcomes [51,52]. The imbalance of the redox system affects the female reproductive organs and results in oxidative stress, which impacts the function of the ovaries, the salpinx, the placenta, and the uterus. ROS may act as important mediators in hormone signaling, oocyte maturation, ovarian steroidogenesis, ovulation, luteolysis, luteal maintenance in pregnancy, implantation, compaction, blastocyst development, germ cell function, and corpus luteum formation [38].
3.2. Pathological Roles of OS in Female Reproductive Tract
It is also known that OS causes lipid damage and inhibits protein synthesis and TP depletion. These processes were described in the background of common obstetrical situations, e.g., the preterm premature rupture of the membranes, since oxidant stress caused by elevated ROS levels and simultaneous antioxidant depletion may damage collagen, resulting in premature membrane rupture [53]. Hypoxia causes altered placental function, leading to preeclampsia and fetal growth restriction. Particularly, in late gestation, elevated oxidative stress was detected in pregnancies complicated by diabetes, intrauterine growth restriction, and preeclampsia in association with increased trophoblast apoptosis and deportation and impaired placental vascular reactivity. OS was detected by increased lipid peroxides and isoprostanes and declined antioxidant expression and activity [54].
Normal endometrium has decreased SOD activity and increased ROS levels in the late secretory phase. An OS-induced autoantibody titer increase was detected in the peritoneal fluid of patients diagnosed with endometriosis. Elevated lysophosphatidyl choline—a known chemotactic factor of T lymphocytes—was observed in the same peritoneal fluid samples [55]. Expression of SOD, Mn and Cu-Zn dismutases, lipid peroxides, or glutathione peroxidase were detected in normal ovarian cycling [9,56]. Ovarian steroidogenesis is presumably associated with OS, while their expression is correlated with Ad4-binding protein—which serves as a general regulator of steroidogenic P450 genes—and superoxide dismutase expression [56]. In cell cultures, hydrogen peroxide resulted in reduced progesterone and estradiol hormones [57]. Suzuki et al. [56] also hypothesize that luteal Cu-Zn SOD has a supportive function in pregnancy.
OS influences the placenta, as Watson and coworkers [58] found syncytiotrophoblast damage in elevated oxygen levels resulted in microvilli decrease on their surface and a decrease in mitochondria. In another work, the same investigators proved that syncytiotrophoblast cells express antioxidants in early pregnancy [58,59].
Oxidative stress is an undeniable factor in the pathophysiology of other obstetrical diseases including polycystic ovarian disease, different fetal embryopathies, or intrauterine growth retardation, which have been associated with increasing OS. One factor in the background of these disorders is the activation of redox-sensitive transcription factors, such as p53 or NF-κB through the activation of different proinflammatory cytokines, such as interleukin-6 (IL-6), IL-18, or tumor necrosis factor (TNF-alfa), described in polycystic ovary syndrome (PCOS) [60]. Another process is protein oxidation, e.g., PCOS patients had higher plasma-advanced oxidation protein products in serum samples compared to control women [61]. The opening of ion channels has been described in the background since the increased ROS presence leads to Ca2+ ion release from the endoplasmic reticulum and other stores and this dysregulation leads to follicular arrest and reproductive or menstrual dysfunction [62]. Systemic endovascular inflammation detected in preeclampsia is caused by the dysfunction of maternal endothelial cells, leading to proteinuria and hypertension [63]. Oxidative stress participates in the development of intrauterine growth restriction (IUGR) through elevated levels of malondialdehyde, xanthine oxidase in maternal plasma, umbilical cord plasma, and placental tissue compared to the control group. An increased superoxide dismutase activity in maternal plasma and cord blood samples and an elevated glutathione peroxidase activity in maternal plasma and placental tissue were measured, while catalase activity was decreased in cord blood and placental tissue samples in IUGR groups [64].
An increased level of OS has been reported in women of advanced age undergoing in vitro fertilization (IVF) treatment [65]. An increased presence of OS in follicular fluid leads to ovarian senescence. Oocyte maturation is an essential process during IVF and intracytoplasmic sperm injection (ICSI). OS is altered in various reproductive processes such as oocyte maturation and folliculogenesis and is detrimental to natural and assisted fertility. Ovulation is essential for reproduction and is initiated by the luteinizing hormone surge; however, the overabundance of inflammatory precursors following LH surge generates ROS [66]. A previous study showed higher total antioxidant capacity in infertile women aged 30–39 years compared to pregnant women of the same age [67]. The erroneous oocyte mitochondria are responsible for the increased in vivo ROS levels. Age-related processes seem to be associated with ROS aggregation and mitochondrial dysfunction [68]. The repeated ovulation may accumulate, resulting in inflammatory changes in the ovary and promoting oxidative damage [66,69]. Presumably, the elevated presence of ROS disrupts the prooxidant–antioxidant balance in peritoneal fluid and leads to infertility in women, causing damaged or degenerated cytoskeleton fibers. Higher ROS levels directly affect the ovum after its release from the ovary, the development of zygote/embryo, or damage the spermatozoa [70]. Previous investigations comparing the presence of ROS in peritoneal fluid samples of women undergoing laparoscopy under infertility assessment and fertile women operated with tubal ligation showed elevated ROS levels in infertile patients [50]. In the same study, investigators found that infertile patients had significantly reduced levels of antioxidants such as vitamin E and glutathione. Reduced ability to eliminate ROS to neutralize toxic effects causes uncompensated balance [50] and the proposal to use antioxidant treatment in clinical practice. Free radical-induced damage may be partly involved in the age-related fall of follicle reserves [71]. IVF patients in advanced reproductive age may show reduced expression of genes responsible for the dissolution of ROS [65], such as a decline in the SOD1 or SOD2, catalyzing mRNA composition, confirming that reproductive aging may downregulate the protective gene expression of granulosa cells [72]. High follicular fluid ROS levels are associated with negative IVF outcomes, particularly in smokers [38]. There is a growing interest in the examination of OS in the female reproductive system, since it may be a crucial point in investigating the reason for infertility.
Not only are the maternal functions affected by OS and AOX systems. The sensitive fetus is constantly responsive to the maternal milieu, and previous works prove that OS leads to several pregnancy-associated disorders affecting fetal intrauterine development. Moreover, the placenta does not prevent the infiltration of harmful factors and substances from the maternal circulation to the fetus. Previous investigations have also described that environmental toxins affecting the mother can be shifted directly to the fetus during pregnancy [73], leading to the activation, and, in this way, the potential programming of the AOX defense system. It is necessary to resolve whether the epigenetic modification of the AOX system is possible. It is known that a high-fat diet induces epigenetic changes in the fetal epigenome and alters AOX genes, such as hepatic Pon1 gene, a known antioxidant. It is possible that the AOX system can be epigenetically programmed in utero, since investigators observed that the liver of fetuses whose mothers followed an HF diet during their pregnancy did not later develop obesity [74]. Although the supplement and vitamin market has developed exponentially worldwide [75], clinical trials did not clearly prove their beneficial role during fertility treatments. A previous review summarized and analyzed the results of 63 trials focusing on the effects of different antioxidants (L-arginine, vitamin E, myo-inositol, D-chiro-inositol, carnitine, selenium, vitamin B complex, vitamin C, vitamin D + calcium, CoQ10, and omega-3 polyunsaturated fatty acids) with the participation of 7760 women. The authors concluded that trials provide limited evidence about the beneficial and protective effects of antioxidant use [76]. An association was found between antioxidant use and in the development of clinical pregnancy rates among women with PCOS [77]. Vitamin D supplementation was beneficial in menstrual dysfunction [78]. Application of micronutrients positively influenced the pregnancy rate and live birth in case of IVF pregnancies [76,79].
4. Oxidative Stress in Pre-Embryos and Their Surroundings
Energy production, including ATP molecules, starts in parallel with embryonic development. During normal aerobic metabolism-related embryonic development, three free radicals are known to be present: hydrogen peroxide (H2O2), superoxide anion (O2−), and hydroxyl radical (OH−) [80]. The effect of free radicals on embryonic development could be considered complex, as these molecules have a diverse impact, such as deterioration of cell promotion, depending on the number of free radicals, starting from the stage of development (fertilization, cleavage state, compaction, blastulation) and the environment (in vivo or in vitro). ROS are produced by spermatozoa and leukocytes during fertilization, as well as during processes such as sperm-induced oocyte activation and the activation of the embryonic genome [81]. OS can also arise all through in vitro embryo production (IVP), starting from in vitro maturation to the progress of embryo development, as the protective antioxidant mechanisms that function in vivo are absent in vitro [82]. As Argawal et al. [31] reviewed, the metabolic processes of the embryos are causing an increase in the amount of highly toxic ammonia, which may cause damages in the cells through ROS overproduction.
4.1. Sources of ROS during ART
In conventional IVF, the potential cellular origin of ROS differs from those of cells fertilized by ICSI [83]. The 4–5 oocytes in each dish, the few thousand cumulus cells, and the approximately 150–200 × 106 spermatozoa used for insemination in conventional IVF can all produce ROS during co-incubation. Diseases such as PCOS [84] or endometriosis [85] are characterized by elevated levels of ROS in the oocyte environment. In these cases, it is suggested to perform ICSI because cumulus cells are no longer a possible source of ROS since the incubation starts after the oocytes have been depleted of all cumulus cells. Spermatozoa and their injection into the oocytes are two potential biological sources of ROS in the ICSI setup. However, if ICSI is performed in a male indication with a healthy female partner, it is conceivable that the absence of cumulus cells may have the opposite effect, i.e., a reduction in the oocyte’s resistance to ROS [86]. In most IVF labs, rather extended insemination periods (14–16 h) are the standard practice. Although prolonged exposure time of oocytes to spermatozoa might cause oxidative damage [87], several groups have looked into shortening the exposure time. The outcomes have been inconsistent. Several researchers stated that short co-incubation of gametes in IVF (usually 2–4 h) had positive results [88,89], whereas others claimed the contrary [90].
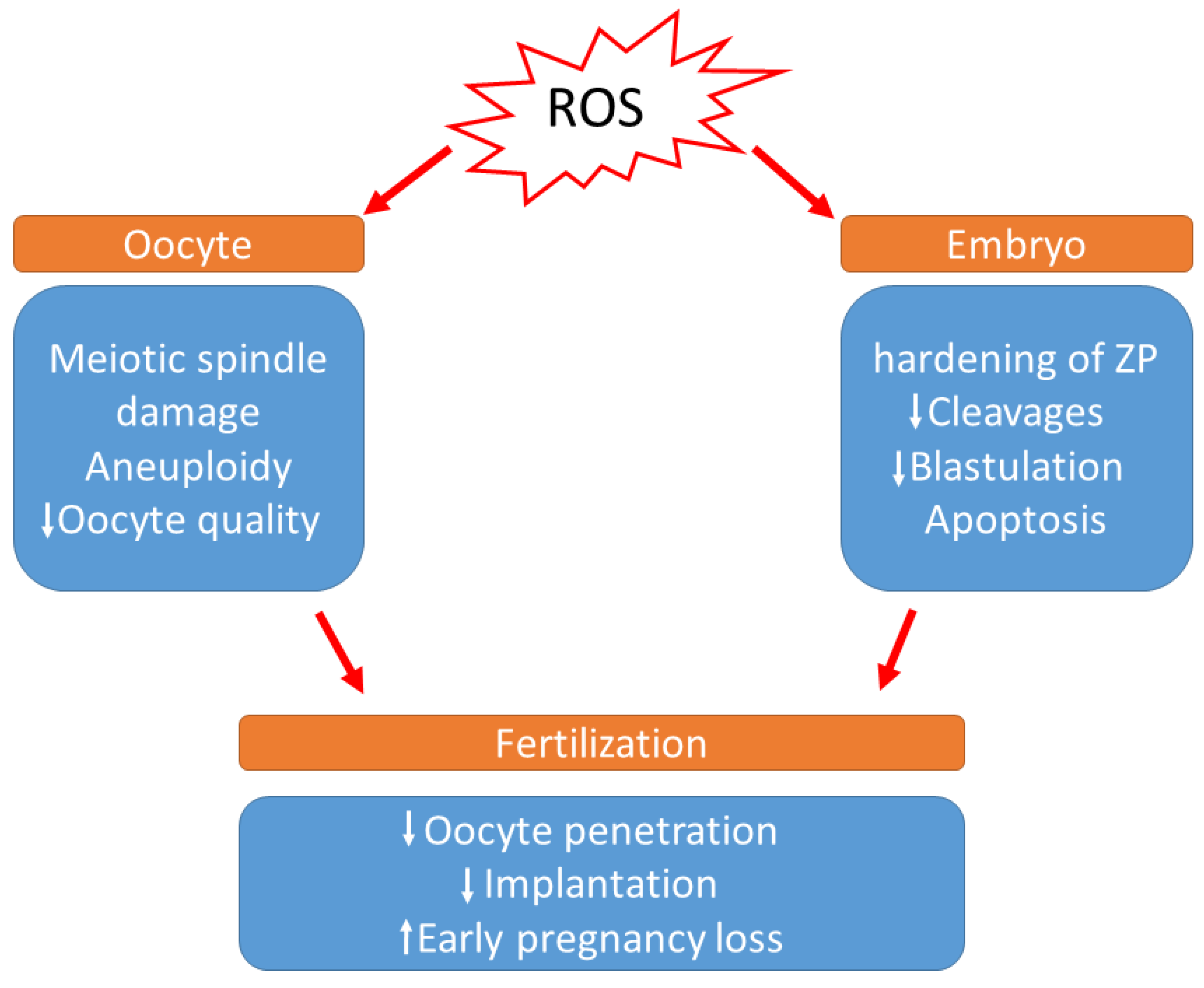
During in vitro development, the absence of non-enzymatic antioxidants in the environment surrounding the oocytes, the difference in O2 concentration between in vivo and in vitro conditions, visible light, and culture media additives can also contribute to ROS generation. According to a previous study, preimplantation embryos are especially vulnerable to conditions that trigger OS [91]. It has been demonstrated that a direct association occurs between increased ROS concentration and programmed cell death (apoptosis), resulting in the degree of embryo fragmentation or the poor rate of blastocyst development [92,93] (Figure 2). Cell necrosis causes swelling and rupture of the cell membrane, whereas fragmentation causes the cell to condense and divide into numerous fragments, resulting in cytoplasmic condensation and condensed nuclei, which are referred to as apoptotic bodies [93]. Moreover, increased ROS levels in the embryo cause mitochondrial changes, cell blockage, ATP depletion, and apoptosis. Mitochondrial DNA is more vulnerable to mutation due to a lack of histones, which also serve to reduce ROS. Defective mitochondrial DNA in embryos can cause metabolic malfunction and, as a result, disrupt in embryo development. These changes may have a variety of effects, including embryo development retardation and arrest, metabolic dysfunction, and possibly apoptosis [71]. According to Várnagy et al. [94], the level of 8-hydroxy-2′-deoxyguanosine (8-OHdG) in the follicular fluid, a biomarker of oxidative DNA damage, has a detrimental impact on the number of good quality embryos. DNA damage caused by oxidative stress may potentially result in early pregnancy loss [95]. Exposure to ROS results in the hardening of the zona pellucida and can weaken the implantation ability of embryos [96].
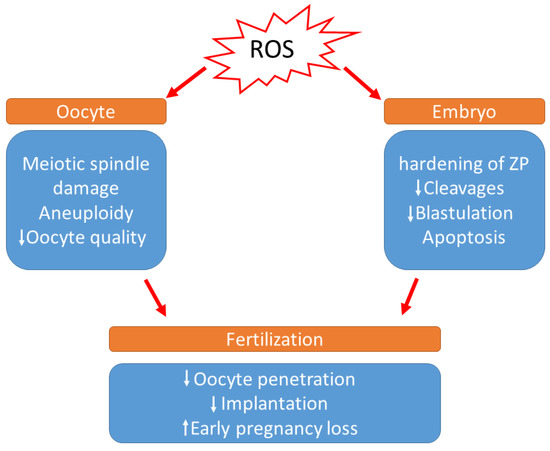
Figure 2.
Effects of increased levels of reactive oxygen species during IVF. (ROS: Reactive oxygen species, ZP: zona pellucida).
The importance of ROS is decreased at the blastocyst stage because the embryo shifts from oxidative phosphorylation to aerobic glycolysis for sustenance protein synthesis and ion transport systems [97,98]. A low level of ROS produced by embryos, on the other hand, is required for development regulation [99].
The reproductive tract not only produces oocytes, but also protects gametes and the embryo from visible light exposure. During the processes of assisted reproduction, (retrieval of oocytes, preparation of the sperm, IVF or ICSI procedure, incubation and microscopic examination of formed embryos, embryo transfer), gametes, zygotes, and embryos are subjected to a variable spectrum of light from different sources, including safety cabinets, microscopes, or time-lapse imaging cameras [100,101].
4.2. The Effect of Light Exposure of Gametes and Embryos
Subdued and filtered light using red filters on laboratory lamps and UV or infrared filters in microscopes to eliminate white and UV light exposure, throughout all work stages, to sperm cells, oocytes, and embryos, resulted in better embryo quality [102]. Because light generates reactive oxygen species (ROS), oxidative stress is considered one of the plausible causes at the origin of the embryonic lesion. The harmful effects of light are associated with the generation of H2O2 in peroxisomes and mitochondria [103], activation of stress genes, or direct DNA damage via ionization [100].
The toxic effects of UV light have long been known for cells. Previous studies showed that not only UV radiation, but also visible light is toxic to mammalian cells [104,105]. However, the toxic effects of visible light (400–800 nm) are less commonly recognized. Still, several studies confirm the harmful effects of visible light on oocytes, sperm, and embryos [101,104,106].
Furthermore, the detrimental effects of visible light depend on the wavelength. Based on studies of light radiation, stress gene activation and DNA damage in embryos can also be triggered. Blue light (400–500 nm) is declared to be orders of magnitude more deleterious than longer wavelengths of the visible spectrum [101,104].
Light-generated ROS formation takes place in cellular flavins that absorb light and in membranal chromophores. ROS generation can cause mitochondrial dysfunction and cellular damage [101] and changes the membrane redox state, which might lead to membrane channel opening [105].
Based on the above, using light filters may reduce detrimental environmental factors in an IVF laboratory. Bognar et al. [105] showed that white light exposure reduced the implantation potential of in vitro cultured mouse embryos. However, if a red optical filter was used the harmful effect of light was reduced. Our recent human study shows how essential it is to reduce the detrimental effects of illumination, thereby preserving the number of viable embryos and minimizing embryo loss during IVF and ICSI [102].
4.3. OS in the Embryo Culture Medium
Metallic ions in culture media, such as Fe2+ and Cu2+, have the ability to speed up cell ROS production by participating in Fenton and Haber–Weiss processes (Figure 3) [80]. As a result, depending on the composition of commercial embryo culture media, variable levels of ROS are generated. However, while endogenous ROS are formed by embryo metabolism, exogenous ROS are formed spontaneously by buffers and different types of additives in the culture medium. Consequently, additional, exogenous antioxidants appear to be required [107]. Therefore, antioxidants are generally added to the embryo culture medium, ensuring that the oxidant and antioxidant balance in the embryos is maintained [82]. However, it has been established that the formation of ROS is higher in more complex culture media compared to simple media [108,109]. To enhance embryo quality and viability in vitro, it appears that the composition of the embryo culture medium must be optimized. It has been suggested that antioxidants be added to the maturation medium to reduce the danger of oxidative stress and subsequent DNA damage [82].
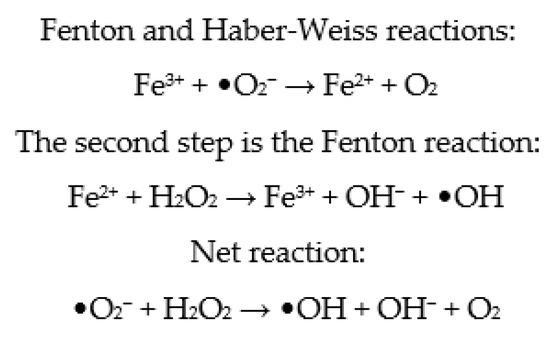
Figure 3.
Fenton and Haber–Weiss reactions.
ROS inducers can also be found among media additives. Because of its significant antioxidant capabilities, serum albumin is an important addition [110]. Serum preparations, which are often added to culture media, include high quantities of amine oxidase, which results in a rise in H2O2 production [111].
4.4. Methods Used to Counteract OS Effects
Human serum albumin (HSA) is currently being used to improve human embryo culture media as a protein supplement with antioxidant properties [112,113]. Copper ions attach to particular binding sites in albumin and have the ability to speed up the decomposition of free radical processes. Pool and Martin [114] were the first to use HSA in human embryo culture conditions, demonstrating that albumin increased embryo growth. For fertilization and embryo development, rHSA was found to be as effective as HSA. Furthermore, employing rHSA in IVF may reduce contamination and the transfer of plasma-derived contaminants. Due to the expensive cost of manufacture, rHSA is not used extensively as an additive in human embryo culture media [2]. Since the primarily discovered add-on with antioxidant effect, it has been found that many more molecules are proved to be effective in reducing OS in embryonic culture droplets. Aitken reviewed in 2020 [115] that molecules such as alpha-lipoic acid, hypotaurine and N-acetyl cysteine, 9-cis-retinoic acid, coenzyme Q10, melatonin, rosmarinic acid, and citrus flavonoids or hesperetin are now commonly used by the manufactures of embryo culture media.
A study on antioxidants is required to determine optimal supplementation levels for human embryo culture media, because many antioxidants have positive effects on embryo growth during ART. Antioxidants are an effective therapeutic method. It is, nevertheless, difficult to identify a superior or optimal culture medium from the others [116], even if we consider that the ROS levels of various commercial cultural media, even those produced by the same company, could differ significantly [116]. As Yang et al. [93] reviewed as a result, adding free radical scavengers and metal chelators, such as SOD, transferrin, ethylenediaminetetraacetic acid (EDTA), and thioredoxin, to the culture media at a low oxygen concentration may lead to improved embryo development.
5. Conclusions
In conclusion, mild levels of ROS have a non-negligible effect on the physiological maturation processes of gametes. As signaling molecules, they have importance in the regulation of cell proliferation rates and apoptosis, as well as in the modulation of gene expression pathways. ROS play a crucial role in the normal functioning of spermatozoa, oocytes, and in the development of the preimplantation embryo. In contrast, OS, due to elevated ROS levels, is one of the most important disorders that can lead to sub- and infertility in both men and women. Whereas in a healthy environment, there is a precisely adjusted balance between ROS and antioxidants. The elevated levels of ROS are extremely harmful due to the damage of lipids, DNA, or proteins. Among the potential ROS sources during ART, we highlighted the importance of reducing photo-oxidative stress by using light filters that can reduce harmful environmental factors in an IVF laboratory. Not only the recognition of the molecular networks between pro- and antioxidant pathways, but also the determination of the biological conditions that either predispose to the bioaccumulation of ROS or promote the biodestruction of oxygen-derived free radicals, could be helpful in undertaking various efforts related to the practical application of assisted reproductive technologies (ARTs) in different mammalian species. These efforts include the improvement of both developmental competence and quality-related parameters of in vitro-produced embryos generated by gamete coincubation or intracytoplasmic sperm injection (ICSI)-mediated IVF [117,118,119,120] or by somatic cell nuclear transfer (SCNT)-based cloning [121,122,123].
Author Contributions
Conceptualization, J.B., E.S., B.N. and P.M.; writing—original draft preparation, P.M., R.A.V. and B.N.; writing—review and editing, J.B., E.S., B.N. and K.K.; supervision, J.B. and E.S.; project administration, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by RRF-2.3.1-21-2022-00012 „National Laboratory on Human Reproduction” program and the Medical School of the University of Pécs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Zarbakhsh, S. Effect of antioxidants on preimplantation embryo development in vitro: A review. Zygote 2021, 29, 179–193. [Google Scholar] [CrossRef]
- Bell, E.L.; Emerling, B.M.; Chandel, N.S. Mitochondrial regulation of oxygen sensing. Mitochondrion 2005, 5, 322–332. [Google Scholar] [CrossRef]
- Bell, E.L.; Chandel, N.S. Mitochondrial oxygen sensing: Regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007, 43, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod. Biomed. Online 2008, 16, 553–569. [Google Scholar] [CrossRef]
- De Giusti, V.C.; Caldiz, C.I.; Ennis, I.L.; Pérez, N.G.; Cingolani, H.E.; Aiello, E.A. Mitochondrial reactive oxygen species (ROS) as signaling molecules of intracellular pathways triggered by the cardiac renin-angiotensin II-aldosterone system (RAAS). Front. Physiol. 2013, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Y.; Li, Y.; Jin, Q.; Sun, J.; Zhiqiang, E.; Gao, Q. Supplementation of kaempferol to in vitro maturation medium regulates oxidative stress and enhances subsequent embryonic development in vitro. Zygote 2020, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Attaran, M.; Pasqualotto, E.; Falcone, T.; Goldberg, J.M.; Miller, K.F.; Agarwal, A.; Sharma, R. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int. J. Fertil. Women’s Med. 2000, 45, 314–320. [Google Scholar]
- Szczepańska, M.; Koźlik, J.; Skrzypczak, J.; Mikołajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Cackler, A.B.; Arnett, M.G. Why should you care about free radicals? RN 2004, 67, 38–42. [Google Scholar]
- Iwayama, K.; Kusakabe, A.; Ohtsu, K.; Nawano, T.; Tatsunami, R.; Ohtaki, K.-I.; Tampo, Y.; Hayase, N. Long-term treatment of clarithromycin at a low concentration improves hydrogen peroxide-induced oxidant/antioxidant imbalance in human small airway epithelial cells by increasing Nrf2 mRNA expression. BMC Pharmacol. Toxicol. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2020, 20, 41–52. [Google Scholar] [CrossRef]
- Carrell, D.T.; Liu, L.; Peterson, C.M.; Jones, K.P.; Hatasaka, H.H.; Erickson, L.; Campbell, B. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch. Androl. 2003, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef]
- Khan, A.U.; Wilson, T. Reactive oxygen species as cellular messengers. Chem. Biol. 1995, 2, 437–445. [Google Scholar] [CrossRef]
- Thompson, A.; Agarwal, A.; du Plessis, S.S. Physiological Role of Reactive Oxygen Species in Sperm Function: A Review. In Antioxidants in Male Infertility: A Guide for Clinicians and Researchers; Parekatil, S.J., Agarwal, A., Eds.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 69–89. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Griveau, J.F.; Le Lannou, D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological role of ROS in sperm function. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Springer: New York, NY, USA, 2020; pp. 337–345. [Google Scholar]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D. Physiological Role of Reactive Oxygen Species in Male Reproduction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Henkel, R., Samanta, L., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 1.8; pp. 65–78. [Google Scholar]
- de Lamirande, E. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar] [PubMed]
- Kumar, N.; Singh, A.K. Reactive oxygen species in seminal plasma as a cause of male infertility. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 565–572. [Google Scholar] [CrossRef]
- Cooper, T.G. The epididymis, cytoplasmic droplets and male fertility. Asian J. Androl. 2010, 13, 130–138. [Google Scholar] [CrossRef]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- Agarwal, A.; Rosas, I.M.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef]
- Aitken, R.J.; Finnie, J.M.; Muscio, L.; Whiting, S.; Connaughton, H.S.; Kuczera, L.; Rothkirch, T.B.; De Iuliis, G.N. Potential importance of transition metals in the induction of DNA damage by sperm preparation medium. Hum. Reprod. 2014, 29, 2136–2147. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; Di Giammarco, N.; Santilli, G.; Francavilla, S.; Francavilla, F. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 2016, 66, 61–67. [Google Scholar] [CrossRef]
- Kotwicka, M.; Skibinska, I.; Jendraszak, M.; Jedrzejczak, P. 17beta-estradiol modifies human spermatozoa mitochondrial function in vitro. Reprod. Biol. Endocrinol. 2016, 14, 50. [Google Scholar]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 1995, 42, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar]
- Twigg, J.P.; Irvine, D.S.; Aitken, R.J. Oxidative damage to DNA in human spermatozoa does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 1864–1871. [Google Scholar] [CrossRef]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Aitken, R.; Krausz, C. Oxidative stress, DNA damage and the Y chromosome. Reproduction 2001, 122, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef]
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa, Mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Humaidan, P.; Haahr, T.; Povlsen, B.B.; Kofod, L.; Laursen, R.J.; Alsbjerg, B.; Elbaek, H.O.; Esteves, S.C. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: A pilot study. Int. Braz. J. Urol. 2022, 48, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sharma, R.K.; Falcone, T.; Goldberg, J.; Agarwal, A. Importance portance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil. Steril. 1997, 68, 826–830. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility–A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28–48. [Google Scholar] [CrossRef]
- Wall, P.D.; Pressman, E.K.; Woods, J.R. Preterm premature rupture of the membranes and antioxidants: The free radical connection. J. Périnat. Med. 2002, 30, 447–457. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.A.; Arlene, N.S.; Morales, J.; Parthasarathy, S. Lysophosphatidyl Choline, a Chemotactic Factor for Monocytes/T-Lymphocytes Is Elevated in Endometriosis. J. Clin. Endocrinol. Metab. 1998, 83, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sugino, N.; Fukaya, T.; Sugiyama, S.; Uda, T.; Takaya, R.; Yajima, A.; Sasano, H. Superoxide dismutase in normal cycling human ovaries: Immunohistochemical localization and characterization. Fertil. Steril. 1999, 72, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Carrasca, I.; Castillo, T.; Troncoso, J.L.; Videla, I.A.; Devoto, L. Functional luteolysis in response to hydrogen peroxide in human luteal cells. J. Endocrinol. 1995, 147, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.L.; Skepper, J.N.; Jauniaux, E.; Burton, G.J. Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta 1998, 19, 27–34. [Google Scholar] [CrossRef]
- Watson, A.l.; Palmer, M.E.; Jauniaux, E.; Burton, G.J. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997, 18, 295–299. [Google Scholar] [CrossRef]
- Kaya, C.; Erkan, A.F.; Cengiz, S.D.; Dünder, I.; Demirel, E.; Bilgihan, A. Advanced oxidation protein products are increased in women with polycystic ovary syndrome: Relationship with traditional and nontraditional cardiovascular risk factors in patients with polycystic ovary syndrome. Fertil. Steril. 2009, 92, 1372–1377. [Google Scholar] [CrossRef]
- Amato, G.; Conte, M.; Mazziotti, G.; Lalli, E.; Vitolo, G.; Tucker, A.T.; Bellastella, A.; Carella, C.; Izzo, A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet. Gynecol. 2003, 101, 1177–1182. [Google Scholar]
- Rashidi, B.; Haghollahi, F.; Shariat, M.; Zayerii, F. The Effects of Calcium-Vitamin D and Metformin on Polycystic Ovary Syndrome: A Pilot Study. Taiwan. J. Obstet. Gynecol. 2009, 48, 142–147. [Google Scholar] [CrossRef]
- Cuffe, J.S.M.; Holland, O.; Salomon, C.; Rice, G.E.; Perkins, A.V. Review: Placental derived biomarkers of pregnancy disorders. Placenta 2017, 54, 104–110. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, I. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wiener-Megnazi, Z.; Vardi, L.; Lissak, A.; Shnizer, S.; Reznick, A.Z.; Ishai, D.; Lahav-Baratz, S.; Shiloh, H.; Koifman, M.; Dirnfeld, M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil. Steril. 2004, 82, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Babuška, V.; Cedíková, M.; Rajdl, D.; Racek, J.; Zech, N.H.; Trefil, L.; Mocková, A.; Ulčová-Gallová, Z.; Novotný, Z.; Králíčková, M. Srovnání vybraných parametrů oxidačního stresu ve folikulární tekutině u žen s poruchou plodnosti a u zdravých fertilních dárkyň oocytů. [Comparison of selective oxidative stress parameters in the follicular fluid of infertile women and healthy fertile oocyte donors]. Ceská Gynekol. Ceská Lékarská Spolecnost. J. Ev. Purkyne 2012, 77, 543–548. [Google Scholar]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sato, E.F.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Inoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free. Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Tarin, J.J. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol. Hum. Reprod. 1996, 2, 717–724. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef]
- Myllynen, P.; Pasanen, M.; Pelkonen, O. Human placenta: A human organ for developmental toxicology research and biomonitoring. Placenta 2005, 26, 361–371. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Pan, Y.-X. In Utero Oxidative Stress Epigenetically Programs Antioxidant Defense Capacity and Adulthood Diseases. Antioxid. Redox Signal. 2012, 17, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Global Supplement Report. Available online: https://store.newhope.com/products/2019-global-supplement-report2019. (accessed on 28 January 2023).
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female sub-fertility. Cochrane Database Syst. Rev. 2020, 8, CD007807. [Google Scholar] [PubMed]
- Elnashar, A. Antioxidants for female infertility: Review of systematic reviews. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, e84. [Google Scholar] [CrossRef]
- Fang, F.; Ni, K.; Cai, Y.; Shang, J.; Zhang, X.; Xiong, C. Effect of vitamin D supplementa-tion on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement. Clin. Pract. 2017, 26, 53–60. [Google Scholar] [CrossRef]
- Arhin, S.K.; Zhao, Y.; Lu, X.; Chetry, M.; Lu, J. Effect of micronutrient supplementation on IVF outcomes: A systematic review of the literature. Reprod. Biomed. Online 2017, 35, 715–722. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Harvey, A.J.; Kind, K.L.; Thompson, J.G. REDOX regulation of early embryo development. Reproduction 2002, 123, 479–486. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T.; Mohamed, M.S.; Aleem, A.A.N.; Sharma, R.K.; Worley, S.E.; Thornton, J.; Agarwal, A. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil. Steril. 2004, 82, 593–600. [Google Scholar] [CrossRef]
- Mohammadi, M. Oxidative stress and polycystic ovary syndrome: A brief review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Irvine, D.S.; Wu, F.C. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am. J. Obstet. Gynecol. 1991, 164, 542–551. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.; Ferraretti, A.P.; Fiorentino, A.; Tosti, E.; Panzella, S.; Dale, B. Reducing the time of sperm-oocyte interaction in human in-vitro fertilization improves the implantation rate. Hum. Reprod. 1996, 11, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.; Lydic, M.L.; Ho, M.; Bastuba, M.; Hendee, F.; Brody, S.A. Confirmation of the Beneficial Effects of Brief Coincubation of Gametes in Human In Vitro Fertilization. Fertil. Steril. 1998, 69, 399–402. [Google Scholar] [CrossRef]
- Boone, W.R.; Johnson, J.E. Clinical Assisted Reproduction: Extending the Coincubation Time of Gametes Improves In Vitro Fertilization. J. Assist. Reprod. Genet. 2001, 18, 18–20. [Google Scholar] [CrossRef]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, M.-S.; Liu, C.-H.; Tsao, H.-M.; Huang, C.-C.; Yang, Y.-S. The Association Between Microenvironmental Reactive Oxygen Species and Embryo Development in Assisted Reproduction Technology Cycles. Reprod. Sci. 2012, 19, 725–732. [Google Scholar] [CrossRef]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef]
- Várnagy, A.; Kőszegi, T.; Györgyi, E.; Szegedi, S.; Sulyok, E.; Prémusz, V.; Bódis, J. Levels of total antioxidant capacity and 8-hydroxy-2′-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: Focusing on endometriosis. Hum. Fertil. 2018, 23, 200–208. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of oxidative stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, J.X.; Liu, W.W.; Gao, Y.; Han, W.; Xiong, S.; Wu, L.H.; Huang, G.N. Time of insemination culture and outcomes of in vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Updat. 2013, 19, 685–695. [Google Scholar] [CrossRef]
- Martin, K.L. Nutritional and metabolic requirements of early cleavage stage embryos and blastocysts. Hum. Fertil. 2000, 3, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Sherman, A.N.M.; Allen, N.W.; McGowan, L.T.; Tervit, H.R. Total protein content and protein synthesis within pre-elongation stage bovine embryos. Mol. Reprod. Dev. 1998, 50, 139–145. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Crawford, S.B.; Folger, S.G.; Jamieson, D.J.; Barfield, W.D. Centers for Disease C and Prevention (2014). Assisted reproductive technology surveillance–United States. MMWR Surveill Summ. 2011, 63, 1–28. [Google Scholar]
- Pomeroy, K.O.; Reed, M. The effect of light on embryos and embryo culture. J. Reprod. Biotechnol. Fertil. 2013, 5, 104–116. [Google Scholar] [CrossRef]
- Ottosen, L.D.; Hindkjaer, J.; Ingerslev, J. Light exposure of the ovum and preimplantation embryo during ART procedures. J. Assist. Reprod. Genet. 2007, 24, 99–103. [Google Scholar] [CrossRef]
- Bódis, J.; Gödöny, K.; Várnagy, Á.; Kovács, K.; Koppán, M.; Nagy, B.; Erostyák, J.; Herczeg, R.; Szekeres-Barthó, J.; Gyenesei, A.; et al. How to Reduce the Potential Harmful Effects of Light on Blastocyst Development during IVF. Med. Princ. Pract. 2020, 29, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Hockberger, P.E.; Skimina, T.A.; Centonze, V.E.; Lavin, C.; Chu, S.; Dadras, S.; Reddy, J.K.; White, J.G. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6255–6260. [Google Scholar] [CrossRef]
- Hirao, Y.; Yanagimachi, R. Detrimental effect of visible light on meiosis of mammalian eggs in vitro. J. Exp. Zool. 1978, 206, 365–369. [Google Scholar] [CrossRef]
- Bognar, Z.; Csabai, T.J.; Pallinger, E.; Balassa, T.; Farkas, N.; Schmidt, J.; Görgey, E.; Berta, G.; Szekeres-Bartho, J.; Bodis, J. The effect of light exposure on the cleavage rate and implantation capacity of preimplantation murine embryos. J. Reprod. Immunol. 2019, 132, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lavi, R.; Ankri, R.; Sinyakov, M.; Eichler, M.; Friedmann, H.; Shainberg, A.; Breitbart, H.; Lubart, R. The plasma membrane is involved in the visible light-tissue interaction. Photomed. Laser Surg. 2012, 30, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, H.; Sharma, R.; Mahfouz, R.; Agarwal, A. l-Carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil. Steril. 2009, 91, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romero, F.J.; Miguel-Lasobras, E.M.; Dominguez-Arroyo, J.A.; Gonzalez-Carrera, E.; Alvarez, I.S. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod. Biomed. Online 2008, 17, 652–661. [Google Scholar] [CrossRef]
- Menezo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983, 29, 548–555. [Google Scholar] [CrossRef]
- Shannon, P. Factors affecting semen preservation and conception rates in cattle. J. Reprod. Fertil. 1978, 54, 519–527. [Google Scholar] [CrossRef]
- Labied, S.; Jouan, C.; Wenders, F.; Ravet, S.; Gaspard, O.; Thonon, F.; Gridelet, V.; Henry, L.; Perrier d’Hauterive, S.; Nisolle, M. Comparison between paraffin and mineral oil covering on early human embryo culture: A prospective randomized study. Syst. Biol. Reprod. Med. 2019, 65, 81–86. [Google Scholar] [CrossRef]
- Lan, K.C.; Lin, Y.C.; Chang, Y.C.; Lin, H.J.; Tsai, Y.R.; Kang, H.Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J. Assist. Reprod. Genet. 2019, 36, 325–334. [Google Scholar] [CrossRef]
- Pool, T.B.; Martin, J.E. High continuing pregnancy rates after in vitro fertilization-embryo transfer using medium supplemented with a plasma protein fraction containing alpha- and beta-globulins. Fertil. Steril. 1994, 61, 714–719. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.F.; Lee, T.H.; Liu, C.H.; Tsao, H.M.; Huang, C.C.; Lee, M.S. Effects of reactive oxygen species levels in prepared culture media on embryo development: A comparison of two media. Taiwan J. Obstet. Gynecol. 2014, 53, 504–508. [Google Scholar] [CrossRef]
- Sills, E.S.; Palermo, G.D. Human blastocyst culture in IVF: Current laboratory applications in reproductive medicine practice. Rom. J. Morphol. Embryol. 2010, 51, 441–445. [Google Scholar]
- Yamanaka, K.I.; Khatun, H.; Egashira, J.; Balboula, A.Z.; Tatemoto, H.; Sakatani, M.; Takenouchi, N.; Wada, Y.; Takahashi, M. Heat-shock-induced cathepsin B activity during IVF and culture compromises the developmental competence of bovine embryos. Theriogenology 2018, 114, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C.S.; Cha, J.H.; Son, W.Y.; Yoon, S.H.; Kim, K.A.; Lim, J.H. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Kharche, S.D.; Pathak, J.; Agarwal, S.; Kushwah, B.; Sikarwar, A. Effect of Ca Ionophore on Blastocyst Production Following Intracytoplasmic Sperm Injection in Caprine Oocytes. Reprod. Domest. Anim. 2016, 51, 611–617. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. Molecular conditions of the cell nucleus remodelling/reprogramming process and nuclear transferred embryo development in the intraooplasmic karyoplast injection technique: A review. Czech J. Anim. Sci. 2005, 50, 185–195. [Google Scholar] [CrossRef]
- Qu, P.; Shen, C.; Du, Y.; Qin, H.; Luo, S.; Fu, S.; Dong, Y.; Guo, S.; Hu, F.; Xue, Y.; et al. Melatonin Protects Rabbit Somatic Cell Nuclear Transfer (SCNT) Embryos from Electrofusion Damage. Sci. Rep. 2020, 10, 2186. [Google Scholar] [CrossRef]
- Wiater, J.; Samiec, M.; Wartalski, K.; Smorąg, Z.; Jura, J.; Słomski, R.; Skrzyszowska, M.; Romek, M. Characterization of Mono- and Bi-Transgenic Pig-Derived Epidermal Keratinocytes Expressing Human FUT2 and GLA Genes–In Vitro Studies. Int. J. Mol. Sci. 2021, 22, 9683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).