PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland
Abstract
1. Introduction
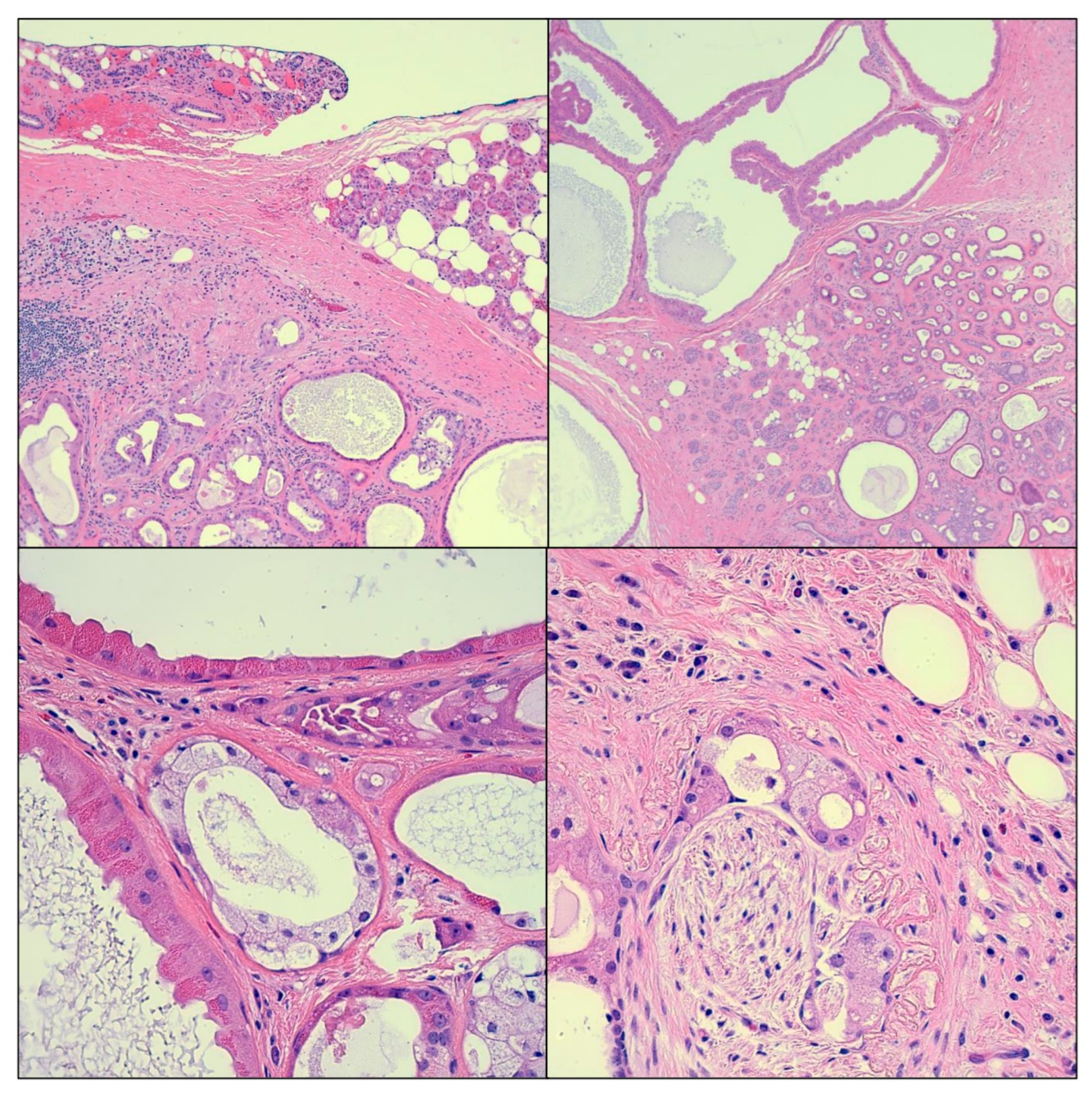
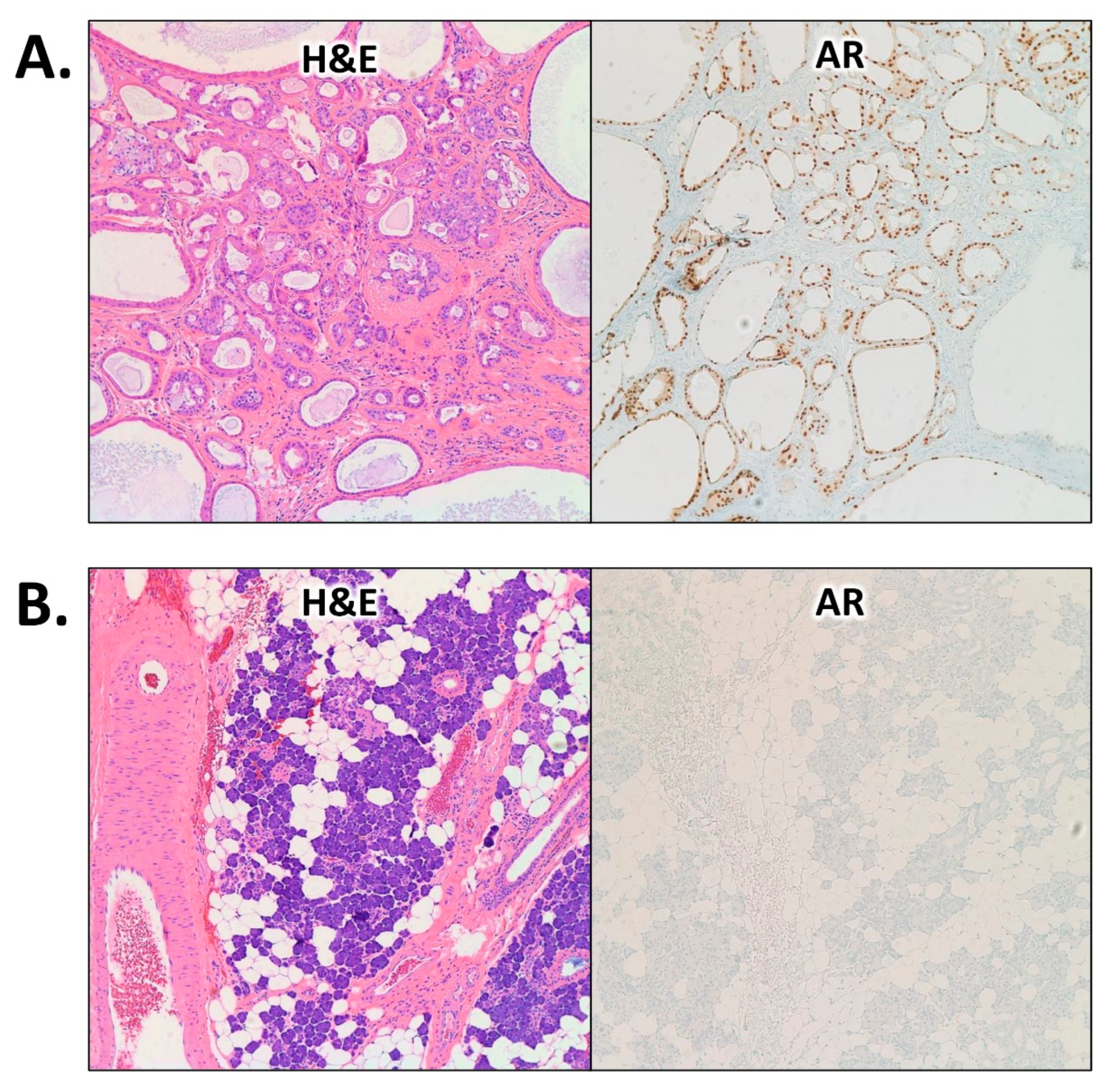
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnepp, D.R. Salivary gland tumor “wishes” to add to the next WHO Tumor Classification: Sclerosing polycystic adenosis, mammary analogue secretory carcinoma, cribriform adenocarcinoma of the tongue and other sites, and mucinous variant of myoepithelioma. Head Neck Pathol. 2014, 8, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Ellis, G.L.; Slater, L.J.; Foss, R.D. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am. J. Surg. Pathol. 1996, 20, 161–170. [Google Scholar] [CrossRef]
- Skálová, A.; Gnepp, D.R.; Simpson, R.H.; Lewis, J.E.; Janssen, D.; Sima, R.; Vanecek, T.; Di Palma, S.; Michal, M. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am. J. Surg. Pathol. 2006, 30, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Michal, M.; Simpson, R.H.; Stárek, I.; Prádná, J.; Pfaltz, M. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ. Report of three cases with immunohistochemical and ultrastructural examination. Virchows Arch. 2002, 440, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gnepp, D.R.; Wang, L.J.; Brandwein-Gensler, M.; Slootweg, P.; Gill, M.; Hille, J. Sclerosing polycystic adenosis of the salivary gland: A report of 16 cases. Am. J. Surg. Pathol. 2006, 30, 154–164. [Google Scholar] [CrossRef]
- Petersson, F. Sclerosing polycystic adenosis of salivary glands: A review with some emphasis on intraductal epithelial proliferations. Head Neck Pathol. 2013, 7 (Suppl. 1), S97–S106. [Google Scholar] [CrossRef]
- Mokhtari, S.; Atarbashi Moghadam, S.; Mirafsharieh, A. Sclerosing polycystic adenosis of the retromolar pad area: A case report. Case Rep. Pathol. 2014, 2014, 982432. [Google Scholar] [CrossRef]
- Su, A.; Bhuta, S.M.; Berke, G.S.; Lai, C.K. A unique case of sclerosing polycystic adenosis of the sinonasal tract. Hum. Pathol. 2013, 44, 1937–1940. [Google Scholar] [CrossRef]
- Canas Marques, R.; Félix, A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014, 464, 621–625. [Google Scholar] [CrossRef]
- Bishop, J.A.; Gagan, J.; Baumhoer, D.; McLean-Holden, A.L.; Oliai, B.R.; Couce, M.; Thompson, L.D.R. Sclerosing Polycystic “Adenosis” of Salivary Glands: A Neoplasm Characterized by PI3K Pathway Alterations More Correctly Named Sclerosing Polycystic Adenoma. Head Neck Pathol. 2020, 14, 630–636. [Google Scholar] [CrossRef]
- Skálová, A.; Gnepp, D.R.; Lewis, J.S., Jr.; Hunt, J.L.; Bishop, J.A.; Hellquist, H.; Rinaldo, A.; Vander Poorten, V.; Ferlito, A. Newly Described Entities in Salivary Gland Pathology. Am. J. Surg. Pathol. 2017, 41, e33–e47. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Chan, J.K.; Grandis, J.R. WHO Classification of Head and Neck Tumours; IARC Publications: Lyon, France, 2017. [Google Scholar]
- Nakazawa, M.; Antonarakis, E.S.; Luo, J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm. Cancer 2014, 5, 265–273. [Google Scholar] [CrossRef]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009, 69, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Bartsch, G. Androgen axis in prostate cancer. J. Cell. Biochem. 2006, 99, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Boudadi, K.; Antonarakis, E.S. Resistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate Cancer. Clin. Med. Insights Oncol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Mitani, Y.; Rao, P.H.; Maity, S.N.; Lee, Y.C.; Ferrarotto, R.; Post, J.C.; Licitra, L.; Lippman, S.M.; Kies, M.S.; Weber, R.S.; et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: Potential therapeutic ramifications. Clin. Cancer Res. 2014, 20, 6570–6581. [Google Scholar] [CrossRef]
- Dalin, M.G.; Watson, P.A.; Ho, A.L.; Morris, L.G. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 2017, 9, 17. [Google Scholar] [CrossRef]
- Der, C.J.; Finkel, T.; Cooper, G.M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell 1986, 44, 167–176. [Google Scholar] [CrossRef]
- Fernández-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Wang, D.; Saba, N.F.; Chen, Z.G. A Historic Perspective and Overview of H-Ras Structure, Oncogenicity, and Targeting. Mol. Cancer Ther. 2020, 19, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Dermime, S.; Uddin, S. RAS-mediated oncogenic signaling pathways in human malignancies. Semin. Cancer Biol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Grünewald, I.; Vollbrecht, C.; Meinrath, J.; Meyer, M.F.; Heukamp, L.C.; Drebber, U.; Quaas, A.; Beutner, D.; Hüttenbrink, K.B.; Wardelmann, E.; et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015, 6, 18224–18237. [Google Scholar] [CrossRef]
- Ross, R.L.; Burns, J.E.; Taylor, C.F.; Mellor, P.; Anderson, D.H.; Knowles, M.A. Identification of mutations in distinct regions of p85 alpha in urothelial cancer. PLoS ONE 2013, 8, e84411. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, B.S.; Janakiraman, V.; Kljavin, N.M.; Chaudhuri, S.; Stern, H.M.; Wang, W.; Kan, Z.; Dbouk, H.A.; Peters, B.A.; Waring, P.; et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 2009, 16, 463–474. [Google Scholar] [CrossRef]
- Ng, P.K.; Li, J.; Jeong, K.J.; Shao, S.; Chen, H.; Tsang, Y.H.; Sengupta, S.; Wang, Z.; Bhavana, V.H.; Tran, R.; et al. Systematic Functional Annotation of Somatic Mutations in Cancer. Cancer Cell 2018, 33, 450–462. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Yao, L.; Kuang, X.Y.; Zuo, W.J.; Li, S.; Qiao, F.; Liu, Y.R.; Cao, Z.G.; Zhou, S.L.; et al. Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat. Commun. 2018, 9, 1357. [Google Scholar] [CrossRef]
- Ettl, T.; Schwarz-Furlan, S.; Haubner, F.; Müller, S.; Zenk, J.; Gosau, M.; Reichert, T.E.; Zeitler, K. The PI3K/AKT/mTOR signalling pathway is active in salivary gland cancer and implies different functions and prognoses depending on cell localisation. Oral Oncol. 2012, 48, 822–830. [Google Scholar] [CrossRef]
- Saintigny, P.; Mitani, Y.; Pytynia, K.B.; Ferrarotto, R.; Roberts, D.B.; Weber, R.S.; Kies, M.S.; Maity, S.N.; Lin, S.H.; El-Naggar, A.K. Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: Implications for targeted therapy. Cancer 2018, 124, 3693–3705. [Google Scholar] [CrossRef]
- Griffith, C.C.; Seethala, R.R.; Luvison, A.; Miller, M.; Chiosea, S.I. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am. J. Surg. Pathol. 2013, 37, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Williams, L.; Griffith, C.C.; Thompson, L.D.; Weinreb, I.; Bauman, J.E.; Luvison, A.; Roy, S.; Seethala, R.R.; Nikiforova, M.N. Molecular characterization of apocrine salivary duct carcinoma. Am. J. Surg. Pathol. 2015, 39, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, I.; Bishop, J.A.; Chiosea, S.I.; Seethala, R.R.; Perez-Ordonez, B.; Zhang, L.; Sung, Y.S.; Chen, C.L.; Assaad, A.; Oliai, B.R.; et al. Recurrent RET Gene Rearrangements in Intraductal Carcinomas of Salivary Gland. Am. J. Surg. Pathol. 2018, 42, 442–452. [Google Scholar] [CrossRef] [PubMed]

| Gene | Alteration | Reference Sequence | Nucleotide Change | VAF |
|---|---|---|---|---|
| AR | AR-V7 | NM_000044.3 | -- | -- |
| HRAS | p.Q61R | NM_005343.2 | c.182A>G | 0.7% |
| PIK3R1 | p.N564D | NM_181523.2 | c.1690A>G | 23.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmad, H.F.; Elhammady, G.; Gass, J.M.; Paramo, J.C.; Poppiti, R.; Alexis, J. PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland. Curr. Issues Mol. Biol. 2023, 45, 954-962. https://doi.org/10.3390/cimb45020061
Bahmad HF, Elhammady G, Gass JM, Paramo JC, Poppiti R, Alexis J. PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland. Current Issues in Molecular Biology. 2023; 45(2):954-962. https://doi.org/10.3390/cimb45020061
Chicago/Turabian StyleBahmad, Hisham F., Gina Elhammady, Jennifer M. Gass, Juan C. Paramo, Robert Poppiti, and John Alexis. 2023. "PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland" Current Issues in Molecular Biology 45, no. 2: 954-962. https://doi.org/10.3390/cimb45020061
APA StyleBahmad, H. F., Elhammady, G., Gass, J. M., Paramo, J. C., Poppiti, R., & Alexis, J. (2023). PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland. Current Issues in Molecular Biology, 45(2), 954-962. https://doi.org/10.3390/cimb45020061







