Effect of Porcine Placental Extract Mixture on Alcohol-Induced Hepatotoxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Groups
2.3. Experimental Procedure
2.4. Analytical Procedures
2.5. Histopathological Examination
2.6. Statistical Analysis
3. Results
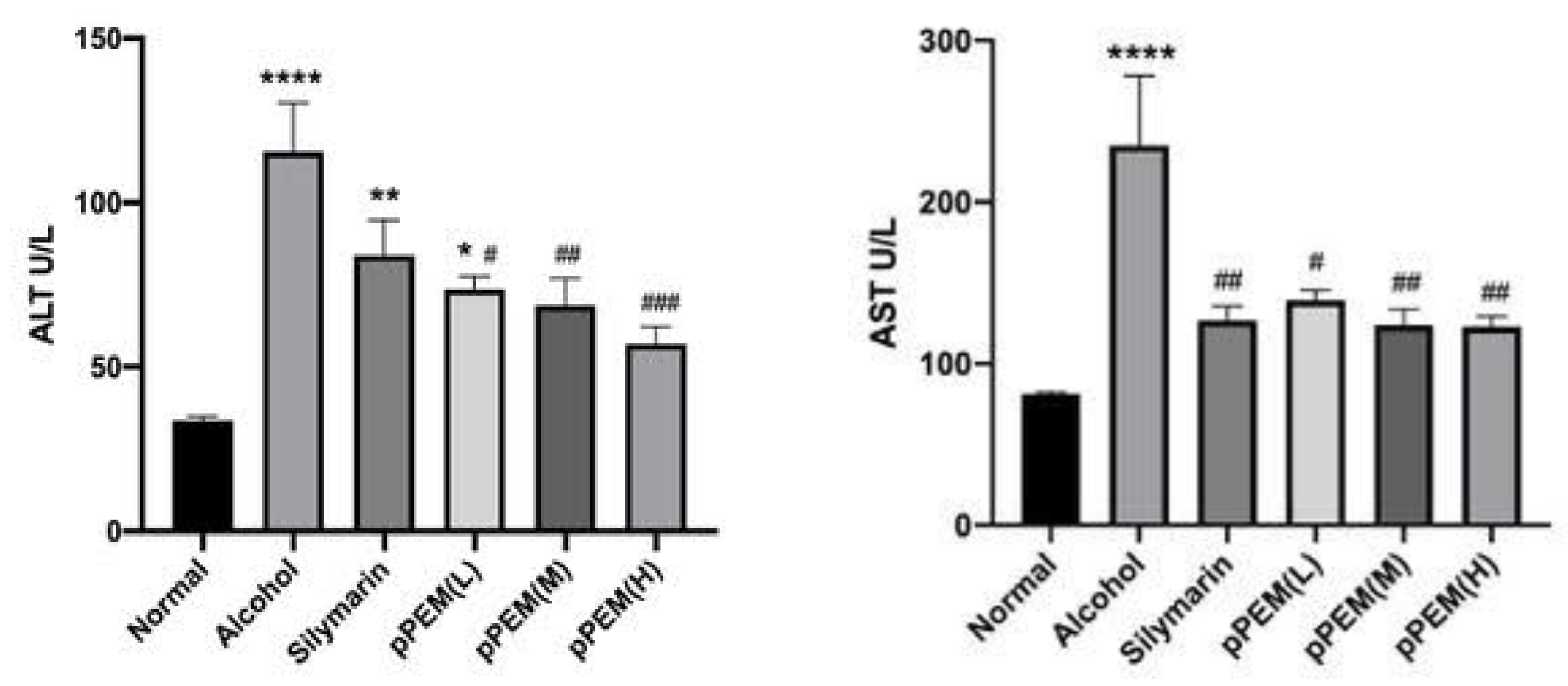
3.1. Blood Chemistry Analysis
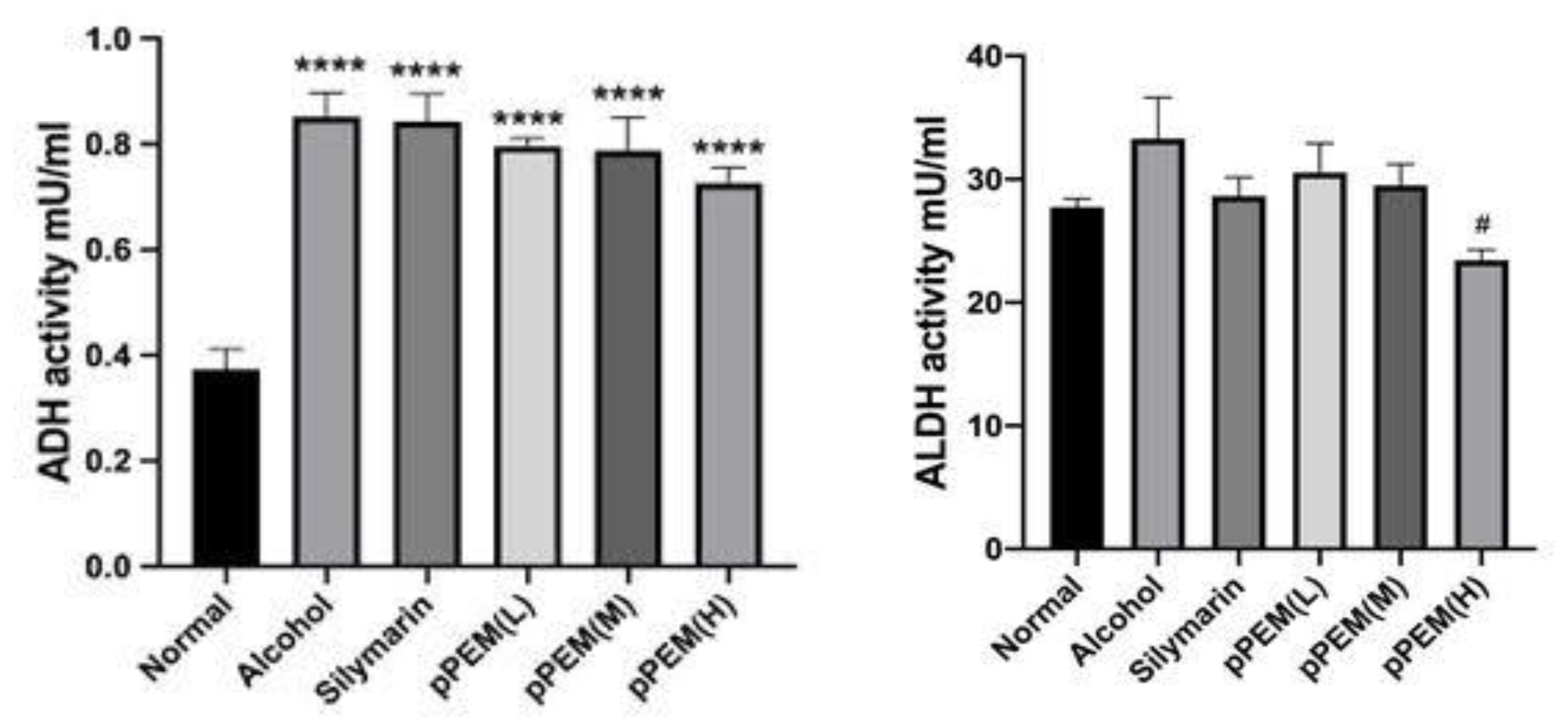
3.2. Effects of pPEM on Hepatic ADH and ALDH Activities
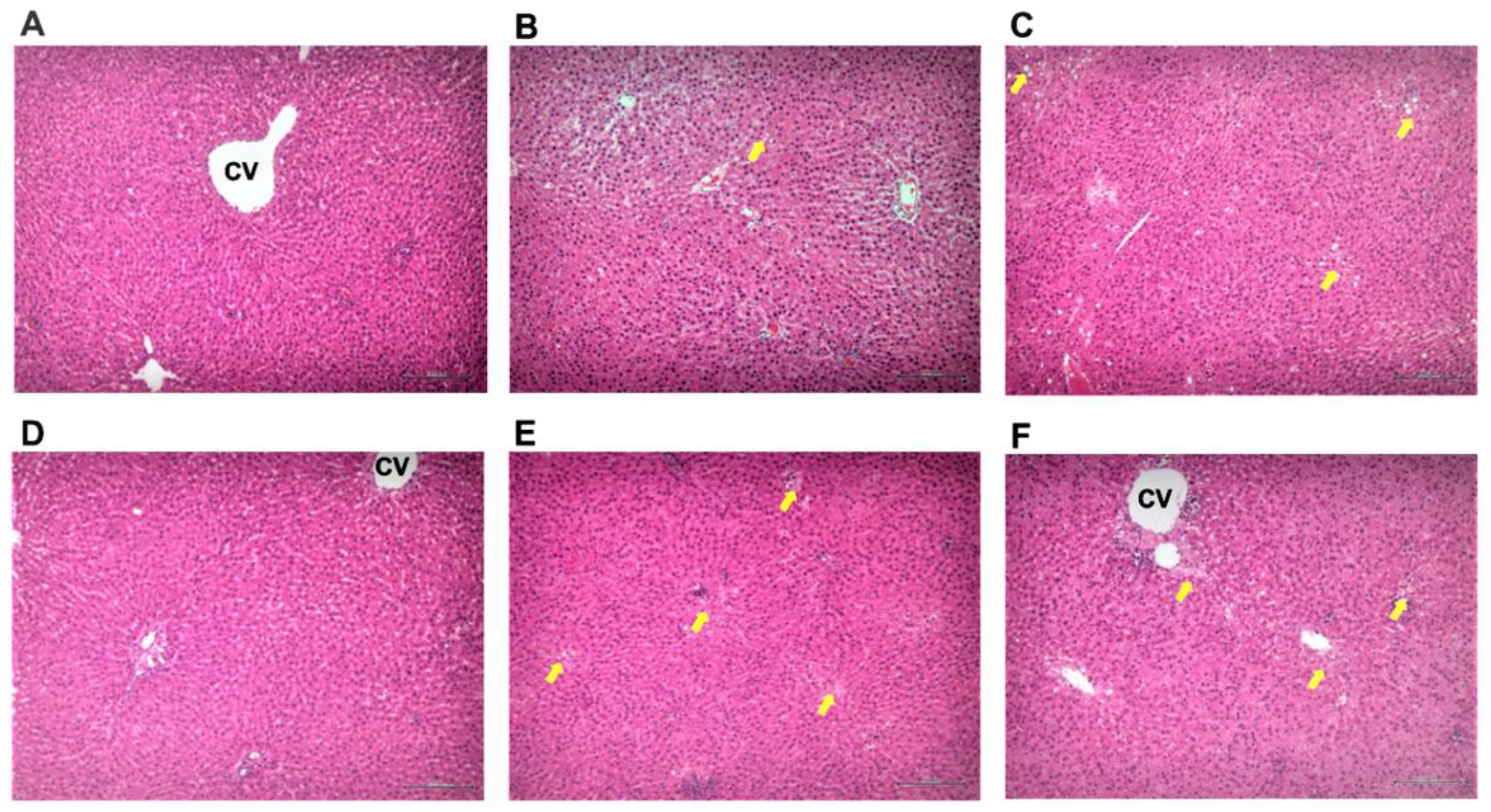
3.3. Effect of pPEM on Hepatic Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cross, J.C. Placental function of development and disease. Reprod. Fertil. Dev. 2006, 18, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; Kato, Y.; Kaku, T.; Sugiyama, Y. Human placental extract stimulates liver regeneration in rats. Biol. Pharm. Bull. 1998, 21, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Tonooka, M.; Abe, K.; Usami, K.; Kasahara, T. Comparative studies on rat primary cultured and isolated hepatocytes in the evaluation of a therapeutic agents of liver disease. Jpn. J. Pharmacol. 1986, 41, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.Y.; Lee, Y.H.; Lee, H.S. The effect of placental extract using microneedle therapy system on acne scars. Korean Soc. Aesth. Cosmetol. 2009, 7, 15–23. [Google Scholar]
- Cho, T.H.; Park, S.S.; Park, K.M. Efficacy of human placental extract pharmacopuncture into Kwanwon on fatigue in students. J. Korean Orient. Med. 2013, 34, 29–34. [Google Scholar]
- Togashi, S.; Takahashi, N.; Iwama, M.; Watanabe, S.; Tamagawa, K.; Fukui, T. Antioxidative collagen-derived peptides in human-placenta extract. Placenta 2002, 23, 497–502. [Google Scholar] [CrossRef]
- Biswas, T.K.; Auddy, B.; Bhattacharya, N.P.; Bhattacharya, S.; Mukherjee, B. Wound healing activity of human placental extracts in rats. Acta Pharmacol. Sin. 2001, 22, 1113–1136. [Google Scholar]
- Park, N.J. Safety, efficacy and limitations of medical use of placental extract. J. Korean Med. Ass. 2005, 58, 1013–1021. [Google Scholar] [CrossRef][Green Version]
- Jung, J.; Lee, H.J.; Lee, J.M.; Na, K.H.; Hwang, S.G.; Kim, G.J. Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int. Immunopharmacol. 2011, 11, 976–984. [Google Scholar] [CrossRef]
- Wu, J.; Yang, T.; Wang, C.; Liu, Q.; Yao, J.; Sun, H.; Kaku, T.; Liu, K.X. Laennec protects murine from concanavalin a-induced liver injury through inhibition of inflammatory reactions and hepatocyte apoptosis. Biol. Pharm. Bull. 2008, 31, 2040–2044. [Google Scholar] [CrossRef]
- Park, S.Y.; Phark, S.; Lee, M.; Lim, J.Y.; Sul, D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta 2010, 31, 873–879. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, K.; Lee, S.M.; Yoo, S.H.; Hwang, S.G.; Choi, J.Y.; Lee, S.W.; Kim, K.K.; Kang, H.C.; Cheon, G.J. Efficacy and safety of human placental extract for alcoholic and nonalcoholic steatohepatitis: An open-label, randomized, comparative study. Biol. Pharm. Bull. 2014, 37, 1853–1859. [Google Scholar] [CrossRef][Green Version]
- Park, Y.R.; Kim, S.R.; Jeon, G.H.; Kim, S.H.; Chae, H.D.; Kim, C.H.; Suh, C.S.; Lee, B.S.; Choi, H.; Park, H.M.; et al. Effect and safety of human placental extract on manopausal symptoms. J. Korean Soc. Menopause. 2009, 15, 178–185. [Google Scholar]
- Koike, K.; Yamamoto, Y.; Suzuki, N.; Yamazaki, R.; Yoshikawa, C.; Takano, F.; Sugiura, K.; Inoue, M. Efficacy of porcine placental extract on shoulder stiffness in climacteric women. Climacteric 2013, 13, 447–452. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Koike, K.; Takano, F.; Sugiur, K.; Suzuki, N. Efficacy of porcine placental extract on winkle widths below the eye in climacteric women. Climacteric 2014, 17, 370–376. [Google Scholar] [CrossRef]
- McGregor, C.G.; Davies, W.R.; Oi, K.; Teotia, S.S.; Schirmer, J.M.; Rosdahl, J.M.; Tazelaar, H.D.; Kremers, W.K.; Walker, R.C.; Byrne, G.W.; et al. Cardiac xenotranplantation: Recent preclinical progress with 3-month median survival. J. Thorac. Cardiovasc. Surg. 2005, 130, 844–851. [Google Scholar] [CrossRef]
- Adachi, M.; Brenner, D.A. Clinical syndromes of alcoholic liver diseases. Dig. Dis. 2005, 23, 255–263. [Google Scholar] [CrossRef]
- Zakhari, S.; Li, T.K. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology 2007, 46, 2032–3039. [Google Scholar] [CrossRef]
- Kotesh, A.; Shi, Q.Y.; Lin, H.Z.; Huang, X.; Diehl, A.M. Chronic ethanol exosure potentiates lipopolysaccharide liver injury despite inhibiting jun N-terminal kinase and caspase 3 activations. J. Biol. Chem. 2002, 44, 13037–13044. [Google Scholar] [CrossRef]
- Ceccanti, M.; Attilia, A.; Balducci, G.; Attilia, F.; Giacomelli, S.; Rotondo, C.; Sasso, G.F.; Xerouchakis, E.; Attilia, M.L. Acute alcoholic hepatitis. J. Clin. Gastroenterol. 2006, 40, 833–841. [Google Scholar] [CrossRef]
- Shukla, S.D.; Pruett, S.B.; Szabo, G.; Arteel, G.E. Binge ethanol and liver: New molecular developments. Alcohol Clin. Exp. Res. 2013, 37, 550–557. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Hajizadeh, M.; Hekmatdoost, A. Dietary supplementation in patients with alcoholic liver disease: A review on current evidence. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 348–360. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Morse, A.C.; Schulteis, G.; Holloway, F.A.; Koob, G.F. Conditioned place aversion to the “Hangover” phase of acute ethanol administration in the rat. Alcohol 2000, 22, 19–24. [Google Scholar] [CrossRef]
- Yang, C.H.; Ting, W.J.; Day, C.H.; Ju, D.T.; Yeh, Y.L.; Chung, L.C.; Tsai, F.J.; Tsai, C.H.; Tsai, Y.; Huang, C.Y. SHSST cyclodextrin complex prevents the fibrosis effect on CCl4-induced cirrhotic cardiomyopathy in rats through TGF-β pathway inhibition effects. Int. J. Mol. Sci. 2014, 15, 8037–8048. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Natta, M.V.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Sakhuja, P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J. Gastroenterol. 2014, 20, 16464–16479. [Google Scholar] [CrossRef]
- Jung, J.; Moon, J.W.; Choi, J.H.; Lee, Y.W.; Park, S.H.; Kim, G.J. Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced liver injury. Int. J. Stem Cells. 2015, 8, 79–89. [Google Scholar] [CrossRef]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denozière, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1353–1362. [Google Scholar] [CrossRef]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Maity, P.P.; Manchikanti, P.; Chaudhury, K.; Dhara, S. Silk sponges ornamented with a placenta-derived extracellular matrix augment full-thickness cutaneous wound healing by stimulating neovascularization and cellular migration. ACS Appl. Mater. Interfaces 2018, 10, 16977–16991. [Google Scholar] [CrossRef]
- Shimokobe, H.; Sumida, Y.; Tanaka, S.; Mori, K.; Kirtamura, Y.; Fukumoto, K.; Kakutani, A.; Ohno, T.; Kanemasa, K.; Imai, S.; et al. Human placental extract treatment for non-alcoholic steatohepatitis non-responsive to lifestyle intervention: A pilot study. Hepatol. Res. 2015, 45, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Kim, K.N.; Sung, E.; Lee, S.Y.; Shin, H.C. Human placental extract as a subcutaneous injection is effective in chronic fatigue syndrome: A multi-center, double-blind, randomized, placebo-controlled study. Biol. Pharm. Bull. 2016, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.D.; Das, B.C.; Hazam, R.K.; Kumar, A.; Medhi, S.; Kar, P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J. Gen. Virol. 2014, 95, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Kamiyoshi, A.; Koyama, T.; Iinuma, N.; Yamaguchi, S.; Miyazaki, H.; Hirano, E.; Kaku, T.; Shindo, T. Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells. Heliyon 2017, 3, e00416. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Bak, D.H.; Na, J.T.; Choi, M.J.; Lee, B.C.; Oh, C.T.; Kim, J.Y.; Han, H.J.; Kim, M.J.; Kim, T.H.; Kim, B.J. Anti-apoptotic effects of human placental hydrolysate against hepatocyte toxicity in vivo and in vitro. Int. J. Mol. Med. 2018, 42, 2569–2583. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.S.; Kang, S.; Park, S. Hepatoprotective effects of pig placental hydrolysates on liver damage induced rats by injecting carbon tetrachloride. J. Appl. Biol. Chem. 2012, 55, 109–115. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Kim, S.M.; Seo, J.S.; Bae, G.W.; Kim, K.N.; Kang, J.S. Effect of single-dose. Oral enzymatic porcine placental extract on pharmacokinetics of alcohol and liver function in rats. Alcohol Clin. Exp. Res. 2020, 44, 1018–1024. [Google Scholar] [CrossRef]
- Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The Protective effect of sheep placental extract on Concanavalin A-induced liver injury in mice. Molecules 2019, 24, 28. [Google Scholar] [CrossRef]
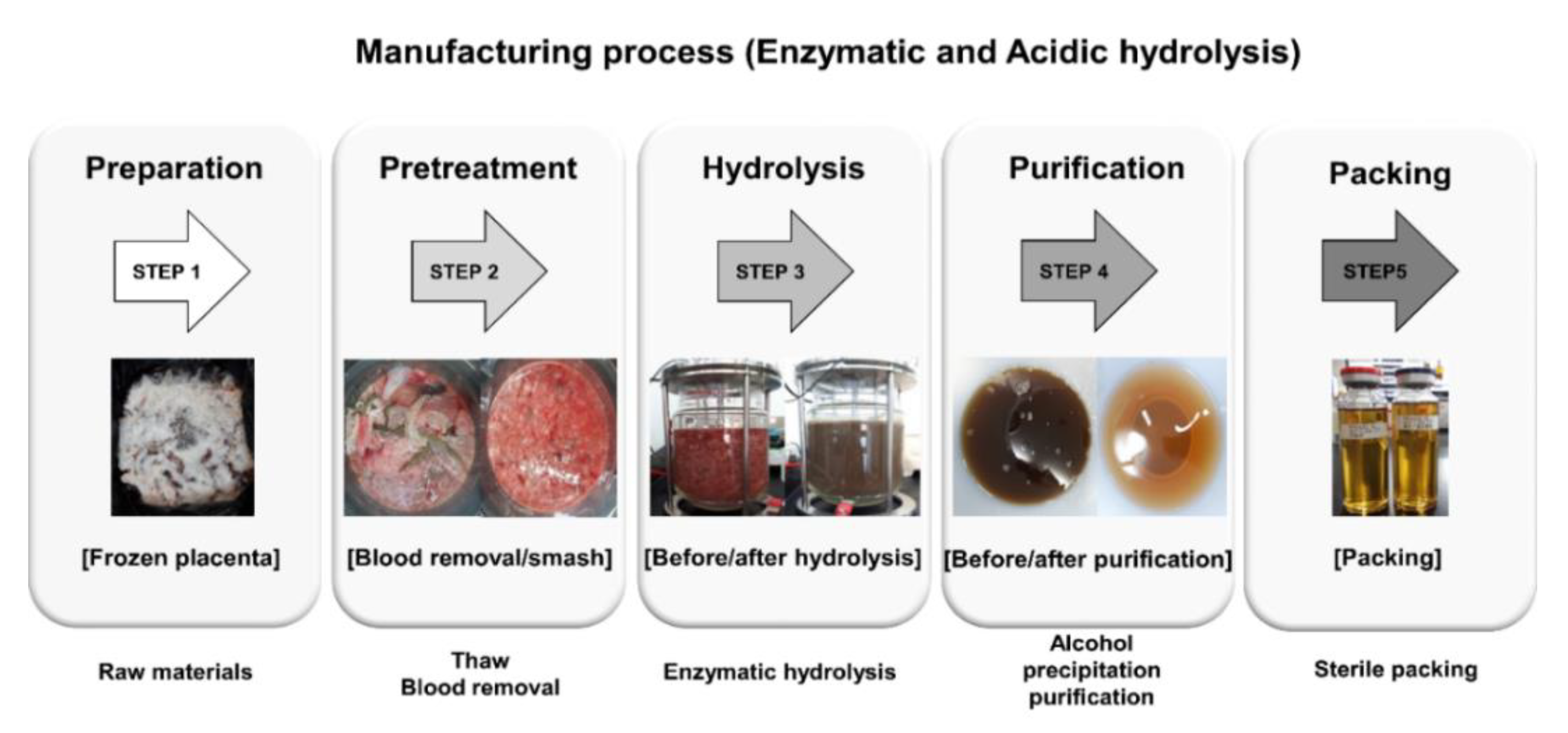
| Test Samples | Total Nitrogen * | Amino Acids | Peptides |
|---|---|---|---|
| Enzymatic Extract | 46.8 mg/g | 42.5% | 57.5% |
| Acid Extract | 35.8 mg/g | 81.2% | 18.8% |
| pPEM (E/A = 1/3) | 39.0 mg/g | 62.0% | 38.0% |
| Groups (n = 10)/ Parameters | LDH | TG | Albumin | ALP | Total Bilirubin | Cholesterol | Total Protein |
|---|---|---|---|---|---|---|---|
| (IU/L) | (mg/dL) | (g/dL) | (IU/L) | (mg/dL) | (mg/dL) | (g/dL) | |
| Normal | 820.4 ± 127.98 a | 41.23 ± 28.94 a,b,c,d | 2.24 ± 0.13 | 483.6 ± 119.02 a | 0.14 ± 0.01 a,b | 0.14 ± 0.01 a | 5.32 ± 0.31 |
| Alcohol | 1162.64 ± 904.36 a,b | 32.97 ± 10.96 a,b | 2.38 ± 0.08 | 633.73 ± 91.97 b | 0.15 ± 0.01 a,b | 0.15 ± 0.01 b,c | 5.44 ± 0.14 |
| Silymarin | 646.7 ± 340.96 a | 26.2 ± 14.27 a | 2.37 ± 0.13 | 555.63 ± 108.75 a,b | 0.14 ± 0.02 a | 0.14 ± 0.02 c | 5.24 ± 0.28 |
| pPEM (L) | 695.97 ± 220.62 a | 39.44 ± 22.68 a,b,c | 2.33 ± 0.22 | 552.89 ± 94.56 a,b | 0.15 ± 0.02 a,b | 0.15 ± 0.02 a,b,c | 5.19 ± 0.46 |
| pPEM (M) | 818.59 ± 292.64 a | 68.21 ± 26.9 d,e | 2.34 ± 0.18 | 490.23 ± 82.2 a | 0.14 ± 0.01 a | 0.14 ± 0.01 c | 5.22 ± 0.34 |
| pPEM (H) | 783.81 ± 174.14 a | 64.56 ± 28.05 c,d,e | 2.39 ± 0.12 | 568.11 ± 85.03 a,b | 0.14 ± 0.02 a,b | 0.14 ± 0.02 a,b,c | 5.32 ± 0.27 |
| Lesions/Groups | Normal | Alcohol | Silymarin | pPEM (L) | pPEM (M) | pPEM (H) |
|---|---|---|---|---|---|---|
| Lobular inflammation | 0 | 2 | 0 | 1 | 0 | 0 |
| Fatty degeneration | 0 | 2 | 1 | 1 | 1 | 1 |
| Steatosis | 0 | 1 | 0 | 0 | 0 | 0 |
| Total score | 0 | 5 | 1 | 2 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-M.; Diao, W.-J.; An, W.; Kim, H.-J.; Lim, H.-J.; Kim, K.-N.; Bae, G.-W.; Kang, J.-S. Effect of Porcine Placental Extract Mixture on Alcohol-Induced Hepatotoxicity in Rats. Curr. Issues Mol. Biol. 2022, 44, 2029-2037. https://doi.org/10.3390/cimb44050137
Kim S-M, Diao W-J, An W, Kim H-J, Lim H-J, Kim K-N, Bae G-W, Kang J-S. Effect of Porcine Placental Extract Mixture on Alcohol-Induced Hepatotoxicity in Rats. Current Issues in Molecular Biology. 2022; 44(5):2029-2037. https://doi.org/10.3390/cimb44050137
Chicago/Turabian StyleKim, Se-Mi, Wen-Jing Diao, Wen An, Hyun-Jin Kim, Ha-Jong Lim, Keun-Nam Kim, Gun-Won Bae, and Ju-Seop Kang. 2022. "Effect of Porcine Placental Extract Mixture on Alcohol-Induced Hepatotoxicity in Rats" Current Issues in Molecular Biology 44, no. 5: 2029-2037. https://doi.org/10.3390/cimb44050137
APA StyleKim, S.-M., Diao, W.-J., An, W., Kim, H.-J., Lim, H.-J., Kim, K.-N., Bae, G.-W., & Kang, J.-S. (2022). Effect of Porcine Placental Extract Mixture on Alcohol-Induced Hepatotoxicity in Rats. Current Issues in Molecular Biology, 44(5), 2029-2037. https://doi.org/10.3390/cimb44050137







