Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Groot, A.C.; Schmidt, E. Essential Oils, Part I: Introduction. Dermatitis 2016, 27, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, K.; Schroter, C.; Brüggmann, D.; Tinneberg, H.-R.; von Georgi, R. Use of Complementary and Alternative Medicine in Departments of Obstetrics in Germany. Komplementmed 2009, 16, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, R. Developmental Toxicology, Placental Toxicity; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1301–1325. [Google Scholar]
- Williams, W. Pre-Conception Care and Aromatherapy in Pregnancy. J. Clin. Aromather. 2005, 2, 15–19. [Google Scholar]
- Arévalo, L.; Campbell, P. Placental Effects on the Maternal Brain Revealed by Disrupted Placental Gene Expression in Mouse Hybrids. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, E.; Bulmer, J.N.; Ayis, S.; Lyall, F.; Robson, S.C. Late Sporadic Miscarriage Is Associated with Abnormalities in Spiral Artery Transformation and Trophoblast Invasion. J. Pathol. 2006, 208, 535–542. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Placental Oxidative Stress: From Miscarriage to Preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Wohl, D.; Yagel, S. Regulation of Trophoblast Invasion: From Normal Implantation to Pre-Eclampsia. Mol. Cell Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Sebire, N.J.; Foskett, M.; Fisher, R.A.; Rees, H.; Seckl, M.; Newlands, E. Risk of Partial and Complete Hydatidiform Molar Pregnancy in Relation to Maternal Age. BJOG 2002, 109, 99–102. [Google Scholar] [CrossRef]
- Turbeville, H.R.; Sasser, J.M. Preeclampsia beyond Pregnancy: Long-Term Consequences for Mother and Child. Am. J. Physiol. Renal. Physiol. 2020, 318, F1315–F1326. [Google Scholar] [CrossRef]
- Henley, D.V.; Lipson, N.; Korach, K.S.; Bloch, C.A. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med. 2007, 356, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, A.; Luque, L.; Badar, Z.; Kornic, S.; Danon, M. Prepubertal Gynecomastia and Chronic Lavender Exposure: Report of Three Cases. J. Pediatric. Endocrinol. Metab. 2016, 29, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; Coons, L.A.; Diaz, A.; Korach, K.S. Lavender Products Associated With Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Dunne, E.; Baker, C. The Relationship between Lavender and Tea Tree Essential Oils and Pediatric Endocrine Disorders: A Systematic Review of the Literature. Complement. Ther. Med. 2020, 49, 102288. [Google Scholar] [CrossRef] [PubMed]
- IPCS Global Assessment of the State-of-the-Science of Endocrine Disruptor; World Health Organization: Geneva, Switzerland, 2002.
- Grignard, E.; Jesus, K.; Hubert, P. Regulatory Testing for Endocrine Disruptors; Need for Validated Methods and Integrated Approaches. Front. Toxicol. 2022, 3. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 Placental Cells in Toxicology Studies: A Promising Tool to Reveal Pregnancy Disorders. Anat. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life 2021, 11, 314. [Google Scholar] [CrossRef]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells 2022, 11, 495. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 Receptor: A Main Player in Inflammation. Biochem. Pharm. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Zeng, D.; Yao, P.; Zhao, H. P2X7, a Critical Regulator and Potential Target for Bone and Joint Diseases. J. Cell Physiol. 2019, 234, 2095–2103. [Google Scholar] [CrossRef]
- Francistiová, L.; Bianchi, C.; Di Lauro, C.; Sebastián-Serrano, Á.; de Diego-García, L.; Kobolák, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Oliveira-Giacomelli, Á.; Glaser, T.; Arnaud-Sampaio, V.F.; Andrejew, R.; Dieckmann, L.; Baranova, J.; Lameu, C.; Ratajczak, M.Z.; Ulrich, H. Hyperactivation of P2X7 Receptors as a Culprit of COVID-19 Neuropathology. Mol. Psychiatry 2021, 26, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Tsimis, M.E.; Lei, J.; Rosenzweig, J.M.; Arif, H.; Shabi, Y.; Alshehri, W.; Talbot, C.C.; Baig-Ward, K.M.; Segars, J.; Graham, E.M.; et al. P2X7 Receptor Blockade Prevents Preterm Birth and Perinatal Brain Injury in a Mouse Model of Intrauterine Inflammation. Biol. Reprod. 2017, 97, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organisation of Standardization: Geneva, Switzerland, 2009.
- Etzel, T.M.; Calafat, A.M.; Ye, X.; Chen, A.; Lanphear, B.P.; Savitz, D.A.; Yolton, K.; Braun, J.M. Urinary Triclosan Concentrations during Pregnancy and Birth Outcomes. Environ. Res. 2017, 156, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Calafat, A.M.; Cordero, J.F.; Meeker, J.D. A Repeated Measures Study of Phenol, Paraben and Triclocarban Urinary Biomarkers and Circulating Maternal Hormones during Gestation in the Puerto Rico PROTECT Cohort. Environ. Health 2019, 18, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Zhang, B.; He, Y.; Hong, D.; Song, S.; Huang, Y.; Zhang, T. Triclosan and Triclocarbon in Maternal-Fetal Serum, Urine, and Amniotic Fluid Samples and Their Implication for Prenatal Exposure. Environ. Pollut. 2020, 266, 115117. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and Paternal Preconception Exposure to Phenols and Preterm Birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Xu, B.; Tang, R.; Han, X.; Qin, Y.; Xu, B.; Hang, B.; Mao, Z.; Huo, W.; et al. Parental Phenols Exposure and Spontaneous Abortion in Chinese Population Residing in the Middle and Lower Reaches of the Yangtze River. Chemosphere 2013, 93, 217–222. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Garcia, J.M.; Lin, H.; Wang, Y.; Yan, P.; Wang, L.; Tan, Y.; Luo, J.; Qiu, Z.; et al. Phthalate Levels in Cord Blood Are Associated with Preterm Delivery and Fetal Growth Parameters in Chinese Women. PLoS ONE 2014, 9, e87430. [Google Scholar] [CrossRef] [Green Version]
- Bahado-Singh, R.O.; Oz, A.U.; Kingston, J.M.; Shahabi, S.; Hsu, C.D.; Cole, L. The Role of Hyperglycosylated HCG in Trophoblast Invasion and the Prediction of Subsequent Pre-Eclampsia. Prenat. Diagn. 2002, 22, 478–481. [Google Scholar] [CrossRef]
- Einerson, B.D.; Straubhar, A.; Soisson, S.; Szczotka, K.; Dodson, M.K.; Silver, R.M.; Soisson, A.P. Hyperglycosylated HCG and Placenta Accreta Spectrum. Am. J. Perinatol 2019, 36, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngala, R.A.; Fondjo, L.A.; Gmagna, P.; Ghartey, F.N.; Awe, M.A. Placental Peptides Metabolism and Maternal Factors as Predictors of Risk of Gestational Diabetes in Pregnant Women. A Case-Control Study. PLoS ONE 2017, 12, e0181613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, R.M.; Donatelli, M.; Bucalo, M.L.; Bellomonte, M.L.; Bompiani, G.D. Placental Lactogen, Progesterone, Total Estriol and Prolactin Plasma Levels in Pregnant Women with Insulin-Dependent Diabetes Mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984, 16, 393–401. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational Diabetes and Adverse Perinatal Outcomes from 716,152 Births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Möllers, L.S.; Yousuf, E.I.; Hamatschek, C.; Morrison, K.M.; Hermanussen, M.; Fusch, C.; Rochow, N. Metabolic-Endocrine Disruption Due to Preterm Birth Impacts Growth, Body Composition, and Neonatal Outcome. Pediatr. Res. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fodor, P.; White, B.; Khan, R. Inflammation-The Role of ATP in Pre-Eclampsia. Microcirculation 2020, 27, e12585. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B.; Illes, P. P2X7 Receptor: An Emerging Target in Central Nervous System Diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 Receptor Mediates NLRP3 Inflammasome Activation in Depression and Diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for Estrogen and Androgen Receptor Activities in 200 Pesticides by in Vitro Reporter Gene Assays Using Chinese Hamster Ovary Cells. Environ. Health Perspect. 2004, 112, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, P.; Palma, V.L.; Matos, C.; Fernandes, R.M.; Bohn, A.; Soares, A.M.V.M.; Barbosa, I.R. Assessment of the Pesticides Atrazine, Endosulfan Sulphate and Chlorpyrifos for Juvenoid-Related Endocrine Activity Using Daphnia Magna. Chemosphere 2009, 76, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Iida, M.; Yabushita, H.; Matsuda, T.; Kojima, H. In Vitro Screening for Aryl Hydrocarbon Receptor Agonistic Activity in 200 Pesticides Using a Highly Sensitive Reporter Cell Line, DR-EcoScreen Cells, and in Vivo Mouse Liver Cytochrome P450-1A Induction by Propanil, Diuron and Linuron. Chemosphere 2008, 74, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Cutuli, D.; Colonnello, V.; Cardona, D.; Ricceri, L.; Calamandrei, G. Neonatal Exposure to Chlorpyrifos Affects Maternal Responses and Maternal Aggression of Female Mice in Adulthood. Neurotoxicol. Teratol. 2008, 30, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Fillâtre, Y.; Gray, F.-X.; Roy, C. Pesticides in Essential Oils: Occurrence and Concentration in Organic and Conventional Orange Essential Oils from Eleven Geographical Origins. Anal. Chim. Acta 2017, 992, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Turco, V.L.; Rando, R.; Arena, G.; Pollicino, D.; Luppino, R.R.; Dugo, G. Pesticide and Plasticizer Residues in Citrus Essential Oils from Different Countries. Nat. Prod. Commun. 2010, 5, 1934578X1000500838. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Li, G.; Feng, W.; Liu, L.; Zhang, J.; Wu, W.; Xu, L.; Yan, Y. Chlorpyrifos Is Estrogenic and Alters Embryonic Hatching, Cell Proliferation and Apoptosis in Zebrafish. Chem. Biol. Interact. 2015, 239, 26–33. [Google Scholar] [CrossRef]
- Grünfeld, H.T.; Bonefeld-Jorgensen, E.C. Effect of in Vitro Estrogenic Pesticides on Human Oestrogen Receptor Alpha and Beta MRNA Levels. Toxicol. Lett. 2004, 151, 467–480. [Google Scholar] [CrossRef]
- Natsch, A.; Hostettler, L.; Haupt, T.; Laue, H. A Critical Assessment of the Estrogenic Potency of Benzyl Salicylate. Toxicol. Rep. 2021, 8, 1002–1007. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of in Vitro and in Vivo Estrogenic Activity of UV Filters in Fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Fent, K. Multiple Hormonal Activities of UV Filters and Comparison of in Vivo and in Vitro Estrogenic Activity of Ethyl-4-Aminobenzoate in Fish. Aquat. Toxicol. 2006, 79, 305–324. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kawaguchi, M.; Miyazaki, K.; Nakamura, M. Estrogenic Activity of Tissue Conditioners in Vitro. Dent. Mater. 2003, 19, 341–346. [Google Scholar] [CrossRef]
- Charles, A.K.; Darbre, P.D. Oestrogenic Activity of Benzyl Salicylate, Benzyl Benzoate and Butylphenylmethylpropional (Lilial) in MCF7 Human Breast Cancer Cells in Vitro. J. Appl. Toxicol. 2009, 29, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, I.; Molina-Molina, J.M.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Real, M.; Sáenz, J.M.; Fernández, M.F.; Olea, N. Simultaneous Determination of the UV-Filters Benzyl Salicylate, Phenyl Salicylate, Octyl Salicylate, Homosalate, 3-(4-Methylbenzylidene) Camphor and 3-Benzylidene Camphor in Human Placental Tissue by LC-MS/MS. Assessment of Their in Vitro Endocrine Activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 936, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, C.; Hu, Y.; Sun, L.; Jiao, J.; Zhao, L.; Zhu, D.; Li, J.; Tian, Y.; Bai, H.; et al. The Estrogenic Potential of Salicylate Esters and Their Possible Risks in Foods and Cosmetics. Toxicol. Lett. 2012, 209, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ishii, H.; Sakuma, Y. Identification of Novel Splicing Events and Post-Transcriptional Regulation of Human Estrogen Receptor α F Isoforms. Mol. Cell Endocrinol. 2011, 333, 55–61. [Google Scholar] [CrossRef]
- Widmaier, E.P. Metabolic Feedback in Mammalian Endocrine Systems. Horm. Metab. Res. 1992, 24, 147–153. [Google Scholar] [CrossRef]
- Hiller-Sturmhöfel, S.; Bartke, A. The Endocrine System. Alcohol. Health Res. World 1998, 22, 153–164. [Google Scholar]
- Rat, P.; Olivier, E.; Tanter, C.; Wakx, A.; Dutot, M. A Fast and Reproducible Cell- and 96-Well Plate-Based Method for the Evaluation of P2X7 Receptor Activation Using YO-PRO-1 Fluorescent Dye. J. Biol. Methods 2017, 4, e64. [Google Scholar] [CrossRef] [Green Version]

| EO | Component | Relative Proportion (%) |
|---|---|---|
| niaouli | 1–8 cineol | 56.34 |
| orange | limonene | 95.18 |
| tea tree | 4-terpineol | 36.98 |
| wintergreen | methyl salicylate | 94.56 |
| ylang-ylang | benzyl salicylate | 2.16 |
| Niaouli EO | 1,8-cineol | |
|---|---|---|
| Cell viability | approx. 100% | approx. 100% |
| Progesterone | ↗ (×2.21 at 0.17 × 10−2%) | approx. ×1 |
| Estradiol | approx. ×1 | ↘ (×0.51 at 0.94 × 10−4% and 0.94 × 10−2%) |
| h-hCG | ↗ (×1.57 at 0.17 × 10−1%) | approx. ×1 |
| hPL | ↗ (×1.21 at 0.17 × 10−2%)↗ (×1.49 at 0.17 × 10−1%) | ↘ (×0.89 at 0.94 × 10−3%)↘ (×0.76 at 0.94 × 10−2%) |
| P2X7 receptor activation | approx. ×1 | approx. ×1 |
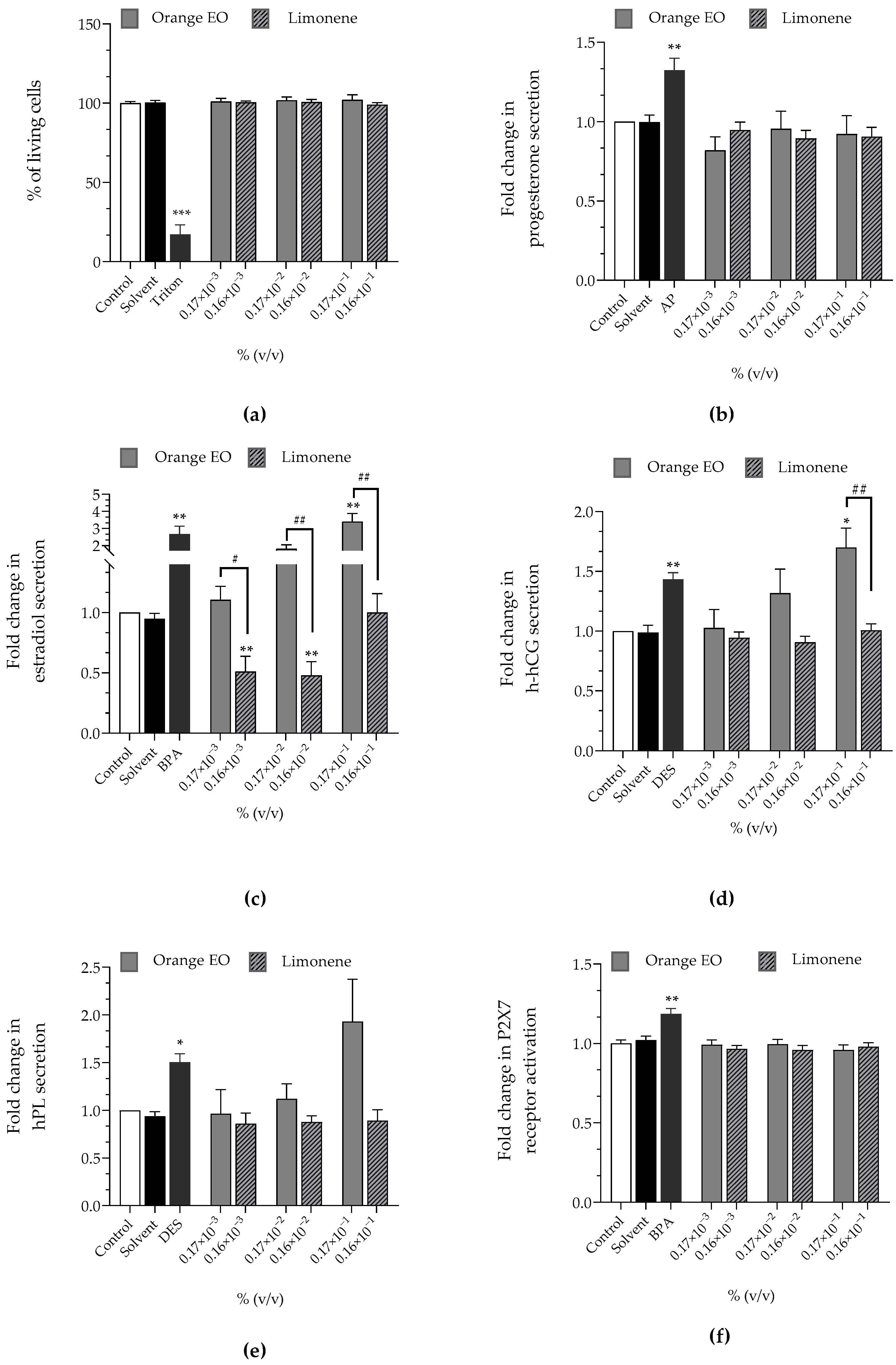
| Orange EO | Limonene | |
|---|---|---|
| Cell viability | approx. 100% | approx. 100% |
| Progesterone | approx. ×1 | approx. ×1 |
| Estradiol | ↗ (×1.81 at 0.17 × 10−2%)↗ (×3.41 at 0.17 × 10−1%) | ↘ (×0.51 at 0.16 × 10−3%)↘ (×0.48 at 0.16 × 10−2%) |
| h-hCG | ↗ (×1.70 at 0.17 × 10−1%) | approx. ×1 |
| hPL | ↗ (×1.93 at 0.17 × 10−1%) | approx. ×1 |
| P2X7 receptor activation | approx. ×1 | approx. ×1 |
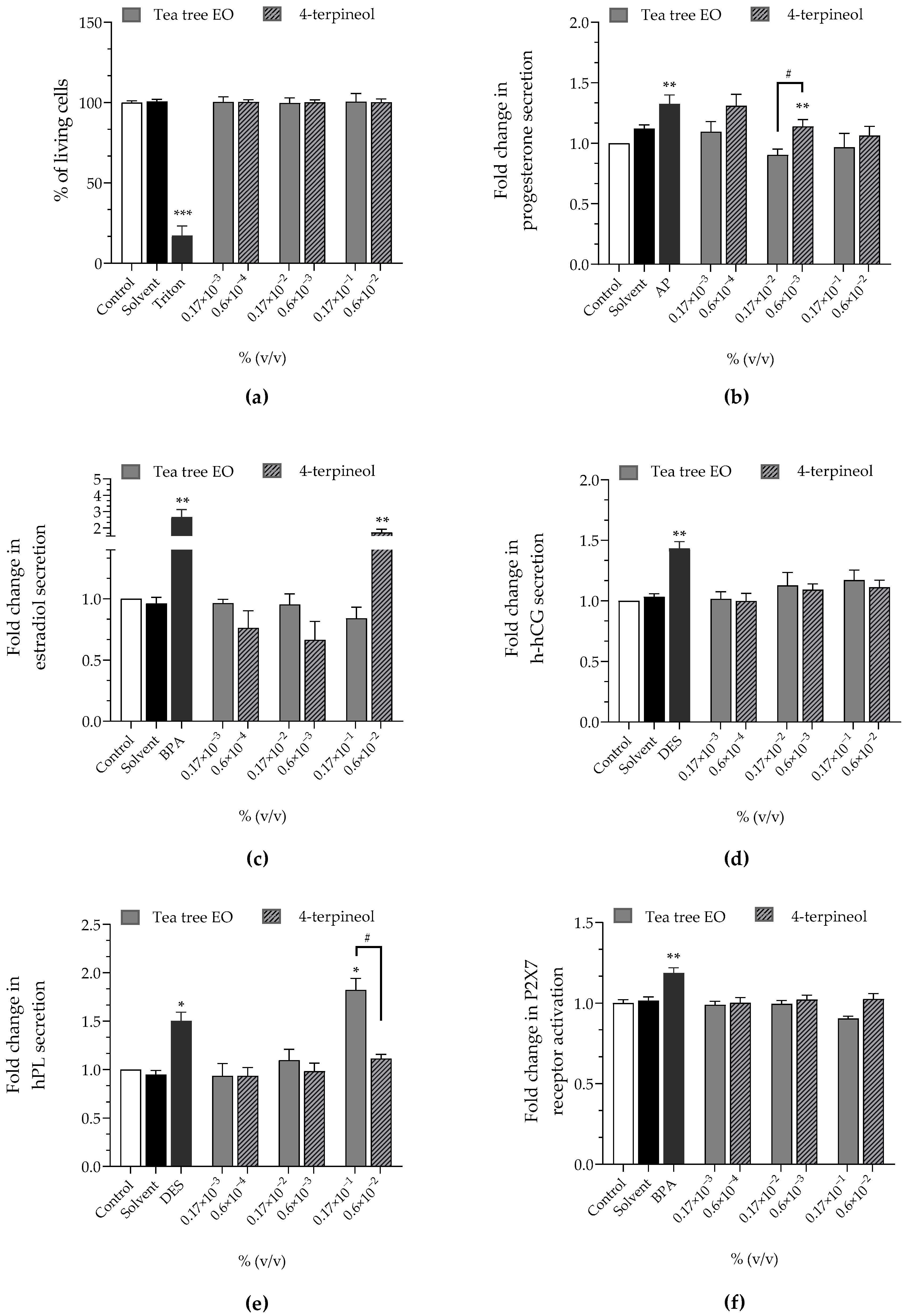
| Tea Tree EO | 4-Terpineol | |
|---|---|---|
| Cell viability | approx. 100% | approx. 100% |
| Progesterone | approx. ×1 | ↗ (×1.31 at 0.6 × 10−4%)↗ (×1.14 at 0.6 × 10−3%) |
| Estradiol | approx. ×1 | ↗ (×1.71 at 0.6 × 10−2%) |
| h-hCG | approx. ×1 | approx. ×1 |
| hPL | ↗ (×1.38 at 0.17 × 10−1%) | approx. ×1 |
| P2X7 receptor activation | approx. ×1 | approx. ×1 |
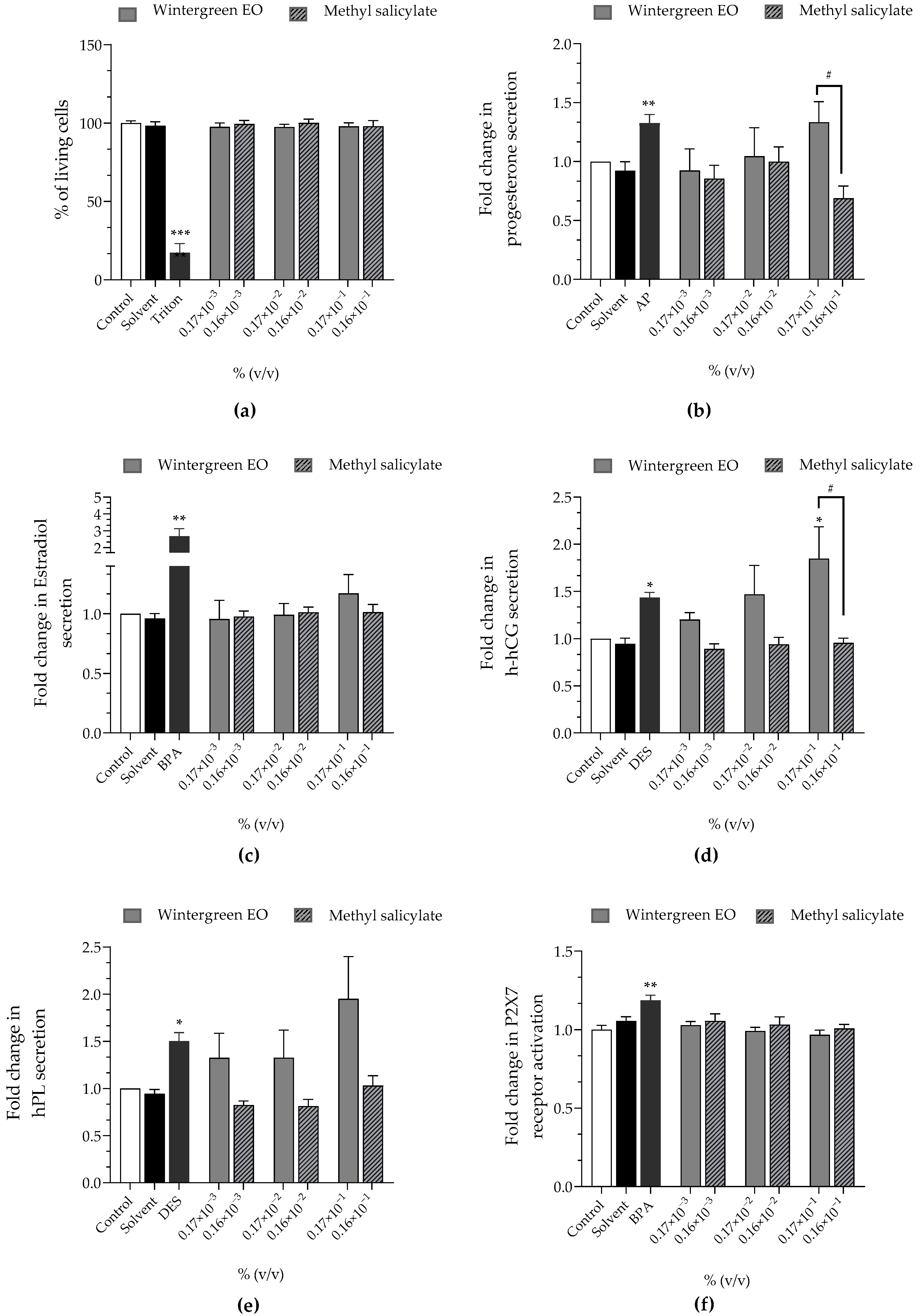
| Wintergreen EO | Methyl Salicylate | |
|---|---|---|
| Cell viability | approx. 100% | approx. 100% |
| Progesterone | ↗ (×1.33 at 0.17 × 10−1%) | ↘ (×0.69 at 0.16 × 10−1%) |
| Estradiol | approx. ×1 | approx. ×1 |
| h-hCG | ↗ (×1.47 at 0.17 × 10−2%)↗ (×1.85 at 0.17 × 10−1%) | approx. ×1 |
| hPL | ↗ (×1.32 at 0.17 × 10−3%)↗ (×1.32 at 0.17 × 10−2%)↗ (×1.95 at 0.17 × 10−1%) | approx. ×1 |
| P2X7 receptor activation | approx. ×1 | approx. ×1 |
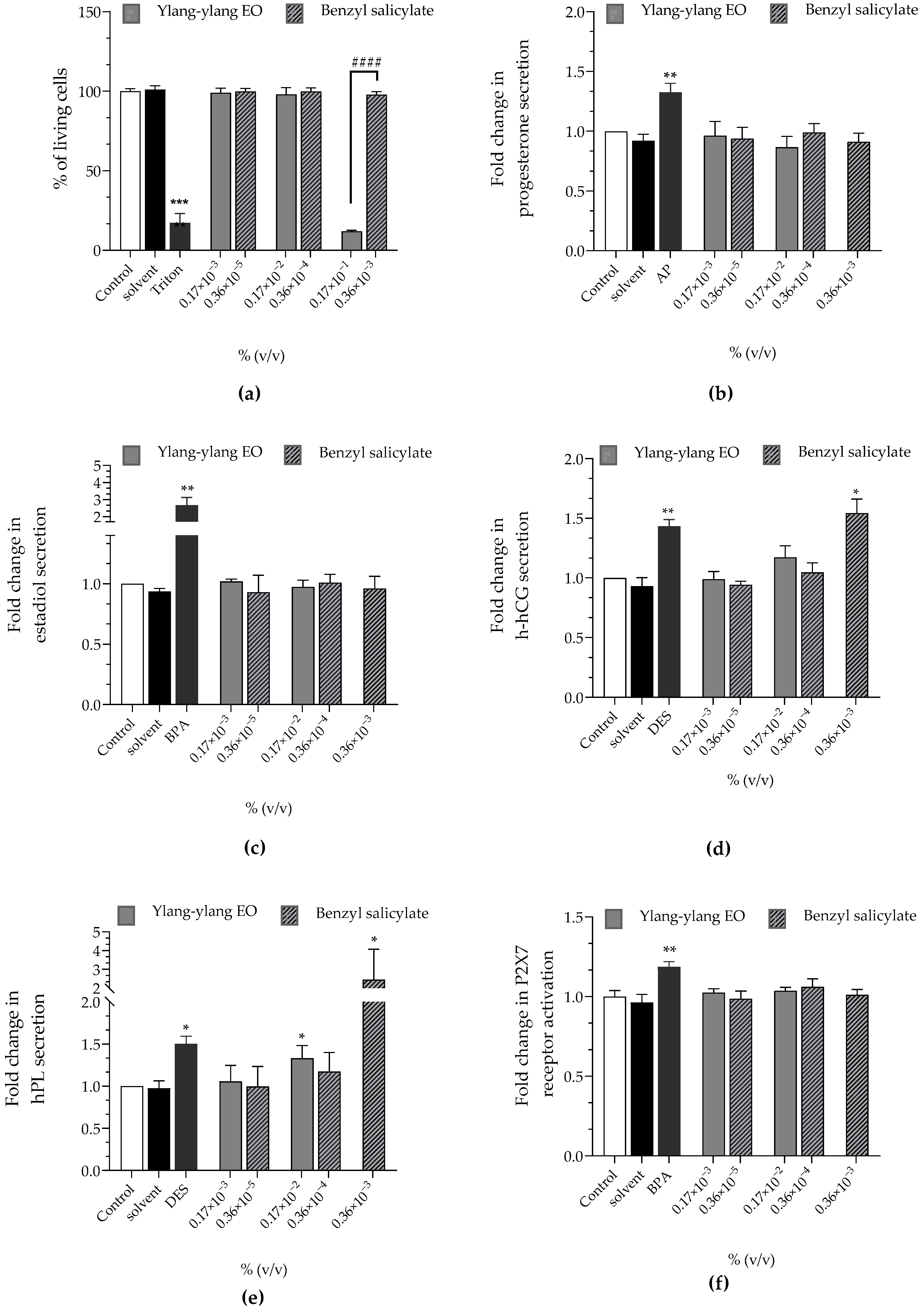
| Ylang-ylang EO | Benzyl Salicylate | |
|---|---|---|
| Cell viability | approx. 100% | approx. 100% |
| Progesterone | approx. ×1 | approx. ×1 |
| Estradiol | approx. ×1 | approx. ×1 |
| h-hCG | approx. ×1 | ↗ (×1.54 at 0.36 × 10−3%) |
| hPL | ↗ (×1.33 at 0.17 × 10−2%) | ↗ (×2.45 at 0.36 × 10−3%) |
| P2X7 receptoractivation | approx. ×1 | approx. ×1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects. Curr. Issues Mol. Biol. 2022, 44, 2794-2810. https://doi.org/10.3390/cimb44070192
Fouyet S, Olivier E, Leproux P, Dutot M, Rat P. Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects. Current Issues in Molecular Biology. 2022; 44(7):2794-2810. https://doi.org/10.3390/cimb44070192
Chicago/Turabian StyleFouyet, Sophie, Elodie Olivier, Pascale Leproux, Mélody Dutot, and Patrice Rat. 2022. "Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects" Current Issues in Molecular Biology 44, no. 7: 2794-2810. https://doi.org/10.3390/cimb44070192
APA StyleFouyet, S., Olivier, E., Leproux, P., Dutot, M., & Rat, P. (2022). Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects. Current Issues in Molecular Biology, 44(7), 2794-2810. https://doi.org/10.3390/cimb44070192






