The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Collection and Processing
2.3. DNA Extraction
2.4. PCR Amplification
2.5. Library Preparation and Sequencing
2.6. Data Preparation
2.7. Pre-Processing and Quality Control (QC)
2.8. Data Exploration
2.9. Data Analysis
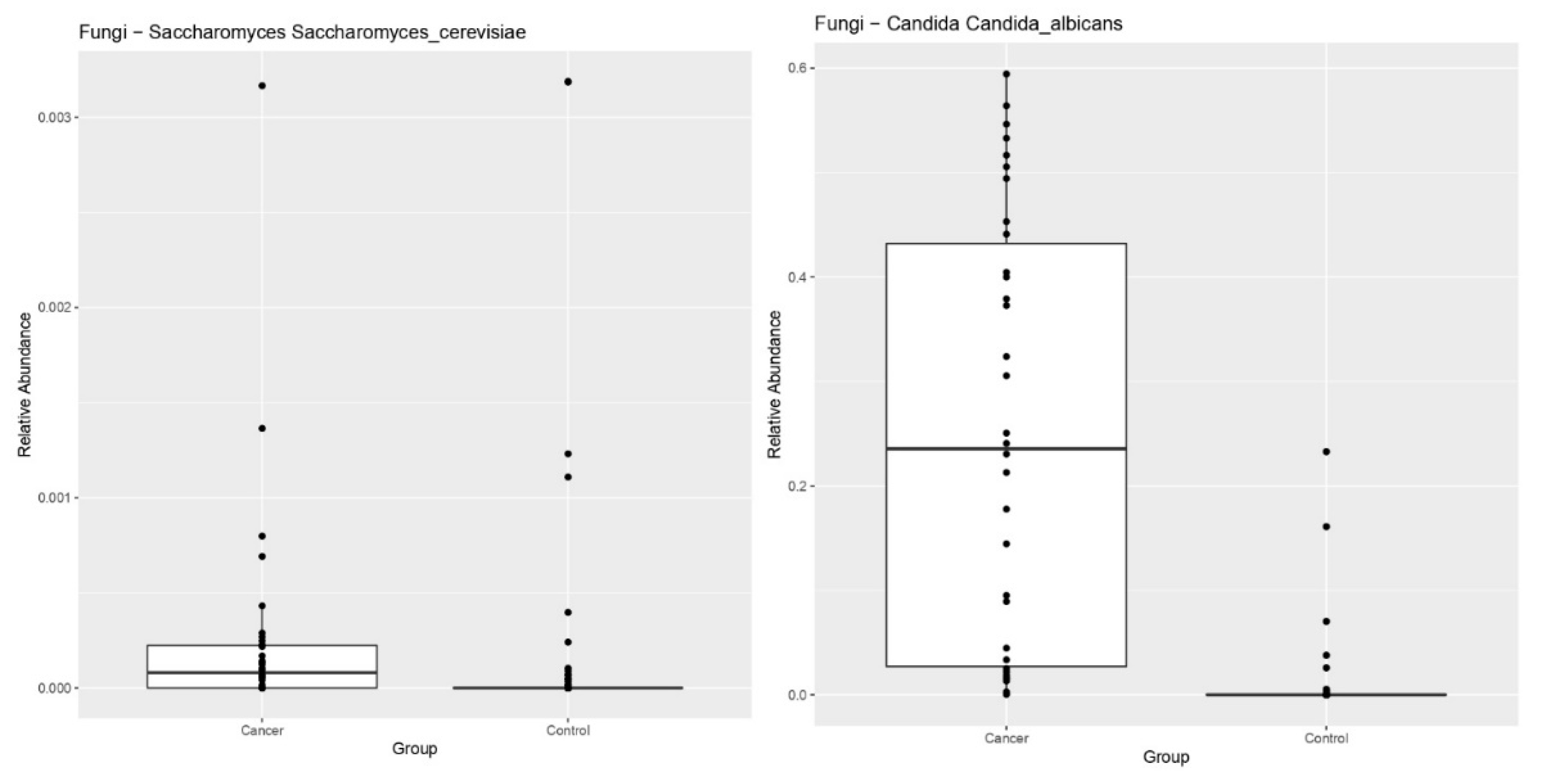
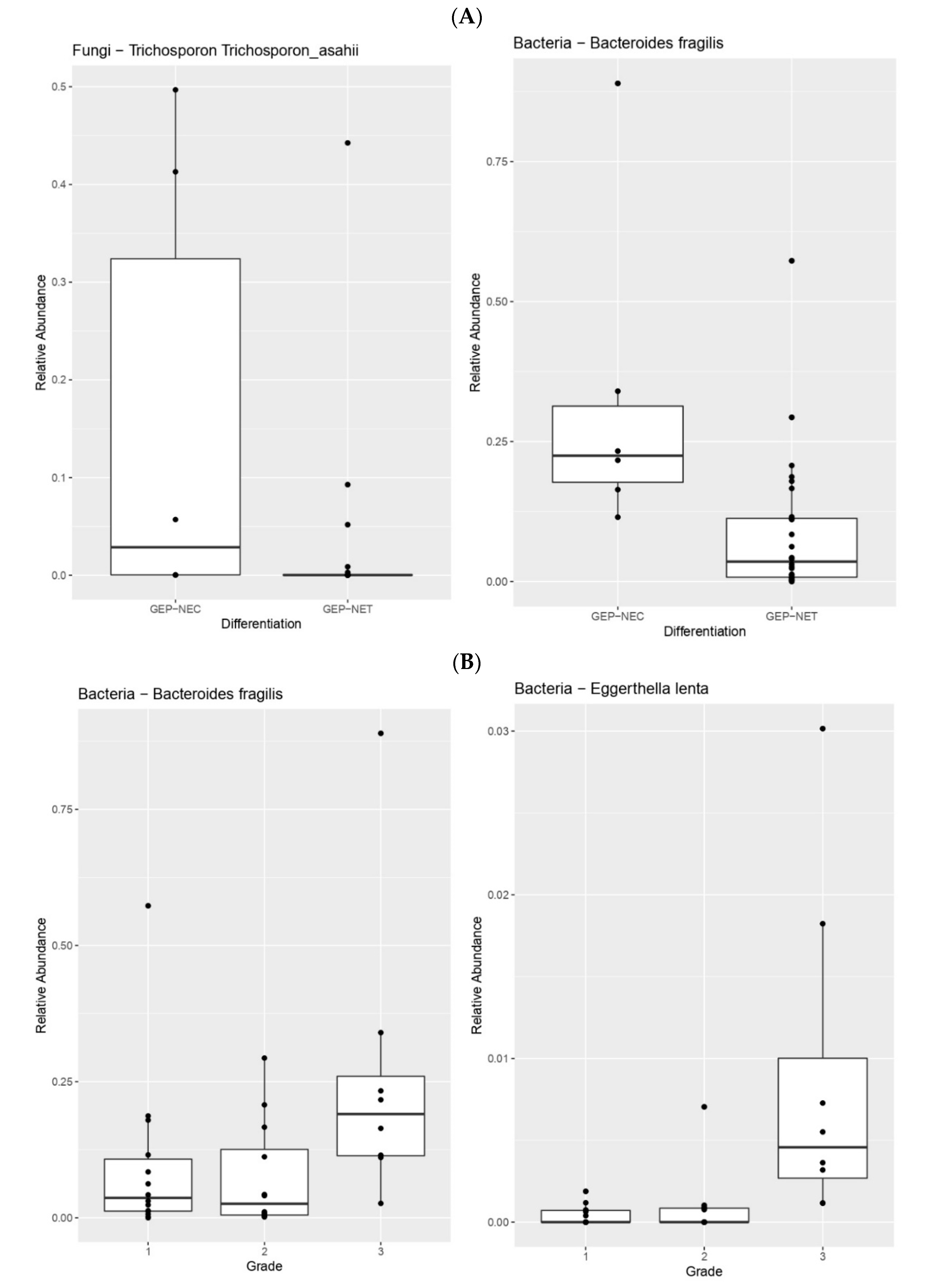
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearse, A.G.; Polak, J.M. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 1971, 12, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Sorbye, H.; Baudin, E.; Borbath, I.; Caplin, M.; Chen, J.; Cwikla, J.B.; Frilling, A.; Grossman, A.; Kaltsas, G.; Scarpa, A.; et al. Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology 2019, 108, 54–62. [Google Scholar] [CrossRef]
- Cénit, M.C.; Matzaraki, V.; Tigchelaar, E.F.; Zhernakova, A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim. Biophys. Acta 2014, 1842, 1981–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Shui, L.; Yang, X.; Li, J.; Yi, C.; Sun, Q.; Zhu, H. Gut Microbiome as a Potential Factor for Modulating Resistance to Cancer Immunotherapy. Front. Immunol. 2020, 10, 2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liang, X.F.; He, S.; Chen, X.; Wang, J.; Li, J.; Zhu, Q.; Zhang, Z.; Li, L.; Alam, M.S. Effects of High Carbohydrate Diet-Modulated Microbiota on Gut Health in Chinese Perch. Front. Microbiol. 2020, 11, 575102. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Lee, H.B.; Kim, Y.; Park, H.Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Vimal, J.; Himal, I.; Kannan, S. Role of microbial dysbiosis in carcinogenesis & cancer therapies. Indian J. Med. Res. 2020, 152, 553–561. [Google Scholar] [PubMed]
- Li, J.J.; Zhu, M.; Kashyap, P.C.; Chia, N.; Tran, N.H.; McWilliams, R.R.; Bekaii-Saab, T.S.; Ma, W.W. The role of microbiome in pancreatic cancer. Cancer Metastasis. Rev. 2021, 40, 777–789. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Dhar, R.; Pethusamy, K.; Seethy, A.; Srivastava, T.; Sah, R.; Sharma, J.; Karmakar, S. Exploring the Role of Gut Microbiome in Colon Cancer. Appl. Biochem. Biotechnol. 2021, 193, 1780–1799. [Google Scholar] [CrossRef]
- Gupta, H.; Youn, G.S.; Shin, M.J.; Suk, K.T. Role of Gut Microbiota in Hepatocarcinogenesis. Microorganisms 2019, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.S.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers 2021, 13, 3149. [Google Scholar] [CrossRef] [PubMed]
- Blagojevic, M.; Camilli, G.; Maxson, M.; Hube, B.; Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candidalysin triggers epithelial cellular stresses that induce necrotic death. Cell Microbiol. 2021, 23, e13371. [Google Scholar] [CrossRef] [PubMed]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef] [Green Version]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell Infect. Microbiol. 2020, 9, 449. [Google Scholar] [CrossRef]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. 2020, 27, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, K.S.; Dakshinamurthy, A.; Goldberg, P.; Blackburn, J.M. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS ONE 2015, 10, e0119462. [Google Scholar] [CrossRef] [Green Version]
- Yurdakul, D.; Yazgan-Karataş, A.; Şahin, F. Enterobacter Strains Might Promote Colon Cancer. Curr. Microbiol. 2015, 71, 403–411. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wan, X.; Wu, X.; Zhang, C.; Liu, J.; Hou, S. Eubacterium rectale contributes to colorectal cancer initiation via promoting colitis. Gut Pathog. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [Green Version]
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Lucas, C.; Barnich, N.; Nguyen, H.T.T. Microbiota, Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef] [Green Version]
- Vitale, G.; Dicitore, A.; Barrea, L.; Sbardella, E.; Razzore, P.; Campione, S.; Faggiano, A.; Colao, A.; Albertelli, M.; Altieri, B.; et al. From microbiota toward gastro-enteropancreatic neuroendocrine neoplasms: Are we on the highway to hell? Rev. Endocr. Metab. Disord. 2021, 22, 511–525. [Google Scholar] [CrossRef]
- Strowski, M.Z.; Cramer, T.; Schäfer, G.; Jüttner, S.; Walduck, A.; Schipani, E.; Kemmner, W.; Wessler, S.; Wunder, C.; Weber, M.; et al. Helicobacter pylori stimulates host vascular endothelial growth factor-A (vegf-A) gene expression via MEK/ERK-dependent activation of Sp1 and Sp3. FASEB J. 2004, 18, 218–220. [Google Scholar] [CrossRef]
| Characteristics | Neuroendocrine Neoplasms | Healthy Controls |
|---|---|---|
| Numbers | 34 | 34 |
| Median Age | 64 (57–70) | 67 (56–74) |
| Male % | 35% | 41% |
| Primary site | 22 small bowel | None |
| 10 pancreatic | ||
| 1 gallbladder | ||
| 1 unknown primary | ||
| Histological Differentiation | NA | |
| NET | 29 | |
| G1 | 14 | |
| G2 | 12 | |
| G3 | 3 | |
| NEC | 5 | |
| Dietary habits | Number of patients | |
| Red meat/Times per week | ||
| 0 | 4 | |
| 1 | 15 | |
| 2 | 11 | |
| 3 | 2 | |
| 4 | 2 | |
| Chicken/Times per week | ||
| 0 | 1 | |
| 1 | 8 | |
| 2 | 8 | |
| 3 | 8 | |
| 4 | 6 | |
| 5 | 2 | |
| 6 | 1 | |
| Fish/Times per week | ||
| 0 | 2 | |
| 1 | 22 | |
| 2 | 6 | |
| 3 | 1 | |
| 5 | 1 | |
| 7 | 1 | |
| Vegetables/Times per week | ||
| 2 | 1 | |
| 3 | 2 | |
| 4 | 2 | |
| 5 | 2 | |
| 7 | 25 | |
| 14 | 2 | |
| Sweets/Times per week | ||
| 0 | 2 | |
| 1 | 4 | |
| 2 | 9 | |
| 3 | 4 | |
| 4 | 1 | |
| 5 | 1 | |
| 6 | 1 | |
| 7 | 12 | |
| Antibiotic Use in last 6 months | NA | |
| Yes | 9 | |
| No | 25 |
| Microbiome Diversity | p-Value/Rho | |
|---|---|---|
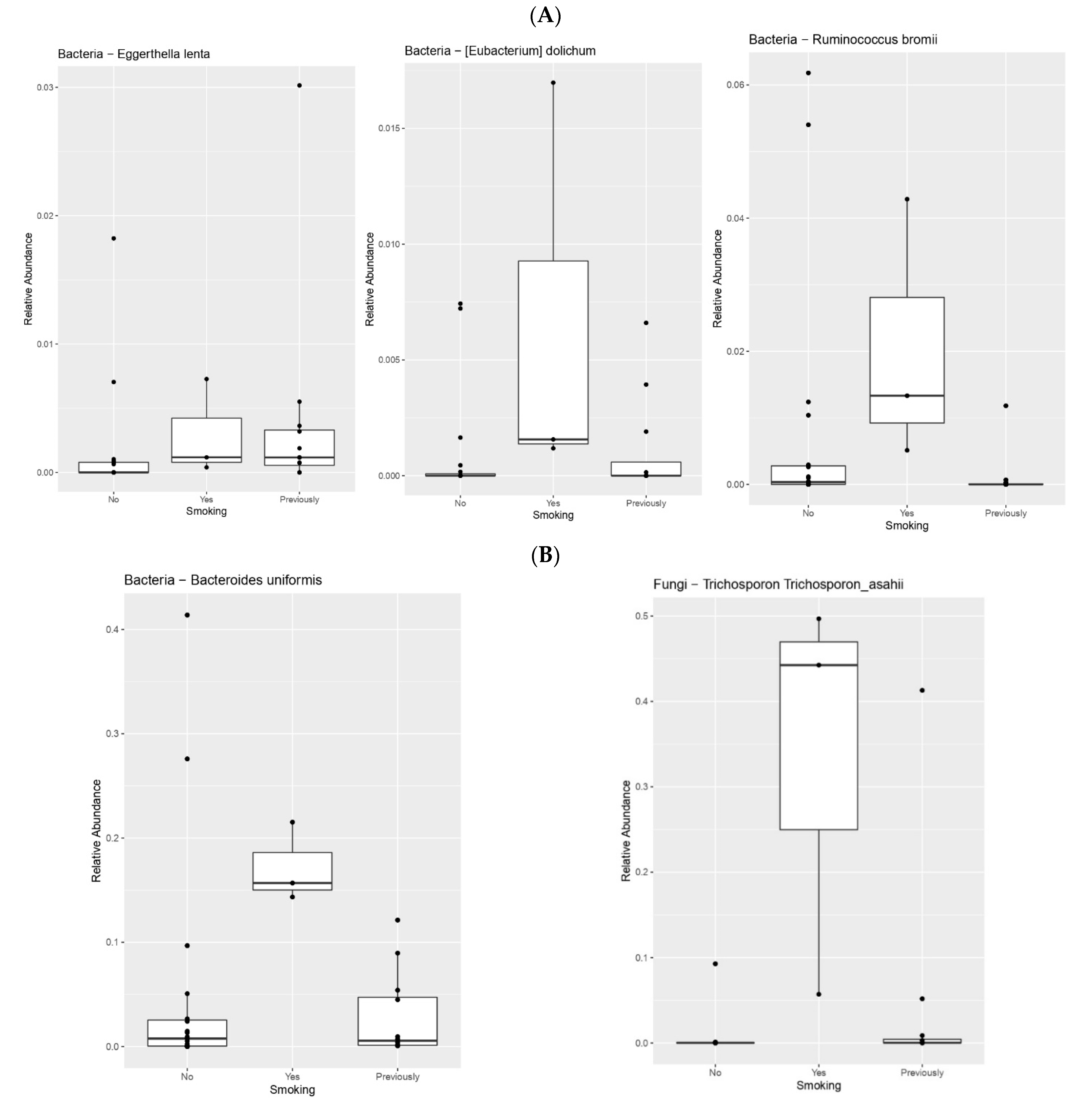
| Smoking | Eggerthella lenta | 0.03171159609 |
| Bacteroides uniformis | 0.04712580917 | |
| Parabacteroides distasonis | 0.02032498682 | |
| Ruminococcus bromii | 0.01317982135 | |
| Eubacterium dolichum | 0.04625801676 | |
| Shigella flexneri | 0.03093423221 | |
| Trichosporon asahii | 0.005986598077 | |
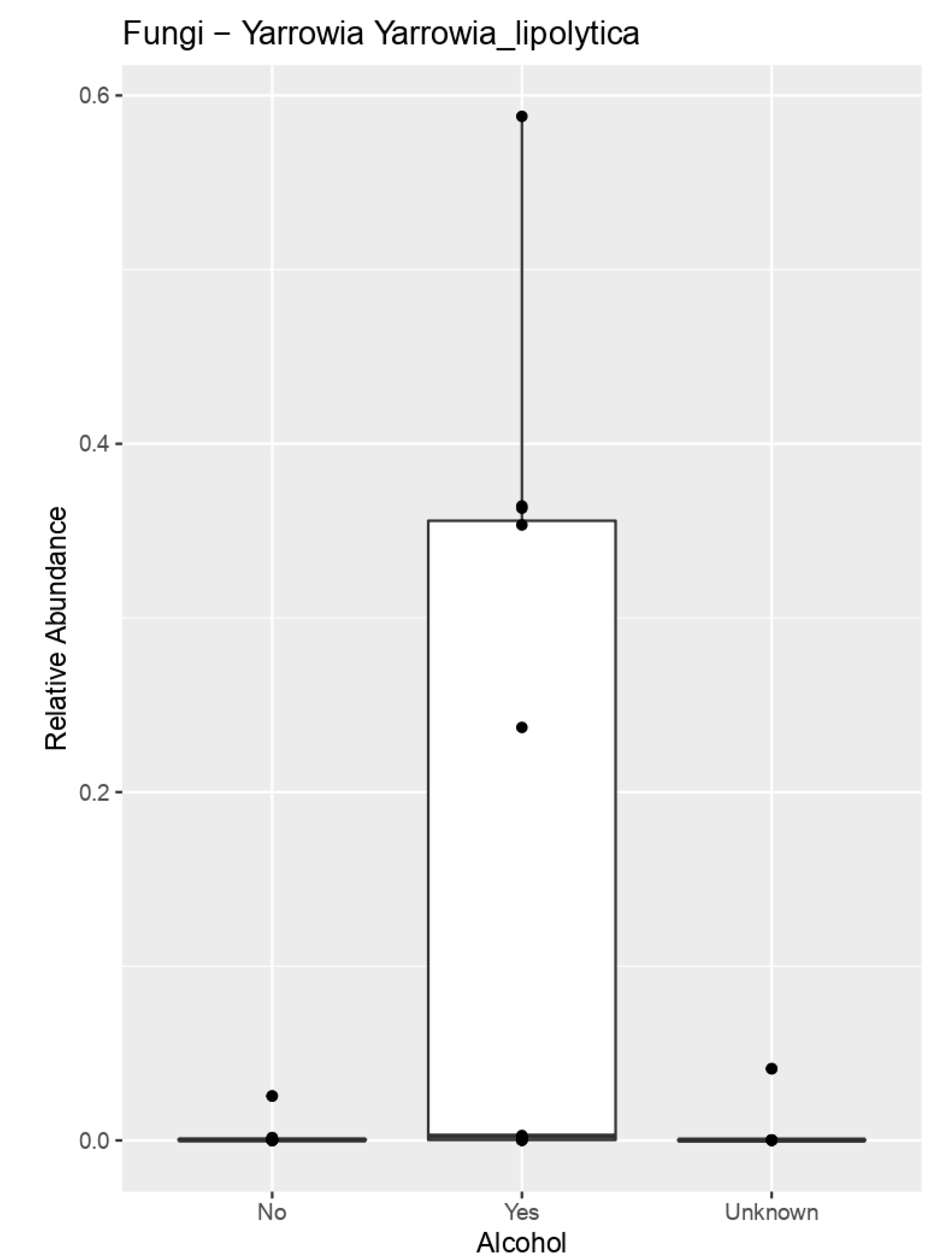
| Alcohol | Collinsella aerofaciens | 0.0370489641 |
| Yarrowia lipolytica | 0.02290117753 | |
| Trichosporon asahii | 0.03762051 | |
| Antibiotic Use | Candida albicans | 0.007496000617 |
| Candida dubliniensis | 0.004912107598 | |
| Candida Tropicalis | 0.005475873728 | |
| Candida sp. | 0.008415458738 | |
| Trichosporon mucoides | 0.01966306396 | |
| Probiotic Use | Bacteroidetes Prevotella | 0.01892686135 |
| Lactobacillus zeae | 0.01330485808 | |
| Anoxybacillus kestanbolensis | 0.04702981161 | |
| Metarhizium anisopliae | 0.002430735173 | |
| Trichosporon mucoides | 0.009765045696 | |
| Red Meat | Bacteroides eggerthii | 0.065/−0.31 |
| Staphylococcus epidermidis | 0.070/−0.31 | |
| Ruminococcus bromii | 0.077/0.30 | |
| Citrobacter gillenii | 0.030/−0.37 | |
| Fish | Eubacterium dolichum | 0.037/−0.358 |
| Cronobacter sakazakii | 0.087/−0.289 | |
| Haemophilus parainfluenzae | 0.072/−0.311 | |
| Vegetables | Roseburia faecis | 0.096/−0.28 |
| Faecalibacterium prausnitzii | 0.080/−0.304 | |
| Ruminococcus bromii | 0.066/−0.318 | |
| Veillonella parvula | 0.002/−0.496 | |
| Candida albicans | 0.0028/−0.496 | |
| Sweets | Bifidobacterium adolescentis | 0.056/0.329 |
| Blautia producta | 0.060/0.329 | |
| Agrobacterium sullae | 0.078/0.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Asa, S.L.; McCormick, T.; Al-Shakhshir, H.; Dasari, A.; Mauricio, R.; Salem, I.; Ocuin, L.M.; Bajor, D.; Lee, R.T.; et al. The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs). Curr. Issues Mol. Biol. 2022, 44, 2015-2028. https://doi.org/10.3390/cimb44050136
Mohamed A, Asa SL, McCormick T, Al-Shakhshir H, Dasari A, Mauricio R, Salem I, Ocuin LM, Bajor D, Lee RT, et al. The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs). Current Issues in Molecular Biology. 2022; 44(5):2015-2028. https://doi.org/10.3390/cimb44050136
Chicago/Turabian StyleMohamed, Amr, Sylvia L. Asa, Thomas McCormick, Hilmi Al-Shakhshir, Arvind Dasari, Retuerto Mauricio, Iman Salem, Lee M. Ocuin, David Bajor, Richard T. Lee, and et al. 2022. "The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs)" Current Issues in Molecular Biology 44, no. 5: 2015-2028. https://doi.org/10.3390/cimb44050136
APA StyleMohamed, A., Asa, S. L., McCormick, T., Al-Shakhshir, H., Dasari, A., Mauricio, R., Salem, I., Ocuin, L. M., Bajor, D., Lee, R. T., Selfridge, J. E., Kardan, A., Lee, Z., Avril, N., Kopp, S., Winter, J. M., Hardacre, J. M., Ammori, J. B., & Ghannoum, M. A. (2022). The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs). Current Issues in Molecular Biology, 44(5), 2015-2028. https://doi.org/10.3390/cimb44050136








