Abstract
Fruits along with vegetables are crucial for a balanced diet. These not only have delicious flavors but are also reported to decrease the risk of contracting various chronic diseases. Fruit by-products are produced in huge quantity during industrial processing and constitute a serious issue because they may pose a harmful risk to the environment. The proposal of employing fruit by-products, particularly fruit peels, has gradually attained popularity because scientists found that in many instances peels displayed better biological and pharmacological applications than other sections of the fruit. The aim of this review is to highlight the importance of fruit peel extracts and natural products obtained in food industries along with their other potential biological applications.
1. Introduction
Approximately 89 million tons of food waste is produced in the European Union, and this figure is anticipated to increase by a factor of 40 in the future [1]. Fruits and vegetables are considered to be basically used food products being either fully cooked, nominally cooked or uncooked [1]. It has been found that the processing of vegetables and fruits alone produces a notable waste of 25–30% of the total product. Furthermore, peels, pomace, rind and seeds are considered to be among the most common wastes. In spite of this, material contains valuable biologically active molecules including enzymes, carotenoids, oils, polyphenols and vitamins. In point of fact, these bioactive molecules demonstrated their significant industrial application including as a food to generate edible films along with probiotics and other industrial applications to develop value-added products [1].
It has been reported that large amounts of secondary metabolites are present in fruit and vegetable wastes and these waste materials have been studied for phenolic molecules, dietary fibers and other biologically active metabolites by extraction [1,2]. Scientific investigations revealed that phytochemicals and essential nutrients are largely present in the peels, seeds, fruits and vegetables [1]. For example, the skin of grapes, avocados, lemons along with seeds of mangoes and jackfruits comprise up to a 15% larger phenolic content than fruit pulp [3,4]. The fruit and vegetable wastes could thus be employed to obtain biologically active metabolites that could be utilized in food industries, cosmetics, food, pharmaceutical and textile industries. The proper utilization of fruit peels will not only resolve the large number of environmental problems, but this strategy will improve health through enriched food products comprising health-enhancing molecules. To the best of our knowledge no comprehensive review has been published about natural products which were isolated from fruit peels and our review mainly focus on the natural products which are purely isolated and their biological effects but not detected via mass techniques. Indeed, some minireviews have been published about the fruit peel crude extracts and biological activities or invidual reviews of fruit peels and their natural products [1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19].
2. Traditional Uses of Fruit Peels
Citrus (C) peels are not fully utilized and these waste materials contain highly valuable bioactive molecules [20]. In addition, fruits and their peels are folklorically used to treat cough, digestive problems, infection, muscle pain and skin inflammation [21,22]. These folkloric preparations are formulated from the peel, fruit and flowers [23]. Notably, peels of C. reticulata and C. unshiu are used under the trade name “Chimpi” in Japan as crude drugs. Similarly, C. aurantium dried peels are employed as the popular folkloric drug “Touhi.” [20,24]. The “tangerine”, a citrus fruit, is considered as one of the most popular foods in many countries around the world. Citrus peel, named “Chenpi” in China, has been employed as a medicine to treat gastrointestinal and respiratory diseases. In addition, the peel of a tangerine is used in drinks and baking in Western countries as an aromatic spice [25,26,27]. Citrus fruit peels are reported to be used in Chinese medicine to treat muscle pain, stomachache, cough, skin inflammation and high blood pressure [28]. Wampee (Clausena lansium) peels are used to treat bronchitis and stomachache in China and Indian folk medicine [29]. The fruit peels of wampee (Clausena lansium) are reported to be utilized for stomachic, bronchitis and as a vermifuge [29] while fruit peels of Jaboticaba (Plinia peruviana) are employed for diarrhea, skin irritation, hemoptysis and asthma [30].
3. Coumarins
Furanocoumarins citrusosides B-D (1–3) (Figure 1), oxypeucedanin (4), oxypeucedanin hydrate (5), 6′-hydroxy-7′-methoxybergamottin (6), isoimparatorin (7), bergomottin (8), 6′,7′-dihydroxybergamottin and (9) were isolated from the fruit peels of Citrus hystrix [31]. Furanocoumarins 7 and 8 exerted significant butyrylcholinesterase inhibition with IC50: 11.2 and 15.4 µM, respectively. On the other hand, furanocoumarins 4 and 6 were also active, but to a lesser extent towards butyrylcholinesterase enzyme with inhibitions of IC50: 63.0 and 23.1 µM, respectively. In addition, coumarin 1 possesses a weak inhibition with IC50: 339 µM while compounds 3, 5 and 9 were not active [31]. Coumarins 5 and 8 are also reported from the fruit peels of Citrus hystrix (Kaffir lime) and it was demonstrated that 5 exhibited iNOS production in RAW264.7 cells, lipopolysaccharide-interferon gamma-induced NO and COX-2 production in HCT116 and HT-29 cells with IC50: 22.5, 18.2, 23.2 and 22.2 μg/mL, respectively, whereas compound 8 had IC50 values of 18.6, 16.1, 17.5 and 18.1 μg/mL, respectively [32].
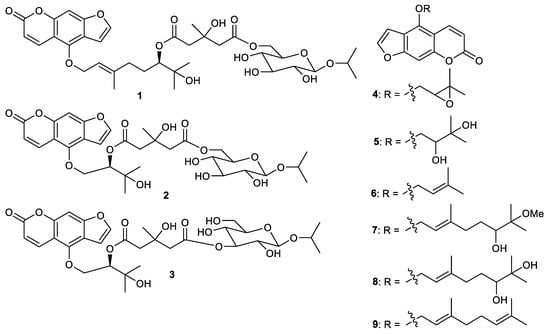
Figure 1.
Structures of coumarins 1–9.
A literature survey illustrated that the peels of C. grandis fruits are employed in traditional Chinese medicine to treat exhaustion, cancer and the common cold [33]. Coumarin 8, auraptene (10) (Figure 2), osthenol (11), isomeranzin (12), marmin (13), epoxyaurapten (14), meranzin hydrate (15), 7-hydroxy-8-(2′-hydroxy-3′-methylbut-3′-enyl) coumarin (16), bergaptol (17), columbianetin (18) and yuehgesin-C (19) are all produced by C. grandis peels. It is interesting to note that coumarins 8, 12 and 14 inhibited the generation of the superoxide anion and elastase activity with IC50: 6.02, 3.89 and 7.57 µM, respectively [34].
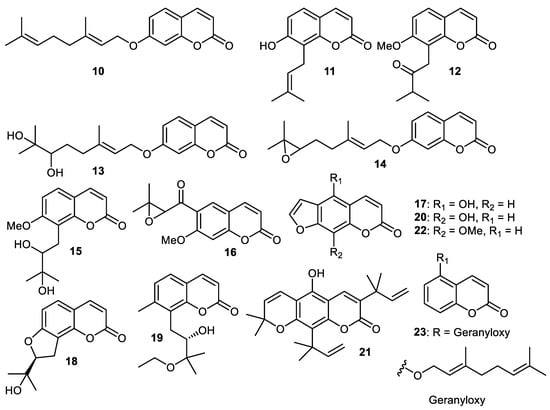
Figure 2.
Structures of coumarins 10–23.
Wampee fruit (Clausena lansium) produces 8-hydroxypsoralen (20) which exerted good antioxidant effects. It also exhibited potent cytotoxic effects towards human liver cancer (HepG2: IC50: 0.34 µg/mL), lung cancer (A549: IC50: 28.2 µg/mL) and cervical cancer (HELA: IC50: 0.013 µg/mL) [29]. Coumarin 21 was isolated from C. reticulata fruit peels [35] while coumarins 22 and 23 were produced by peels of C. reticulate [36].
Dihydromyric (24) and 2″,3″-dihydroxyanisolactone (25) (Figure 3) were reported from the peels of Clausena lansium and coumarin 24 illustrated α-glucosidase effects while compound 25 illustrated antibacterial effects towards Staphylococcus aureus [37]. The peels of Clausena lansium produced clauslactone V (26), clauslactoneW (27), clausenalansimin B (28), anisolactone (29) and wampetin (30) and all compounds demonstrated better α-glucosidase effects (IC50: 0.12 to 0.72 mM) than standard acarbose (IC50: 1.55 mM) [38].
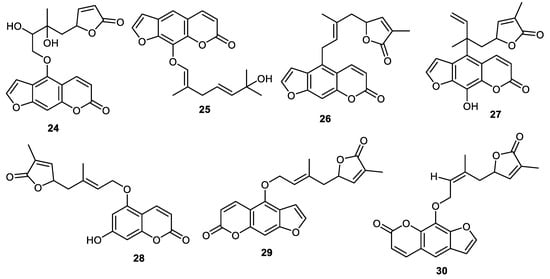
Figure 3.
Structures of coumarins 24–30.
4. Quinone and Phenolic Glycosides
Quinone 31 (Figure 4) is produced by the fruit peel of Elaeagnus rhamnoides [39] and later this same compound was also reported from Rumex aquaticus [40]. It was found that compound 31 displayed antiviral effects at a 50 µM concentration [39]. Eleutheroside B (32) and phlorin (33) are produced by the peels of C. grandis [34] and scorazanone (34) was reported from peels of Goniothalamus scortechinii [41]. 3′,5′-Di-C-β-glucopyranosylphloretin (35) was produced by the peels of Fortunella japonica [42] while arbutin (36) and malaxinic acid (37) were reported from Pyrus pyrifolia (Asian pear) [43].
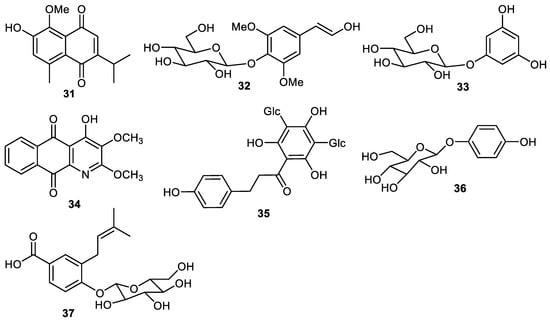
Figure 4.
Structures of quinone and phenolic glycosides 31–38.
5. Sesquiterpenes
Elaeagnus rhamnoides was reported to contain nor-sesquiterpene 39 (Figure 5) from its fruit peel and its absolute configuration was measured via the TDDFT-ECD method. In antiviral screening, compound 39 was found to cause a 2 log10 reduction in HSV-2 yield and additionally possesses antiviral effects as determined in the qPCR method [39]. A new sesquiterpene glycoside 40 was found to be produced by the fruit peels of C. limon [44]. Spathulenol (41) was isolated from the peels of Annona squamosal [45] while crytomeriodiol (42) was produced by peels of Goniothalamus scortechinii [41].
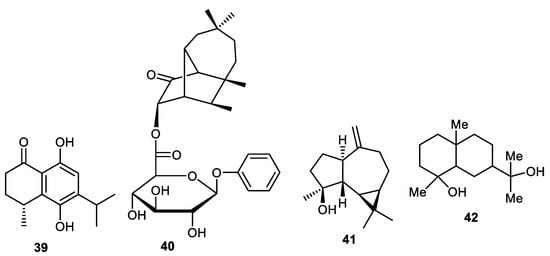
Figure 5.
Structures of sesquiterpenes 39–42.
6. Naphthalene and Betacyanins
Betacyanins, 2′-O-glucosylbetanin (43), 2′-O-apiosylbetanin (44) and 2′-O-apiosylphyllocactin (45) (Figure 6) are produced by the fruit peels of Hylocereus sp. [46]. Musizin (46), a naphthalene derivative, was isolated from the peel of Elaeagnus rhamnoides and this compound exerted antiviral effects and caused a 2.33 log10 reduction at a 12.5 µM concentration [39]. Interestingly enough, the anti-HSV-2 effect of musizin (46) was the same as that reported by Gescher et al. [47].
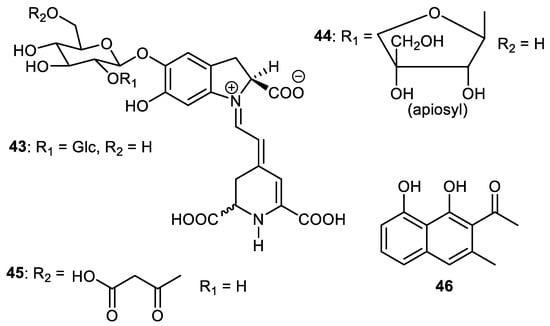
Figure 6.
Structures of naphthalene and betacyanins 43–46.
7. Alkaloids
Caulilexin C (47) (Figure 7) was reported from the peel of Elaeagnus rhamnoides and this metabolite is included in the family of the phytoanticipins. Indol glucosinolates are the biosynthetic precursors of caulilexins [39]. Previously, this compound was reported from the cauliflower [48] as well as from Brassica rapa [49]. In another report, the pyrrolizine alkaloid named punicagranine (48) was reported from the peels of Punica granatum (pomegranate) and its structure was confirmed through an X-ray analysis. Punicagranine (48) is a very unusual pyrrolizine-type alkaloid featuring both a furan-2-carbonyl and carboxylic acid. Biosynthetically, this metabolite may be considered to be constructed through the condensation of the pyrrolizine alkaloid and 2-furoic acid. In a biological assay, punicagranine (48) exerted anti-inflammatory effects with an IC50 of 22.8 μM while the same compound did not show cytotoxicity towards RAW 264.7 cells [50].
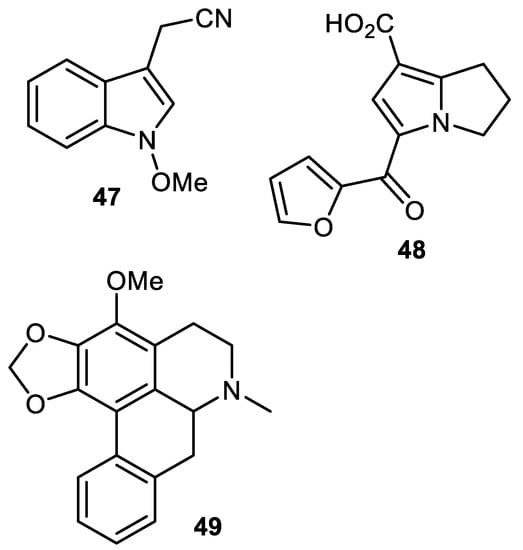
Figure 7.
Structures of alkaloids 47–49.
Justino et al. [51] demonstrated that the polyphenol-enriched fraction of the fruit peel of Annona crassiflora illustrated promising antioxidant effects and these fractions can be employed in clinical applications to treat diabetes complications. Further study indicated that ca. 15 mg kg−1 day−1 could be considered to be an initial dose to undertake all important human studies. Stephalagine (49) was isolated from the fruit peel of A. crassiflora. The EtOH extract of A. crassiflora peels inhibited pancreatic lipase (PL) with IC50: 104.5 µg/mL and notably the stephalagine showed higher PL inhibition with IC50: 8.35 µg/mL [52]. In addition, this alkaloid exerted significant antinociceptive effects in vivo [53].
Steroidal alkaloids named solasodine (50), solamargine (51) and solasonine (52) (Figure 8) were isolated from the fruit peels of Solanum melongena. Alkaloids 50–52 exerted cytotoxic effects towards HCT116 (colon cancer), HEPG2 (liver cancer), HEP2 (larynx cancer), HELA (cervix cancer) and MCF7 (breast cancer), with IC50 values ranging from 2.1 to 9.0 µM [54]. Further study revealed that alkaloids 50–52 induced potent cytotoxic effects towards two liver cancer cells (HepG2 and Huh7) which were associated with S-phase cell cycle arrest. In addition, these molecules induced potent apoptosis in Huh7 cells [55].
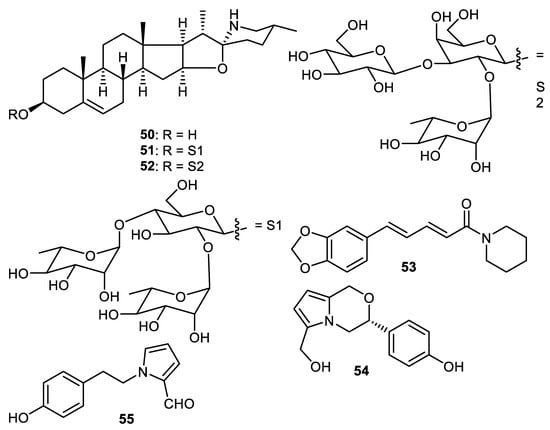
Figure 8.
Structures of alkaloids 50–55.
Piperine (53) was isolated form the Punica granatum fruit peel and inhibited cathepsin D protease with IC50: 12.0 µg/mL [56]. The pyrrole alkaloid, strychnuxin (54) was reported from Strychnos nuxblanda fruit peels and showed no effect on α-glucosidase enzyme [57]. A potent elicitor, reticine A (55), was isolated from the fruit peel of C. reticulate. and the in vivo experiments of this alkaloid suggested it had significant control effects and this molecule was more potent than the elicitor benzothiadiazole (standard compound) [35].
Carbazole alkaloids, claulansine K (56), claulansine J (57), carbazole-3-carboxylic acid (58), methyl 8-hydroxycarbazole-3-carboxylate (59), methyl carbazole-3-carboxylate (60), O-demethylmurrayanine (61) and mukonal (62) (Figure 9) were produced by peels of Clausena lansium. Alkaloid 56 illustrated α-glucosidase effects with the IC50: 0.11 mM. On the other hand, alkaloid 57 demonstrated moderate antibacterial potential towards Staphylococcus aureus [58]. Another alkaloid, clausenamide (63), was isolated from peels of Clausena lansium and illustrated α-glucosidase effects [37].
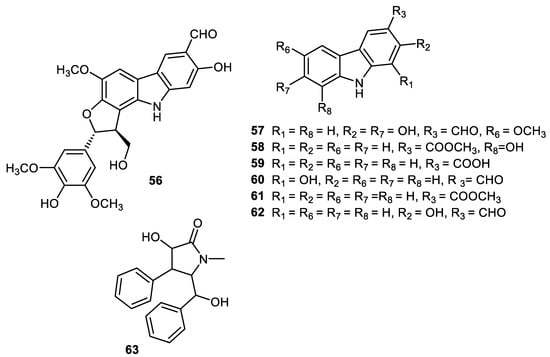
Figure 9.
Structures of alkaloids 56–63.
8. Benzoyltyramines
Benzoyltyramines, atalantums A-G (64–70) along with molecules 71–75 (Figure 10) were isolated from Atalantia monophylla fruit peels. Among these benzoyltyramines, atalantum (64) displayed the strongest cytotoxic effects towards cholangiocarcinoma cells (KKU-M156) with IC50: 1.9 μM. Notably, this compound was 4.7-fold more active than the standard ellipticine (IC50: 9.9 μM). On the other hand, atalantum A (64) demonstrated potent cytotoxic effects on KKU-M214 cells (IC50: 3.0 μM) and this activity was slightly higher than another standard 5-fluorouracil (IC50: 3.76 μM). In addition, benzoyltyramines 64 and 70 exhibited cytotoxicity towards KKU-M214 cells, with IC50: 8.44 and 7.37 μM, respectively. In addition, molecules 65, 67 and 74 displayed significant effects on KKU-M213 cells with IC50: 2.3, 5.6 and 2.7 μM, respectively. The effects of these compounds were higher than ellipticine (IC50: 6.5 μM). In contrast, molecule 66, which featured the longest hydrocarbon chain, exerted weak cytotoxic effects (IC50: 12–29 μM) [59].
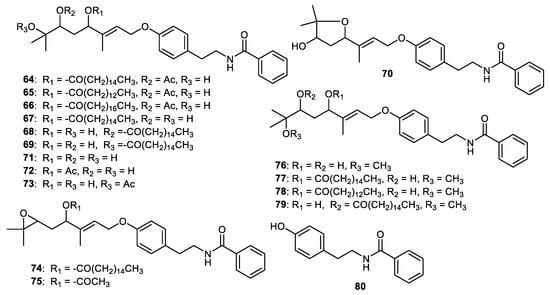
Figure 10.
Structures of alkaloids 64–80.
Among the diols 67–69, molecule 67 possessed potent cytotoxic effects toward the KKU-M156 and KKU-M213 cells (IC50: 2.8 and 5.6 μM) while molecule 68 displayed significant effects on KKU-M156 and KKU-M214 (IC50: 1.9 and 8.4 μM, respectively). On the other hand, compound 69 demonstrated weak effects towards three cancer cells (IC50: 16.1–31.4 μM) and these findings indicated that the palmitoyloxy group’s regiochemistry plays a crucial role. Concerning molecules 64 and 67, the authors proposed that the C-6 acetate group enhances the cytotoxic effects ca. 9-fold toward the KKU-M214 cells. In addition, the 6,7-dihydroxy analog 67 displayed ca. 7- and 11-times stronger effects compared to the corresponding 6-acetoxy analog (compound 64) towards KKU-M213 and KKU-M156 cells, respectively. The cytotoxic effects of compound 68 displayed a 1.42-fold stronger effect towards KKU-M156 cells than compound 67. Interestingly, diols 67 and 68 were selective towards KKU-M156 cells, whereas diol 69 exerted weaker effects. The above findings suggested that the relative position of the hydroxy groups could play a critical role in the degree of cytotoxicity. Comparing the cytotoxic effects of compounds 74 (IC50: 2.7 μM) and 75 (IC50: 11.1 μM), 74 exhibited much better effects towards the KKU-M213 cell line, suggesting that the C-4 palmitoyloxy group better enhanced the effects compared to the acetoxy group [59].
Benzoyltyramines, atalantums H-K (76–79) along with atalantums D-G (67–70) and 80 were reported form the fruit peels of Atalantia monophylla. Biological studies showed that molecules 67 and 68 exerted cytotoxic effects towards cervical (HeLa), breast (MCF-7) and colon (HCT116) cancer with IC50s: ranging from 16–25 and 15–18 µg/mL, respectively. On the other hand, benzoyltyramine 69 was slightly more active than compound 67 (IC50s: ranging from 16–25 µg/mL), whereas compound 70 was slightly less active with IC50s: ranging from 20–35 µg/mL [60].
9. Flavones, Flavanone and Condensed Tannins
Flavones 81–93 (Figure 11) were isolated from the fruit peels of Wisteria floribunda and screened for PDGF-induced VSMC proliferation. Flavones 81, 88, 91 and 92 were the most promising compounds with IC50s: 3.6, 6.7, 4.3 and 4.6 µM, respectively. Of the flavones, 82–87, 90 and 93 possessed moderate inhibition (IC50s: 19.3–39.5 μM). On the other hand, compound 89 was only weakly active (IC50 > 50 μM) [61].
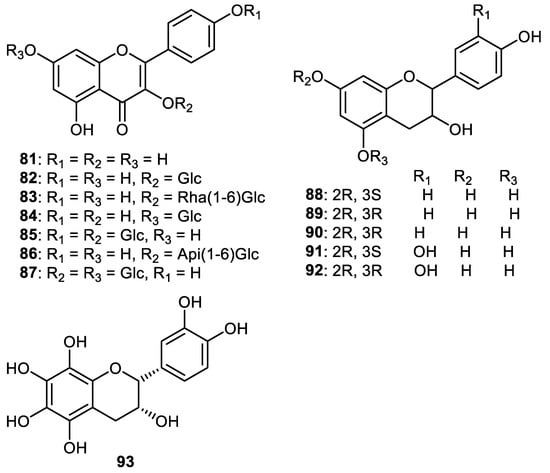
Figure 11.
Structures of flavonoids 81–93.
Hesperidin (94) (Figure 12) is largely present in citrus species such as Citrus sinensis (sweet orange peel) and tangerine (C. reticulata) [62,63]. In addition, hesperidin (94) and naringin are present in orange juice and these natural products are reported to be present in human plasma after diets involving grapefruit and orange as food sources. Hesperidin (94) exerted potential anti-inflammatory, anticarcinogenic, antimicrobial and antioxidant effects [63] and has additionally been successfully employed as a supplemental dietary agent since it has been found that a deficiency thereof causes weakness, aches and night leg cramps. Supplemental hesperidin has been used to treat excess swelling of the legs and oedema [63].
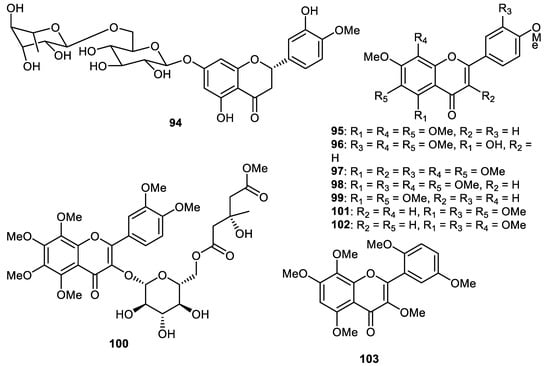
Figure 12.
Structures of flavonoids 94–103.
Hesperidin (94) along with the polymethoxy flavones 95–98 were isolated from the fruit peels of Citrus ‘Hebesu’ and tested for their biological effects. Flavones 96–98 exerted potent anti-neuroinflammatory effects through the inhibition of the expression of IL-1β mRNA [20]. Notably, polymethoxyflavonoids (PMFs) are found to be largely present in citrus plants. It is thus not surprising that citrus peels are one of the richest sources of PMFs including C. sinensis (sweet orange) and C. reticulata (mandarin) [64]. PMFs have demonstrated a plethora of biological effects viz., anticancer, antioxidative, antiviral and anti-inflammatory effects [64,65,66,67].
Tangeretin (95), in anti-arenaviral screening, demonstrated a reduction (65%) in pseudotype infectivity with EC50: 6.0 µM [53]. LASV-GP/HIV-luc infectivity was also decreased by tangeretin (95) in different cell lines, such as U-87 MG cells (glioblastoma), A549 cells (lung cancer), Vero E6 cells (African green monkey kidney cell), with EC50: 4.5, 8.8 and 2.7 μM, respectively [27]. Tangeretin (95) also had significant effects against VSV with an EC50 of 0.72 μM. Further study demonstrated that tangeretin (95) with an EC50 of 0.26 μM, exerted a significant inhibitory effect on LASV infection. In addition, flavone 95 prevented LASV-GP/HIV-luc infectivity along with pseudotype viruses such as LUJV, WWAV, SABV GTOV, MACV, CHPV and JUNV with EC50: ranging from 2.3 μM to 11.4 μM [27,68].
Nobiletin (98), isolated from citrus fruit peel, demonstrated antioxidant potentials. In addition, this flavone is also isolated from C. reticulate (mandarin oranges), C. sinesis (sweet oranges), C. miaray (Miaray mandarins) [69], C. depressa (flat lemons) [70,71], C. tangerine (tangerines), C. aurantium (bitter oranges) [72], C. unshiu (Satsuma mandarins) [73,74], C. reshni (Cleopatra mandarins) [75], C. tachibana, C. leiocarpa (Koji Oranges), C. tardiva (Natsu Mikans), C. succosa (Jimikan), C. Kinokuni (Kinokuni Mandarins), C. erythrosa (Fukushu) and C. sunki (Sunkat) [76].
Zhang et al. [62] demonstrated in vivo studies on the protective effects of flavone 98 in the mediation of cardiac hypertrophy in which it was demonstrated to inhibit aortic banding (AB)-induced cardiac hypertrophy, as measured by cardiomyocytes cross-sectional area, cardiac weight-to-body weight ratio, cardiac function and through gene expression of markers of hypertrophy. The 98 supplementation in cardiac hypertrophy inhibited NOX4 and NAPDH oxidase (NOX)2 expression and alleviated myocyte apoptosis and endoplasmic reticulum (ER) stress. Further studies suggested that the administration of flavone 98 decreased cardiomyocyte hypertrophic response in neonatal rat as stimulated through phenylephrine (PE) and decreased ER stress. However, this study indicated that 98 significantly reduced NOX2 expression but did not affect NOX4 expression in vitro. The authors suggested that the inhibition of oxidative and ER stress by flavone 98 in the cardiac muscle may indicate an effective therapy for the management of cardiac hypertrophy [77].
Nobiletin (98) has additionally been reported to possess potential anti-tumor and anti-inflammatory potential [78,79]. In addition, this flavone has been reported to inhibit NF-κB activation mediated by LPS in macrophages in mice [80]. However, it is still unknown how nobiletin (98) prevents the activation of NF-κB. It is however known that it reduces the activation of NF-κB by inhibiting its DNA-binding potential. In addition, flavone 98 blunted NF-κB transactivation and DNA-binding capacity of p50/p65 and was mediated by LPS [78]. Additional results demonstrated that it neither altered LPS-mediated phosphorylation and IκBα degradation, nor the NF-κB translocation to the nucleus. These findings, therefore, show that the inhibitory action of nobiletin (98) on proinflammatory mediator’s expression may involve the inhibition of NF-κB activity [78].
Nobiletin (98) exerted cytotoxic effects towards HT-29 with IC50: 4.7 µM [81]. This flavone showed protective effects towards a variety of cancers in in vivo studies [73,82,83,84,85,86,87]. Phytochemical investigations of the peels of C. unshiu (Hallabong) resulted in the isolation and purification of tetramethyl-O-scutellarin (99) and this material demonstrated anti-inflammatory effects with IC50: 57.4 μM. Further study revealed that this flavone inhibited the generation of proinflammatory mediators such as IL-1β, TNF-α, PGE2 and IL-6 [28]. Polymethoxylated flavonol glucoside derivatives, citrusunshitin A (100), along with flavones 101–103 were produced by the fruit peels of C. reticulate [88]. In another report, the flavones naringin (104), rhoifolin (105), naringenin 7-rutinoside (106), melitidin (107), vitexicarpin (108), chrysosplin (109) and rubranonoside (110) (Figure 13) were isolated from the peels of C. grandis [34].
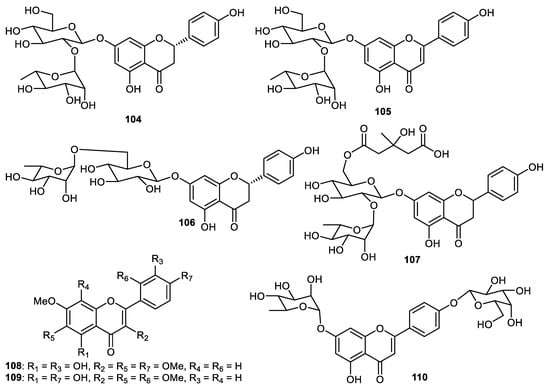
Figure 13.
Structures of flavonoids 104–110.
Paucatalinones C–E (111–113) (Figure 14), representing geranylated flavonoids, are produced by the fruit peel of Paulownia catalpifolia. It was found that these flavonoids exerted good antioxidant effects [89]. Paucatalinones F-K (114–119) along with the geranylated flavanones 120–126 are produced by the Paulownia catalpifolia fruit peel [90]. Notably, paucatalinone F (114) featured an oxygenated cyclogeranyl group while interestingly, paucatalinone H (116) contains a pyranoidal geranyl group. These flavonoids were screened for their protective potential on human umbilical vein endothelial cells (HUVECs) injury. The flavones 117, 122 and 125 exerted proliferative effects in a dose-dependent way from 1 to 50 μmol/L. On the other hand, metabolites 114, 115 and 123 at 50 μmol/L and flavones 126 at 20 and 50 μmol/L decreased their proliferative effects. These results indicated that the above flavonoids demonstrated their cytotoxic effects to HUVECs at these concentrations and flavones 116, 118 and 119 exerted better proliferative effects at only ≥20 μmol/L concentrations. Interestingly, flavonoids 114 possessed antiapoptotic effects [90].
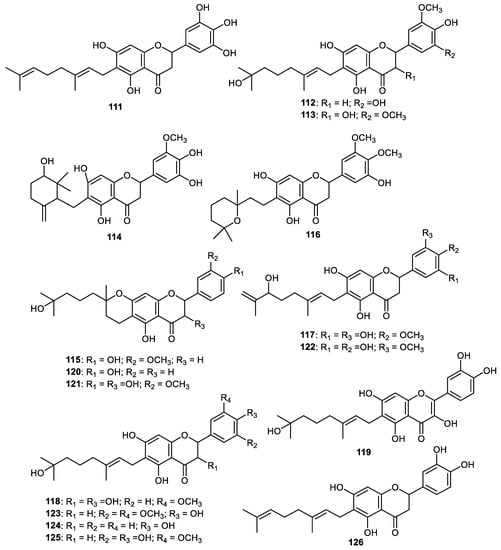
Figure 14.
Structures of flavonoids 111–126.
Flavanone 127 (Figure 15) was isolated from Citrus reticulata fruit peels [91]. It is well known that proanthocyanidins are produced by fruits, beans and nuts [92]. The proanthocyanidin dimers (−)-epicatechin gallate-(4β→8)-(−)-epicatechin (128), procyanidin B2 (129), have been reported from Pyrus pyrifolia (pear) fruit peels [93,94,95]. Pear fruit peels also produced cinnamtannins B1 (130), aesculitannin A (131) and (−)-epicatechin-(4β→6)-(−)-epicatechin-(4β→8, 2β→O→7)-(−)-epicatechin (132) [95] and these proanthocyanidin trimers displayed anticancer, antibacterial and antioxidative effects [96,97,98,99].
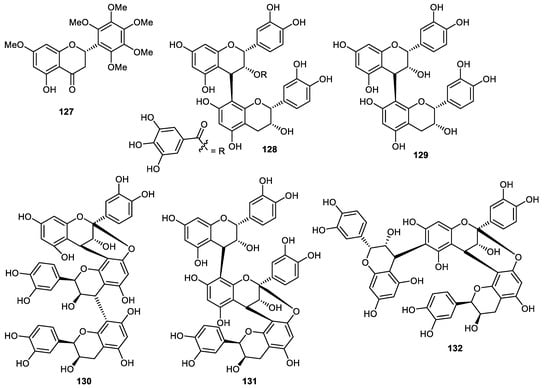
Figure 15.
Structures of flavonoids 127–132.
The pear (Pyrus ussuriensis) peels produced flavones, including rutin (133), (−)-catechin (134), tricin 4′-O-[threo-β-guaiacyl-(7″-O-methyl)-glyceryl] ether (135), tricin 4′-O-[threo-β-guaiacyl-(7″-O-methyl-9″-O-acetyl)-glyceryl] ether (136) and quercetin (137) (Figure 16). The flavones 135 and 136 illustrated antioxidant effects with IC50: 43.5 and 39.1 µg/mL, respectively [100]. Astragalin (138), quercetin 5,4′-dimethyl ether (139), isorhamnetin-3-O-glucoside (140) and isorhamnetin (141) were produced by Opuntia ficus-indica peels and flavone 139 demonstrated significant antibacterial effects towards Streptococcus pneumoniae, Stenotrophomonas maltophilia, Moraxella catarrhalis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Legionella pneumophila. In addition, flavone 140 illustrated good antibacterial potential towards Streptococcus pneumoniae, Stenotrophomonas maltophilia, Moraxella catarrhalis, Klebsiella pneumoniae and Pseudomonas aeruginosa [101].
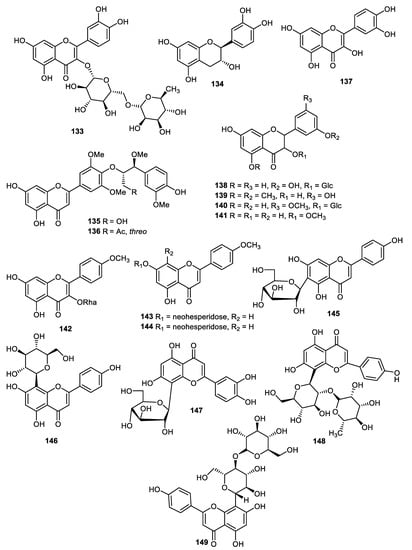
Figure 16.
Structures of flavonoids 133–149.
Kaempferide-3-O-rhamnopyranoside (142), acacetin-7-O-neohesperidoside (143) and acacetin-7-C-neohesperidoside (144) were reported from fruit peels of Fortunella japonica [42]. It was found that isovitexin (145), vitexin (146), orientin (147), vitexin-2″-O-rhamnoside (148) and vitexin-4″-O-glucoside (149) were reported from Benincasae exocarpium fruit peels and screened for antidiabetic effects. Flavones 145–149 inhibit AGE better than AMG (IC50: 178.8 μM, positive control) with IC50: 20.2, 19.5, 9.6, 29.5 and 14.5 μM, respectively. In addition, flavones 145–149 inhibit the α-glucosidase enzyme better than the standard acarbose (IC50: 49.4 μM, except flavone 149) with IC50: 48.1, 52.2, 36.1, 12.8 and 65.3 μM, respectively [102].
10. Lignans
Two lignans named syringaresinol (150) and (−)-pinoresinol (151) (Figure 17) were reported from the fruit peels of Wisteria floribunda and exerted PDGF-induced VSMC proliferation with IC50: > 50 and 46.6 µM, respectively [61]. Sesamin (152) and sesamolin (153) were produced by Strychnos nuxblanda fruit peels. Notably, metabolites 152 and 153 exhibited α-glucosidase with IC50: 447 and 215 μM, respectively, while the activities of these same lignans were higher than acarbose (standard compound; IC50: 526 μM). On the other hand, these compounds were not active as acetylcholinesterase enzymes [57]. Furofuran-type lignans named pinoresinol (154) and medioresinol (155) were isolated from Annona squamosa fruit peels and both compounds illustrated anti-inflammatory effects with IC50: 45.4 and 6.2 µM, respectively [45].
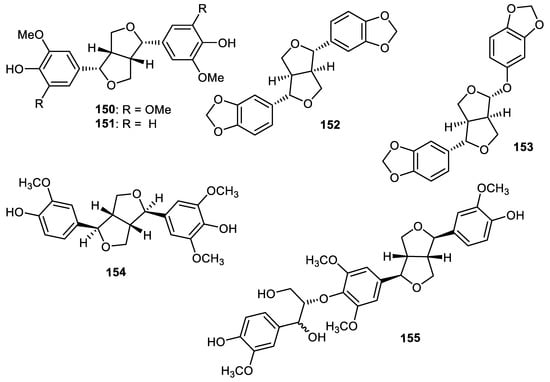
Figure 17.
Structures of lignans 150–155.
11. Hydrolyzable Tannins
Punicalagin (156) (Figure 18) was isolated from the peel of the pomegranate and exerted antifungal effects towards Trichophyton rubrum with an MIC of 62.5 μg/mL, whereas the same compound was cytotoxic on Vero cell (90%) [103]. Punicalagin (156), punicalin (157) and ellagic acid (158) were isolated from pomegranate fruit peels. Tannins 156–158 prevent protease-mediated effects in vitro through acting on HCV NS3/4A protease directly (with IC50: < 0.1 µM for tannins 156 and 157 and compound 158 has IC50: 1.0 µM). Notably, these compounds are all reported to be associated with no toxic effects side effects ex vivo and were quite safe at 5000 mg/kg when given acutely in BALB/c mice. Pharmacokinetics data indicated that compounds 156–158 are readily bioavailable [104].
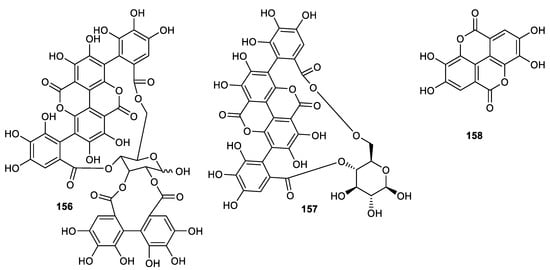
Figure 18.
Structures of tannins 156–158.
Literature revealed that tannins can form complexes with metal ions as well as target macromolecular polysaccharides and proteins [105]. Hence, these compounds may interact with enzymes through their interaction with any zinc moiety thus preventing its activity. In addition, some studies have significantly proven their selective binding of PGN, PLN and EA to NS3 protease enzymes at their substrate binding sites and this is further supported by molecular docking studies [23,104]. It is noted that molecules 156 and 157 possess galloyl residues that provide further evidence for their inhibitory effects against NS3/4A protease.
Further study revealed that compounds 156–158 demonstrated biological effects towards RNA replication of HCV and the exact mechanism of this activity is yet to be investigated. Since compounds 156–158 inhibit NS3 protease, thus affecting HCV polyprotein proteolytic processing which in turn leads to a decreased active viral RNA-dependent RNA polymerase level. This eventually causes a decreasing level of HCV RNA [104]. Of note is that tannin 158 results in apoptosis in the cell line of human prostatic cancer by decreasing antiapoptotic factors including HuR (human antigen R), HO-1 (Heme oxygenase-1) and SIRT1 (silent information regulator). It also decreases apoptotic markers including IL-6 (interleukin-6) and TGF-β (transforming growth factor -β) in cell line of prostatic cancer [106]. Compound 158 also mediates the apoptotic process in cancer cells of the pancreas through inhibiting NF-kB and the mitochondrial depolarization process [107] and demonstrated an anticancer effect through decreasing the expression of genes involved in oxidative stress [108].
12. Phloroglucinol
Myrciarone A (159) and rhodomyrtone (160) (Figure 19) are produced by the fruit peels of Myrciaria dubia and were screened for their antimicrobial effects where it was found that these compounds displayed potent antimicrobial effects towards Bacillus subtilis and B. cereus with MIC: 1.56 and 0.78 µg/mL, respectively. Notably, the activities of metabolite 159 against B. subtilis and Streptococcus aureus were equal to the standard kanamycin, whereas the effects of the same compound against B. cereus and Micrococcus luteus were 4-fold higher than the standard kanamycin (MIC: 6.25 µg/mL) [109].
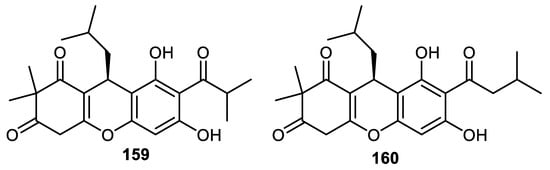
Figure 19.
Structures of phloroglucinol 159 and 160.
The activities of rhodomyrtone (160) towards B. cereus and M. luteus (MIC: 0.78 µg/mL) were 8-fold higher than kanamycin (MIC: 6.25 µg/mL). The antimicrobial effects of molecule 160 against S. mutans (MIC: 1.56 µg/mL) were equal to the standard kanamycin, whereas the same compound was 2-fold more active towards S. aureus (MIC: 0.78 µg/mL) and S. epidermidis. Myrciarone A (159) displayed the same level of activities against S. epidermidis and S. mutans with MIC: 3.13 µg/mL and its effects were 2-fold less than kanamycin (MIC: 1.56 µg/mL). Metabolites 159 and 160 were unfortunately not active towards Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, Candida albicans and Saccharomyces cerevisiae [109].
13. Triterpenoids
13.1. Onocerane Triterpenoids
The fruit peel of Lansium domesticum produced onocerane triterpenoids named lansiosides A-C (161–163) (Figure 20) along with lansic acid (164) and all were effectively shown to decrease leukotriene D4-mediated contraction of guinea pig ileum [110]. In another report, lamesticumin G (165), lansionic acid (166), 3β-hydroxyonocera-8(26),14-dien-21-one (167), compound 168 and lansiolic acid (169) were isolated from the fruit peels of Lansium parasiticum. Lamesticumin G (165) demonstrated α-glucosidase inhibition with IC50: 2.27 mM [111]. In another report, methyl lansioside C (170), lansiosides B (171) and C (172) were reported from the fruit peels of Lansium parasiticum. Triterpenoids 165 (SC50: 14.5 mM) and 171 (SC50: 13.7 mM) exerted moderate antioxidant effects, whereas triterpenoid 172 possessed weaker antioxidant effects (SC50: 23.6 mM) while compounds 170–172 were not active in α-glucosidase screening [112].
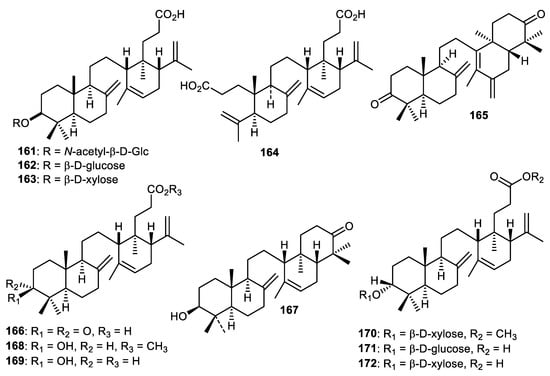
Figure 20.
Structures of onocerane triterpenoids 161–172.
Phytochemical investigation of Lansium domesticum fruit peels resulted in the isolation of an onoceranoid named onoceradienedione (173) (Figure 21) which displayed cytotoxic effects towards T47D, HeLa and A459 cells with IC50: 30.6, 32.3 and 13.7 µg/mL, respectively [113]. In another report, the same fruit peels produced another onoceranoid triterpenoid named lamesticumin A (174) which possessed cytotoxic effects towards epithelial breast cancer (T47D) with IC50: 15.6 μg/mL [114]. Tanaka et al. [115] also investigated L. domesticum fruit peels and reported finding onoceranoid triterpenoids 175–177 all of which displayed moderate toxicity towards Artemia salina. The molecule described as α,γ-onoceradienedione (178) was produced by Lansium domesticum fruit peels and possessed moderate effects towards C. albicans and A. niger [116].
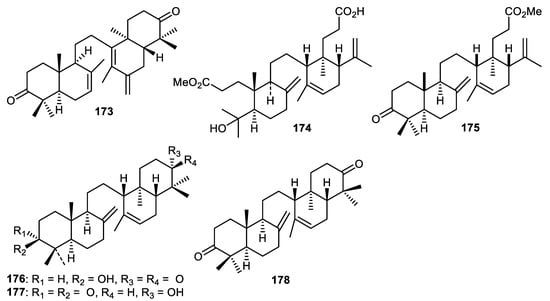
Figure 21.
Structures of onocerane triterpenoids 173–178.
13.2. Miscellaneous Triterpenoids
Lupeol (179), taraxerone (180) and taraxerol (181) (Figure 22) were reported from the fruit peels of Wisteria floribunda and were shown to exert PDGF-induced VSMC proliferation with IC50: 5.4, 7.5 and >50 µM, respectively [61]. Fruit peels of Punica granatum produced ursolic acid (182) which was demonstrated to inhibit cathepsin D with IC50: 8.3 µg/mL [56]. Friedelin (183) was isolated from C. reticulata fruit peels [35]. Cycloartane triterpenes 184–188 [117] and 189–192 [118] were produced by Musa sapientum fruit peels (banana). Lupenone (193), β-amyrin (194) and α-amyrin (195) were obtained from fruit peels of Fortunella japonica [42].
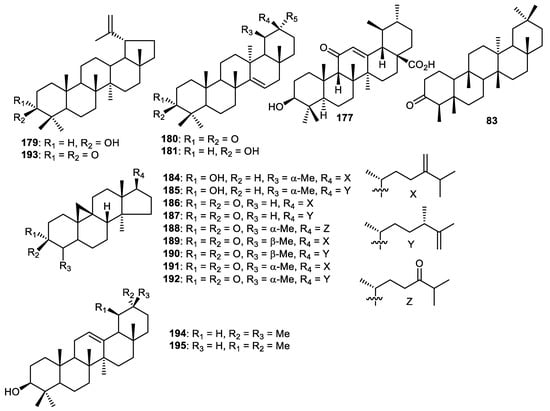
Figure 22.
Structures of triterpenoids 179–195.
14. Steroids
Fruit peels of Wisteria floribunda produced β-sitosterol (196) and β-sitosterol glucopyranoside (197) (Figure 23) and exerted PDGF-induced VSMC proliferation with IC50: 19.8 and >50 µM, respectively [46]. The steroid 197 and β-stigmasterol-3-O-β-D-glucoside (198) were produced by the fruit peels of Solanum melongena [54], whereas steroid 199 was isolated from C. reticulata fruit peels [35]. Steroids 197 and 198 demonstrated cytotoxic effects towards HCT116 (colon cancer), HEP2 (larynx cancer), MCF7 (breast cancer), HEPG2 (liver cancer) and HELA (cervix cancer) with IC50 ranging from 2.2 to 13.4 µM [54]. In another report, steroids 200–202 were reported from the fruit peel of Annona squamosal and illustrated anti-inflammatory effects with IC50: 5.0, 5.2 and 5.4 µM, respectively [45].
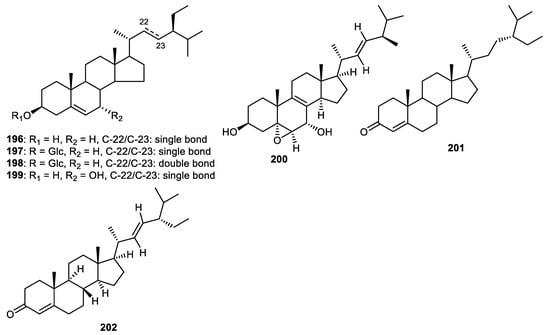
Figure 23.
Structures of steroids 196–202.
15. Peptides
Pepstatin A (203) (Figure 24) was reported from Punica granatum fruit peel [56]. This peptide is a potent inhibitor of aspartic proteases (AP) with an inhibition constant (Ki) of 0.1 nM. Notably, semisynthetic derivatives of pepstatin have led to the discovery of potent AP inhibitors. Research showed that the statyl part of this peptide is thought to be responsible for the pepsin inhibition [119]. Two cyclic peptides 204 and 205 produced by the fruit peels of C. medica had their structures established via extensive spectroscopic techniques [120].
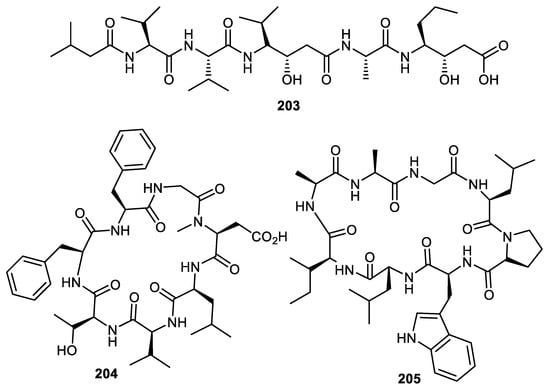
Figure 24.
Structures of peptides 203–205.
16. Miscellaneous
The tetranortriterpenoid, kokosanolide D (206) (Figure 25) was reported from Lansium domesticum fruit peels [121]. Biphenyl ether 207 was isolated from the fruit peel of Elaeagnus rhamnoides. In antiviral screening, compound 207 was found to cause a 3.49 log10 reduction in HSV-2 yield and this metabolite also possesses antiviral effects in the qPCR method [39]. Citrusoside A (208) was isolated from the peels of Citrus hystrix fruits and possesses butyrylcholinesterase inhibition activity with IC50: 376 µM [31].
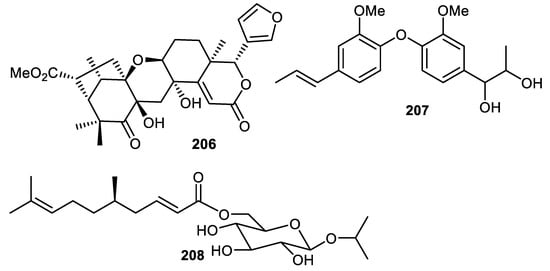
Figure 25.
Structures of compounds 206–208.
Passiflora edulis fruit peels produce edulilic acid (209) (Figure 26) that exhibited an antihypertensive effect [122]. Strychnos nuxblanda fruit peels produced tyrosol (210) which exhibited α-glucosidase effects with an IC50: 295 μM [57]. Compounds 211 and 212 were isolated from C. reticulata fruit peels [35]. Metabolites 213–216 were isolated from Pyrus pyrifolia fruit peels (pear) and 216 displayed higher antioxidant effects than caffeic acid (standard) [123].
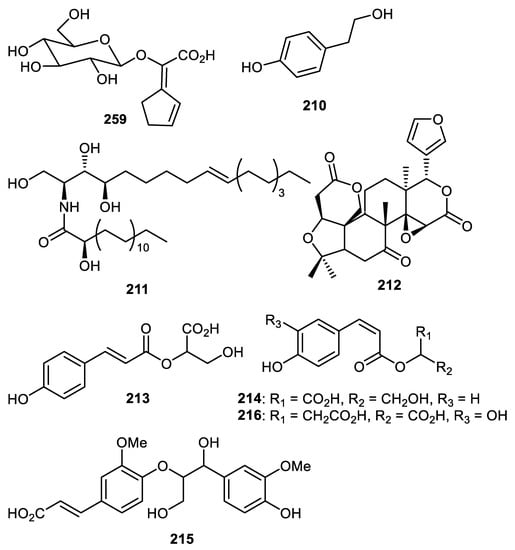
Figure 26.
Structures of compounds 209–216.
Benzaldehyde 217 (Figure 27) was produced by C. reticulate [36] while diterpenes 218–220 were produced by Annona squamosal fruit peel [45] and illustrated anti-inflammatory effects with IC50: 16.3, 19.7 and 38.5 µM, respectively [45]. The pear (Pyrus ussuriensis) peels produced orobol (221), daidzein (222) and possessed antioxidant effects with IC50: 29.1 and 88.5 μg/mL, respectively [100]. The Fortunella japonica fruit peels produced α-tocopherol (223) [42] while Pyrus pyrifolia (pear) fruit peels produced trans-chlorogenic acid (224), cis-chlorogenic acid (225) and 3,5-dicaffeoylquinic acid (226) [43].
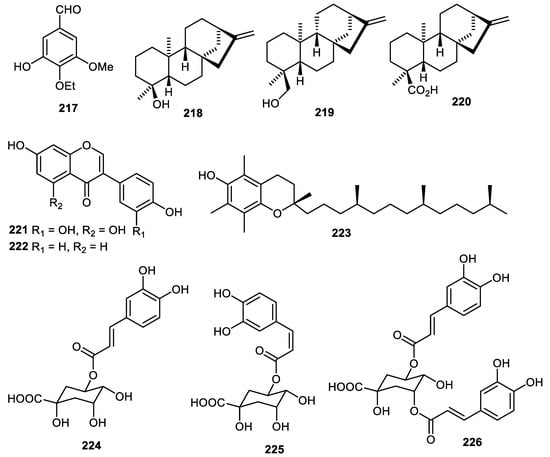
Figure 27.
Structures of compounds 217–226.
Phenolic compounds 227–232 (Figure 28) were isolated from Benincasae exocarpium peels and compounds 228, 231 and 232 inhibited AGE with IC50: 79.7, 191.7 and 54.5 µM, respectively. On the other hand, molecules 228, 229 and 231 illustrated α-glucosidase inhibition with IC50: 48.6, 310.4 and 108.6 µM, respectively [102]. Monoterpenoid 233 was obtained from peels of Clausena lansium [37] while compounds 234–238 were reported from avocado fruit peels [124] and metabolites 239–242 were obtained from Goniothalamus scortechinii fruit peel [41].
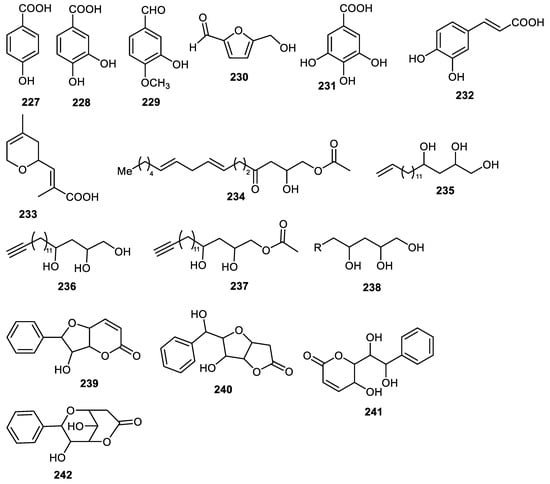
Figure 28.
Structures of compounds 227–242.
17. Fruit Peels and Food Industries
A good number of countries have directed their industry to enhance their food supply sequence effectively because that will decrease food loss and waste. In order to counteract this issue, including active agents viz., antioxidant and antimicrobial molecules or extracts, into packaging materials is considered a feasible solution in order to increase food shelf life, decrease food losses and enhance the wealth of the food industry. Natural products, biopolymers and extracts are reported to maintain the safety and biocompatibility of the material addressing consumer health concerns.
A great number of natural products have been isolated from fruit peels and these compounds illustrated antimicrobial, antioxidant and cytotoxic effects. In addition, Table 1 illustrates that many fruit peel extracts and essential oils possess antimicrobial and antioxidant effects [29,30,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163]. Utilization of fruit peels can become a possible food industry, cosmetic industry and as a source for producing beneficial drugs. With a marked antibacterial potential, fruit-peel-derived natural products, extracts and essential oils could be utilized as antibacterial agents in food processing and storage. For instance, these peel-derived materials could suppress the spoilage bacteria, mainly Pseudomonas antarctica. These peel-derived materials revealed significant antibacterial effects towards pathogenic Staphylococcus aureus which causes food poisoning.
Antioxidants from natural sources are valuable bioactive compounds with well-demonstrated potentials for use in the food industry. Lipid oxidation along with microbial growth are the major cause of spoilage of foods, such as nuts, meats, fish, sauces, milk powders and oils. Fruit-peel-derived natural products, extracts and essential oils demonstrated significant antioxidant capacity and could be employed as a substitute for synthetic antioxidants to enhance the shelf life of foods.
Pomegranate peel is reported to be a source of cellulose [164]. Cellulose is a biodegradable polymer and can be utilized in different food applications since it has been used in biomedical applications, such as carriers in drug delivery. Pectins are carbohydrate macromolecules present in citrus fruit peels, apple pomace and mango peels. Pectins are employed as an ingredient or additive for food in the preparation of jellies, jams and marmalades [164].

Table 1.
Antimicrobial and antioxidant effects of fruit peel extracts and essential oils.
Table 1.
Antimicrobial and antioxidant effects of fruit peel extracts and essential oils.
| Fruit Peels | Antimicrobial and Antioxidant Effects | Ref. |
|---|---|---|
| Wampee (Clausena lansium) | Antioxidant: Extract(s) showed better effect than BHT | [29] |
| Red dragon fruit (Hylocereus polyrhizus) E | Antibacterial: Staphylococcus aureus; Streptococcus mutans; Antifungal: Candida albicans;Aspergillus fumigatus (EO); Antioxidant: E showed good effects | [125,126,127,128,129,130] |
| Melons (Cucumis melo L.) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes | [130] |
| Passion (Passiflora edulis) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes; Antioxidant: E showed good effects | [130,131] |
| Pineapple (Ananas comosus) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes; Pseudomonas aeruginosa; Antioxidant: E showed good effects | [130,132] |
| Carica papaya | Antibacterial: Pseudomonas aeruginosa; Antioxidant: E showed good effects | [132] |
| Dragon Fruit (Hylocereus undatus) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes | [130] |
| Watermelon (Citrullus lanatus) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes; Bacillus subtilis; Pseudomonas species; Staphylococcus aureus; Klebsiella pneumoniae; Protieus mirabilis; Antioxidant: E showed significant effects | [130,133] |
| Mango (Mangifera indica) E | Antibacterial: Staphylococcus epidermidis; Streptococcus pyogenes;Antioxidant: E showed significant effects | [130,134] |
| Mangifera pajang | Antioxidant: E showed significant effects | [135] |
| Citrus reticulata | Antioxidant: EO showed good effects; Antibacterial: Escherichia coli; Staphylococcus aureus; Enterococcus faecalis; Salmonella typhi; Klebsiella pneumoniae; Pseudomonas aeruginosa;Antifungal: Candida albicans | [136,137] |
| Pomelo (Citrus maxima) | Antioxidant: E showed significant effects | [138] |
| Citrus aurantifolia (EO) | Antibacterial: Streptococcus mutans;Lactobacillus casei | [139] |
| Citrus sinensis | Antibacterial: Escherichia coli | [140] |
| Citrus reticulate | Antibacterial: Escherichia coli | [140] |
| Citrus limetta | Antibacterial: Escherichia coli | [140] |
| Citrus medica | Antibacterial: Pseudomonas aeruginosa | [141] |
| Citrus karna EO | Antibacterial: Bacillus subtilis; Pseudomonas aeruginosa | [142] |
| Annona squamosa | Antioxidant: E showed potent effects | [143] |
| Annona reticulate | Antioxidant: E showed potent effects | [143] |
| Apple (Malus domestica) | Antioxidant: E showed good effects | [134] |
| Green sugar apple (Annona squamosa) | Antioxidant: E showed significant effects | [144] |
| Purple sugar apple (Annona squamosa) | Antioxidant: E showed significant effects | [144] |
| Green star apple (Chrysophyllum cainito) | Antioxidant: E showed significant effects | [144] |
| Apricot (Prunus armeniaca) | Antioxidant: E showed good effects | [134] |
| Avocado (Persea americana) | Antioxidant: E showed significant effects | [134] |
| Grapefruit (Citrus x paradisi) | Antioxidant: E showed good effects | [134] |
| Kiwi (Actinidia deliciosa) | Antioxidant: E showed good effects | [134,145] |
| Pomegranate (Punica granatum) | Antibacterial: Escherichia coli; Proteus vulgaris; Pseudomonas aeruginosa; Klebsiella pneumonia; Staphylococcus saprophyticus; Enterococcus faecalis; Streptococcus agalactiae; Klebsiella pneumoniae; Antioxidant: E showed good effects | [146,147] |
| Banana (Musa sp.) | Antibacterial: Escherichia coli; Proteus vulgaris; Pseudomonas aeruginosa; Klebsiella pneumonia; Staphylococcus saprophyticus; Enterococcus faecalis; Streptococcus agalactiae;Antioxidant: E showed significant effects | [146,147] |
| lemon (Citrus limon) | Antibacterial: Escherichia coli; Proteus vulgaris; Pseudomonas aeruginosa; Klebsiella pneumonia; Staphylococcus saprophyticus; Enterococcus faecalis; Streptococcus agalactiae | [146] |
| Solanum melongena | Antioxidant: E showed significant effects | [148] |
| Putranjiva roxburghii | Antibacterial: Bacillus subtelis; Enterobacter xiangfangensis; Antioxidant: E showed significant effects | [149] |
| Pouteria caimito | Antibacterial: Staphylococcus epidermidis; Escherichia coli | [150] |
| Saskatoon berry (Amelanchier alnifolia) | Antioxidant: E showed significant effects | [151] |
| Rambutan (Nephelium lappaceum) | Antibacterial: Salmonella enteritidis; Vibrio parahaemolyticus; Antioxidant: E showed significant effects | [152,153] |
| Ficus carica | Antibacterial: Micrococcus luteus; Proteus vulgaris; Antioxidant: E showed significant effects | [154] |
| Jaboticaba (Plinia peruviana) | Antioxidant: E showed significant effects | [30] |
| Peach (Prunus persica) | Antioxidant: E showed significant effects | [155] |
| Garcia mangostana | Antioxidant: E showed significant effects | [156] |
| Persea americana | Antioxidant: E showed significant effects | [156] |
| Mangifera odorata | Antioxidant: E showed significant effects | [156] |
| Dimocarpus longan | Antioxidant: E showed significant effects | [156] |
| Solanum betaceum | Antioxidant: E showed significant effects | [156] |
| Annona squamosa | Antioxidant: E showed significant effects | [156] |
| Archidendron pauciflorum | Antioxidant: E showed significant effects | [156] |
| Parkia speciosa | Antioxidant: E showed significant effects | [156] |
| Caryocar brasiliense | Antioxidant: E showed significant effects | [157] |
| Leucaena leucocephala | Antioxidant: E showed significant effects | [158] |
| Loquat (Eriobotrya japonica) | Antioxidant: E showed significant effects | [159] |
| Mangosteen (Garcinia mangostana) | Antioxidant: E showed significant effects | [160] |
| Rambutan (Nephelium lappaceum) | Antioxidant: E showed significant effects | [161] |
| Tea (Camellia sinensis) | Antioxidant: E showed significant effects | [162] |
| Syzygium cumini | Antibacterial: Staphylococcus aureus; Enterococcus faecalis; Escherichia coli; Pseudomonas aeruginosa; Proteus vulgaris; Serratia marcescens; Bacillus subtilis; Bacillus cereus; Salmonella typhimurium; Enterobacter aerogenes; Antifungal: Candida albicans; Aspergillus niger | [163] |
| Coconut (Cocos nucifera) | Antioxidant: E showed significant effects | [147] |
E: Extract(s); EO: Essential oils.
18. Fruit-Peel-Based Edible Coatings/Film and Probiotics
Edible coatings are applied as thin layers on the food surface and results in longer food shelf life, retention of food characteristics, properties and functionality at low cost. Shin et al. [165] developed an apple-peel-based edible coating which was used to keep beef patties fresh. This coating was screened for antioxidant effects towards lipid oxidation along with antimicrobial effects towards yeasts, molds and mesophilic aerobic bacteria. Results demonstrated that this coating treatment inhibited lipid oxidation and effectively suppressed the growth of tested microbial entities on raw beef patties. In another study, Al-Anbar et al. [166] established an orange-peel-based edible coating and this protocol was found to enhance the shelf life of cupcakes. It was found that this coating has the ability to enhance storage age, reduce microbial growth and prevent growth of any yeast or mold during storage time. Moghadam et al. [167] established edible films which were derived from mung bean protein supplemented with pomegranate peel. Of note, this film demonstrated significant antioxidant and antibacterial effects and can be used for the packaging of food products. The combined effects of orange peel (Citrus sinensis) essential oil (OPEO) with chitosan film increased the shelf life of fresh shrimps (Parapenaeus longirostris) to 15 days [168].
In another study, the lemon peel essential oil possessed potent antimicrobial effects against Escherichia coli and Bacillus sp. and the addition of this essential oil with an edible coating (sodium alginate and cassava starch) significantly decreases the degradation of fresh strawberry and tofu [169]. In addition, nanoemulsion-based edible coatings comprising OPEO can increase the shelf life [170] of orange slices while a combination of gelatin coating and (OPEO) extended shrimp quality by about 6 days [171]. Probiotic yogurt which was prepared with pineapple peel powder enhances antibacterial activity against Escherichia coli, as well as anticancer and antioxidant effects [172]. It was discovered that the addition of banana, apple and passion fruit peel powder in probiotic yogurt enhances the growth of Lactobacillus casei, L. acidophilus and L. paracasei [173]. In addition, mango peels exhibit a positive effect in milk supplementation [174] and orange, passion fruit and pineapple peel enhance the firmness and consumer acceptability in yogurt [175].
19. Conclusions and Future Perspective
Fruit peels, which form a significant portion as a food processing by-product, have not yet been employed as a useful resource for many health supportive products. It is abundantly clear from this review that the potential possibilities to utilize these bioactive components in the food chain are available to the pharmaceutical industry. One of the possible factors for such an unused resource is an erroneous general misconception that fruit peels are unhealthy and considered an undesired waste. The current review has undoubtedly established that fruit peels are indeed a very rich resource of biologically active natural compounds and will have significant benefits for human and animal health. Investing in the fruit peel processing industry, food processors may expand their usability and flexibility to include a fruit-peel-based novel products business venture to establish a profitable existing enterprise. Producing healthy foods with natural ingredients or with fruit peels could bring so many new advantages via the reduction or elimination of food preservatives, artificial additives and replacing them with cheap natural ingredients. In addition, fruit and vegetable peels are being widely employed as food additives and in the modern era of health-conscious young people, such a venture will add to their repertoire of food buying possibilities.
Author Contributions
Conceptualization, H.H. and A.H.; validation, N.Z.M.; formal analysis, U.H. and A.R.; data curation, I.A.; writing—original draft preparation, writing—review and editing; I.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| qPCR | Quantitative polymerase chain reaction |
| HCV 2 | Herpes simplex virus 2 |
| VSMC | Vascular smooth muscle cells |
| PDGF | Platelet-derived growth factor |
| NOX | (NADPH Oxidase) |
| NF-κB | Nuclear factor kappa B |
| IL-1β | Interleukin 1 beta |
| TNF-α | Tumor necrosis factor-alpha |
| PGE2 | Prostaglandin E2 |
| IL-6 | Interleukin 6 |
References
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Cíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 09–315. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Fahmy, H.A.; Farag, M.A. Ongoing and potential novel trends of pomegranate fruit peel; a comprehensive review of its health benefits and future perspectives as nutraceutical. J. Food Biochem. 2022, 46, e14024. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Izu, G.O.; Chukwuma, M.S.; Samson, M.S.; Makhafola, T.J.; Erukainure, O.L. A review on the medicinal potential, toxicology, and phytochemistry of litchi fruit peel and seed. J. Food Biochem. 2021, 45, e13997. [Google Scholar] [CrossRef]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Sawamoto, A.; Nakajima, M.; Yoshimura, M.; Igase, M.; Fukuda, N.; Tamai, T.; Yoshida, T. Isolation and characterization of neuroprotective components from citrus peel and their application as functional food. Chem. Pharm. Bull. 2021, 69, 2–10. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Umamahesh, K.; Gandhi, A.D.; Reddy, O.V.S. Ethnopharmacological Applications of Mango (Mangiferaindica, L.) Peel—A Review. Curr. Pharm. Biotechnol. 2020, 21, 1298–1303. [Google Scholar] [CrossRef]
- Gullon, P.; Astray, G.; Gullon, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate peel as suitable source of high-added value bioactives: Tailored functionalized meat products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Karle, P.P.; Dhawale, S.C. Manilkara zapota (L.) royen fruit peel: A phytochemical and pharmacological review. Sys. Rev. Pharm. 2019, 10, 11–14. [Google Scholar]
- Choubey, S. Extraction & evaluation of anti-oxidants from different fruit peels: A review. World J. Pharm. Res. 2018, 7, 363–376. [Google Scholar]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of bioactive Compounds: A Review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquezar, B.; Alos, E.; Lado, J.; Zacarias, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Belgacem, I.; Li, D.N.M.G.; Pangallo, S.; Agosteo, G.E.; Schena, L.; Abdelfattah, A.; Benuzzi, M. Pomegranate Peel Extracts as Safe Natural Treatments to Control Plant Diseases and Increase the Shelf-Life and Safety of Fresh Fruits and Vegetables. Plants 2021, 10, 453. [Google Scholar] [CrossRef]
- Parmar, H.S.; Dixit, Y.; Kar, A. Fruit and vegetable peels: Paving the way towards the development of new generation therapeutics. Drug Discov. Ther. 2010, 4, 314–325. [Google Scholar]
- Adhikari-Devkota, A.; Kurauchi, Y.; Yamada, T.; Katsuki, H.; Watanabe, T.; Devkota, H.P. Anti-neuroinflammatory activities of extract and polymethoxyflavonoids from immature fruit peels of Citrus ‘Hebesu’. J. Food Biochem. 2019, 43, e12813. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nishikawa, T.; Furukawa, A.; Itano, S.; Tamura, Y.; Hasei, T.; Watanabe, T. Antimutagenic effects of polymethoxylated flavonoids of Citrus unshiu. Nat. Prod. Commun. 2017, 12, 23–26. [Google Scholar] [PubMed]
- Zhang, C.; Bucheli, P.; Liang, X.; Lu, Y. Citrus flavonoids as functional ingredients and their role in traditional Chinese medicine. Food 2007, 1, 287–296. [Google Scholar]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Kozaki, S. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agricult. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Chai, C.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Effects of storage period and heat treatment on phenolic compound composition in dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J. Pharm. Biomed. Anal. 2011, 54, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; He, S.; Zhang, X.; Guo, J.; Chen, Q.; Yan, F.; Banadyga, L.; Zhu, W.; Qiu, X.; Guo, Y. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antivir. Res. 2018, 160, 87–93. [Google Scholar] [CrossRef]
- Hyun, J.M.; Jo, Y.J.; Kim, J.E.; An, H.J.; Choi, Y.H.; Hyun, C.G.; Lee, N.H. Tetramethyl-O-scutellarin isolated from peels of immature Shiranuhi fruit exhibits anti-inflammatory effects on LPS-induced RAW264.7 cells. Trop. J. Pharm. Res. 2017, 16, 2197–2205. [Google Scholar] [CrossRef]
- Prasad, K.N.; Xie, H.; Hao, J.; Yang, B.; Qiu, S.; Wei, X.; Chen, F.; Jiang, Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010, 118, 62–66. [Google Scholar] [CrossRef]
- Pitz, H.d.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro evaluation of the antioxidant activity and wound healing properties of jaboticaba (Plinia peruviana) fruit peel hydroalcoholic extract. Oxid. Med. Cell. Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef]
- Youkwan, J.; Sutthivaiyakit, S.; Sutthivaiyakit, P. Citrusosides A-D and Furanocoumarins with Cholinesterase Inhibitory Activity from the Fruit Peels of Citrus hystrix. J. Nat. Prod. 2010, 73, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Kidarn, S.; Saenjum, C.; Hongwiset, D.; Phrutivorapongkul, A. Furanocoumarins from Kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem. 2018, 4, 1529259. [Google Scholar] [CrossRef]
- Zhang, T.; Peng, S. Introduction to the origin and evolution of Pomelo and its distribution in China. Chin. J. Ecol. 2000, 19, 58–61. [Google Scholar]
- Kuo, P.C.; Liao, Y.R.; Hung, H.Y.; Chuang, C.W.; Hwang, T.L.; Huang, S.C.; Shiao, Y.J.; Kuo, D.H.; Wu, T.S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 22, 967. [Google Scholar] [CrossRef]
- Wang, D.; Liu, B.; Ma, Z.; Feng, J.; Yan, H. Reticine A, a new potent natural elicitor: Isolation from the fruit peel of Citrus reticulate and induction of systemic resistance against tobacco mosaic virus and other plant fungal diseases. Pest Manag. Sci. 2021, 77, 354–364. [Google Scholar] [CrossRef]
- Saleem, M.; Afza, N.; Anwar, M.A.; Ali, M.S. Aromatic constituents from fruit peels of Citrus reticulata. Nat. Prod. Res. 2005, 19, 633–638. [Google Scholar] [CrossRef]
- Deng, H.D.; Cai, C.H.; Liu, S.; Zeng, Y.B.; Mei, W.L.; He, F.; Hua, M.; Dai, H.F.; Li, S.P. A New Monoterpenoid and a New Flavonoid Glycoside from the Peels of Clausena lansium. Nat. Prod. Commun. 2016, 11, 573–575. [Google Scholar] [CrossRef]
- Deng, H.D.; Mei, W.L.; Guo, Z.K.; Liu, S.; Zuo, W.J.; Dong, W.H.; Li, S.P.; Dai, H.F. Monoterpenoid Coumarins from the Peels of Clausena lansium. Planta Med. 2014, 80, 955–958. [Google Scholar] [CrossRef]
- Rédei, D.; Kúsz, N.; Rafai, T.; Bogdanov, A.; Burián, K.; Csorba, A.; Mándi, A.; Kurtán, T.; Vasas, A.; Hohmann, J. 14-Noreudesmanes and a phenylpropane heterodimer from sea buckthorn berry inhibit Herpes simplex type 2 virus replication. Tetrahedron 2019, 75, 1364–1370. [Google Scholar] [CrossRef]
- Orban-Gyapai, O.; Liktor-Busa, E.; Kúsz, N.; Stefko, D.; Urban, E.; Hohmann, J.; Vasas, A. Antibacterial screening of Rumex species native to the Carpathian Basin and bioactivity-guided isolation of compounds from Rumex aquaticus. Fitoterapia 2017, 118, 101–106. [Google Scholar] [CrossRef]
- Abdullah, A.; Zakaria, Z.; Ahmad, F.B.; Mat-Salleh, K.; Din, L.B. Chemical Constituents from the Fruit Peel of Goniothalamus scortechinii. Sains Malays. 2009, 38, 365–369. [Google Scholar]
- Cho, J.Y.; Kawazoe, K.; Moon, J.H.; Park, K.H.; Murakami, K.; Takaishi, Y. Chemical Constituents from the fruit peels of Fortunella japonica. Food Sci. Biotechnol. 2005, 14, 599–603. [Google Scholar]
- Lee, K.H.; Cho, J.Y.; Lee, H.J.; Park, K.Y.; Ma, Y.K.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; Moon, J.H. Isolation and Identification of Phenolic Compounds from an Asian Pear (Pyrus pyrifolia Nakai) Fruit Peel. Food Sci. Biotechnol. 2011, 20, 1539–1545. [Google Scholar] [CrossRef]
- Sultana, S.; Ali, M.; Ansari, S.H.; Bagri, P. A new sesquiterpene derivatives from the fruit peels of Citrus limon (Linn.) Burm.f. Sci. Pharm. 2007, 75, 165–170. [Google Scholar] [CrossRef][Green Version]
- Dai, W.; Zhang, Y.; Liu, Y.; Jiao, S.; Zhang, M. Chemical Constituents with nitric oxide inhibition from the fruit peel of Annona squamosa. Chem. Nat. Comp. 1983, 57, 1153–1156. [Google Scholar] [CrossRef]
- Wybraniec, S.; Nowak-Wydra, B.; Mitka, K.; Kowalski, P.; Mizrahi, Y. Minor betalains in fruits of Hylocereus species. Phytochemistry 2007, 68, 251–259. [Google Scholar] [CrossRef]
- Gescher, K.; Hensel, A.; Hafezi, W.; Derksen, A.; Kühn, J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antivir. Res. 2011, 89, 9–18. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Sarwar, M.G.; Suchy, M.; Adio, A.M. The phytoalexins from cauliflower, caulilexins A, B and C: Isolation, structure determination, syntheses and antifungal activity. Phytochemistry 2006, 67, 1503–1509. [Google Scholar] [CrossRef]
- Wu, Q.; Bang, M.H.; Lee, D.Y.; Cho, J.G.; Jeong, R.H.; Shrestha, S.; Lee, K.T.; Chung, H.G.; Ahn, E.M.; Baek, N.I. New indoles from the roots of Brassica rapa ssp. Campestris. Chem. Nat. Comp. 2012, 48, 281–284. [Google Scholar] [CrossRef]
- Sun, H.Y.; Ma, N.; Pan, T.; Du, C.L.; Sun, J.-Y. Punicagranine, a new pyrrolizine alkaloid with anti-inflammatory activity from the peels of Punica granatum. Tetrahedron Lett. 2019, 60, 1231–1233. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Peixoto, L.G.; Vilela, D.D.; Caixeta, D.C.; de Souza, A.V.; Teixeira, R.R.; Silva, H.C.G.; de Moura, F.B.R.; Moraes, I.B.; et al. Hepatoprotective Properties of a Polyphenol-Enriched Fraction from Annona crassiflora Mart. Fruit Peel against Diabetes-Induced Oxidative and Nitrosative Stress. J. Agricult. Food Chem. 2017, 65, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.N.; Justino, A.B.; Martins, M.M.; Peixoto, L.G.; Vilela, D.D.; Santos, P.S.; Teixeira, T.L.; da Silva, C.V.; Goulart, L.R.; Pivatto, M.; et al. Stephalagine, an alkaloid with pancreatic lipase inhibitory activityisolated from the fruit peel of Annona crassiflora Mart. Ind. Crops Prod. 2017, 97, 324–329. [Google Scholar] [CrossRef]
- Justino, A.B.; Barbosa, M.F.; Neves, T.V.; Silva, H.C.G.; Brum, E.S.; Fialho, M.F.P.; Couto, A.C.; Saraiva, A.L.; Avila, V.M.R.; Oliveira, S.M.; et al. Stephalagine, an aporphine alkaloid from Annona crassiflora fruit peel, induces antinociceptive effects by TRPA1 and TRPV1 channels modulation in mice. Bioorg. Chem. 2020, 96, 103562. [Google Scholar] [CrossRef] [PubMed]
- Shabana, M.M.; Salama, M.M.; Ezzat, S.M.; Ismail, L.R. In Vitro and In Vivo Anticancer Activity of the Fruit Peels of Solanum melongena L. against Hepatocellular Carcinoma. J. Carcinog. Mutagen. 2013, 4, 149. [Google Scholar]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; Alshehri, O.Y.; Al-Abd, A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Luqman, S.; Dubey, V.; Thakur, J.P.; Saikia, D.; Chanotiya, C.S.; Shanker, K.; Negi, A.S. Inhibition of Cathepsin D protease activity by Punica granatum fruit peel extracts, isolates, and semisynthetic analogs. Med. Chem. Res. 2013, 22, 3953–3958. [Google Scholar] [CrossRef]
- Sichaem, J.; Ingkaninan, K.; Tip-pyang, S. A novel pyrrole alkaloid from the fruit peels of Strychnos nux-blanda. Nat. Prod. Res. 2017, 31, 149–154. [Google Scholar] [CrossRef]
- Deng, H.D.; Mei, W.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Wang, H.; Li, S.P.; Dai, H.F. Carbazole alkaloids from the peels of Clausena lansium. J. Asian Nat. Prod. Res. 2014, 16, 1024–1028. [Google Scholar] [CrossRef]
- Sribuhom, T.; Boueroy, P.; Hahnvajanawong, C.; Phatchana, R.; Yenjai, C. Benzoyltyramine Alkaloids Atalantums A−G from the Peels of Atalantia monophylla and Their Cytotoxicity against Cholangiocarcinoma Cell Lines. J. Nat. Prod. 2017, 80, 403–408. [Google Scholar] [CrossRef]
- Sombatsri, A.; Thummanant, Y.; Sribuhom, T.; Wongphakham, P.; Senawong, T.; Yenjai, C. Atalantums H-K from the peels of Atalantia monophylla and their cytotoxicity. Nat. Prod. Res. 2020, 34, 2124–2130. [Google Scholar] [CrossRef]
- Tai, B.H.; Trung, T.N.; Nhiem, N.X.; Ha, D.T.; Phuong, T.; Thu, N.B.; Luong, H.V.; Bae, K.H.; Kim, Y.H. Chemical Components from the Fruit Peels of Wisteria floribunda and their Effects on Rat Aortic Vascular Smooth Muscle Cells. Bull. Korean Chem. Soc. 2011, 32, 2079–2082. [Google Scholar] [CrossRef][Green Version]
- Aghel, N.; Ramezani, Z.; Beiranvand, S. Hesperidin from Citrus sinensis cultivated in Dezful, Iran. Pak. J. Biol. Sci. 2008, 11, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Lahmer, N.; Belboukhari, N.; Cheriti, A.; Sekkoum, K. Hesperidin and hesperitin preparation and purification from Citrus sinensis Peels. Pharm. Chem. 2015, 7, 1–4. [Google Scholar]
- Nguyen, V.S.; Li, W.; Li, Y.; Wang, Q. Synthesis of citrus polymethoxyflavonoids and their antiproliferative activities on Hela cells. Med. Chem. Res. 2017, 26, 1585–1592. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and itsassociation with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Eun, S.Y. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef]
- Lin, Y.S.; Li, S.; Ho, C.T.; Lo, C.Y. Simultaneous analysis of six polymethoxyflavones and six 5- hydroxyl-polymethoxyflavones by high performance liquid chromatography combined with linear ion trap mass spectrometry. J. Agricult. Food Chem. 2012, 60, 12082–12087. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, X.; Guo, Y. Identification of the dietary supplement capsaicin as an inhibitor of Lassa virus entry. Acta Pharm. Sin. B. 2020, 10, 789–798. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.; Vikram, A.; Patil, B.S. Polymethoxyflavones isolated from the peel of Miaray Mandarin (Citrus miaray) have biofilm inhibitory activity in Vibrio harveyi. J. Agricult. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef]
- Itoh, N.; Iwata, C.; Toda, H. Molecular cloning and characterization of a flavonoid-O-methyltransferase with broad substrate specificity and regioselectivity from Citrus depressa. BMC Plant Biol. 2016, 16, 180. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Charles, A.L.; Kung, H.-F.; Ho, C.-T.; Huang, T.-C. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crops Prod. 2010, 31, 59–64. [Google Scholar] [CrossRef]
- Chen, S.; Cai, D.; Pearce, K.; Sun, P.Y.; Roberts, A.C.; Glanzman, D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife 2014, 3, e03896. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.; Yoshitani, S.-I.; Tsukio, Y.; Murakami, A.; Koshimizu, K.; Yano, M.; Tokuda, H.; Nishino, H.; Ohigashi, H.; Tanaka, T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci. 2001, 69, 901–913. [Google Scholar]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Patil, B.S. Rapid separation method of polymethoxyflavones from citrus using flash chromatography. Sep. Purif. Technol. 2011, 81, 151–158. [Google Scholar] [CrossRef]
- Teruya, T.; Teruya, Y.; Sueyoshi, K.; Yamano, A.; Jitai, Y. Manufacturing Method of Fermentation Treated Products Containing High-Content Nobiletin and Tangeretin. Patent JP 2015202065, 16 November 2015. [Google Scholar]
- Zhang, N.; Wei, W.Y.; Yang, Z.; Che, Y.; Jin, Y.G.; Liao, H.H.; Wang, S.S.; Deng, W.; Tang, Q.Z. Nobiletin, a Polymethoxy Flavonoid, Protects Against Cardiac Hypertrophy Induced by Pressure-Overload via Inhibition of NAPDH Oxidases and Endoplasmic Reticulum Stress. Cell. Phys. Biochem. 2017, 42, 1313–1325. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhou, C.H.; Tao, J.; Li, S.N. Antagonistic effects of nobiletin, a polymethoxyflavonoid, on eosinophilic airway inflammation of asthmatic rats and relevant mechanisms. Life Sci. 2006, 78, 2689–2696. [Google Scholar] [CrossRef]
- Murakami, A.; Shigemori, T.; Ohigashi, H. Zingiberaceous and citrus constituents, 1’-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW 264.7 murine macrophages through different modes of action. J. Nutr. 2005, 135, 2987S–2992S. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agricult. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Zheng, Y.-N.; Tsai, M.-L.; Lai, C.-S.; Ho, C.-T.; Pan, M.-H. 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. J. Food Bioact. 2018, 2, 91–97. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Kohno, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Yano, M.; Tokuda, H.; Nishino, H.; Tanaka, T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biofactors 2004, 21, 111–114. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem.-Biol. Interact. 2010, 183, 276–283. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive e_ects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef]
- Zhong, W.J.; Luo, Y.J.; Li, J.; Wu, Y.P.; Gao, Y.J.; Luo, H.J.; Yang, Y.T.; Jiang, L. Polymethoxylated flavonoids from Citrus reticulata Blanco. Biochem. Syst. Ecol. 2016, 68, 11–14. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Jia, X.H.; Du, C.L.; Tang, W.Z.; Wang., X.J. New antioxidant C-geranylated flavonoids from the fruit peels of Paulownia catalpifolia T. Gong ex D.Y. Hong. Phytochem. Lett. 2017, 21, 169–173. [Google Scholar] [CrossRef]
- Wang, Y.A.; Guo, X.; Jia, X.H.; Xue, J.; Du, H.F.; Du, C.L.; Tang, W.Z.; Wang, X.J.; Zhao, Y.X. Undescribed C-geranylflavonoids isolated from the fruit peel of Paulownia catalpifolia T. Gong ex D.Y. Hong with their protection on human umbilical vein endothelial cells injury induced by hydrogen peroxide. Phytochemistry 2019, 158, 126–134. [Google Scholar]
- Chen, C.Y.; Wang, J.J.; Kao, C.L.; Yeh, H.C.; Song, P.L.; Liu, S.L.; Wu, H.M.; Li, H.T.; Li, W.J. A new flavanone from Citrus reticulate. Chem. Nat. Comp. 2021, 57, 277–279. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L. Occurrrence and biological significance of proanthocyanidins in the American diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Choi, H.J.; Han, H.S.; Park, J.H.; Son, J.H.; Bae, J.H.; Seung, T.S.; An, B.J.; Kim, H.G.; Choi, C. Chemical structure of polyphenol isolated from Korean pear (Pyrus pyrifolia Nakai). Korean J. Food Sci. Technol. 2003, 35, 959–967. [Google Scholar]
- Hamauzu, Y.; Sakai, I. Change in polyphenolic compounds and antioxidant functions in ‘Bartlett’ pear fruit during storage and postharvest ripening. Food Preserv. Sci. 2002, 28, 25–32. [Google Scholar] [CrossRef][Green Version]
- Jeong, D.E.; Cho, J.Y.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Moon, J.H. Isolation of five proanthocyanidins from pear (Pyrus pyrifolia Nakai) fruit peels. Food Sci. Biotechnol. 2017, 26, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Idowe, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, M. Doubly linked, A-type proanthocyanidin trimer and other constituents of Ixora coccinea leaves and their antioxidant and antibacterial properties. Phytochemistry 1993, 71, 2092–2098. [Google Scholar] [CrossRef]
- Morimoto, S.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LIX. Aesculitannins, novel proanthocyanidins with doubly-bonded structures from Aesculus hippocastanum L. Chem. Pharm. Bull. 1987, 35, 4717–4729. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186s–193s. [Google Scholar] [CrossRef]
- Qiu, D.; Guo, J.; Yu, H.; Yane, J.; Yang, S.; Li, X.; Zhang, Y.; Sun, J.; Cong, J.; He, S.; et al. Antioxidant phenolic compounds isolated from wild Pyrus ussuriensis Maxim. fruit peels and leaves. Food Chem. 2018, 241, 182–187. [Google Scholar]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and isolation of anti-pneumonia bioactive compounds from Opuntia ficus-indica fruit waste peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lee, S.J.; Whang, W.K. Isolation of Anti-Diabetic Active Compounds from Benincasae exocarpium (Benincasa cerifera) and Development of Simultaneous Analysis by HPLC-PDA. Molecules 2022, 27, 9. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.G.; Endo, E.H.; Filho, B.P.D. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sudha, G.; Srinivasan, N.; Das, S. Small molecule inhibitors of HCV replication from Pomegranate. Sci. Rep. 2014, 4, 5411. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Giacomo, C.D.; Acquaviva, R.; Barbagallo, T.; Cardile, V.; Kim, D.H.; Abraham, N.G.; Sorrenti, V. Apoptotic markers in a prostate cancer cell line: Effect of ellagic acid. Oncol. Rep. 2013, 30, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, M.; Odinokova, I.; Ohno, I.; Gukovsky, I.; Go, V.L.W.; Pandol, S.J.; Gukovskaya, A.S. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J. Gastroenterol. 2008, 14, 672–3680. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Vinayak, M. Anti-carcinogenic action of ellagic acid mediated via modulation of oxidative stress regulated genes in Dalton lymphoma bearing mice. Leuk. Lymphoma 2011, 52, 2155–2161. [Google Scholar] [CrossRef]
- Kaneshima, T.; Myoda, T.; Toeda, K.; Fujimori, T.; Nishizawa, M. Antimicrobial constituents of peel and seeds of camu-camu (Myrciaria dubia). Biosci. Biotechnol. Biochem. 2017, 81, 1461–1465. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nishide, H.; Kosela, S.; Hayashi, Y. Structure of Lansiosides: Biologically Active New Triterpene Glycosides from Lansium domesticum. J. Org. Chem. 1983, 48, 4462–4466. [Google Scholar] [CrossRef]
- Potipiranun, T.; Worawalai, W.; Phuwapraisirisan, P.; Lamesticumin, G. A new α-glucosidase inhibitor from the fruit peels of Lansium parasiticum. Nat. Prod. Res. 2018, 32, 1881–1886. [Google Scholar] [CrossRef]
- Ramadhan, R.; Worawalai, W.; Phuwapraisirisan, P. New onoceranoid xyloside from Lansium parasiticum. Nat. Prod. Res. 2019, 33, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Labibah, Q.; Tun, K.N.W.; Aminah, N.S.; Kristanti, A.N.; Ramadhan, R.; Takaya, Y.; Abdullah, C.A.C.; Masarudin, M.J. Cytotoxic constituent in the fruit peel of Lansium domesticum. Rasayan J. Chem. 2021, 14, 1336–1340. [Google Scholar] [CrossRef]
- Fadhilah, K.; Wahyuono, S.; Astuti, P. A bioactive compound isolated from Duku (Lansium domesticum Corr) fruit peels exhibits cytotoxicity against T47D cell line. F1000Research 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishibashi, M.; Fujimoto, H.; Okuyama, E.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K. New Onoceranoid Triterpene Constituents from Lansium domesticum. J. Nat. Prod. 2002, 65, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial Terpenoids from Lansium domesticum. Philipp. Agricult. Sci. 2006, 89, 101–105. [Google Scholar]
- Akihisa, T.; Kimura, Y.; Tamura, T. Cycloartane triterpenes from the fruit peel of Musa sapientum. Phytochemistry 1998, 47, 1107–1110. [Google Scholar] [CrossRef]
- Akihisa, T.; Kimura, T.; Kokke, W.C.M.C.; Takase, S.I.; Yasukawa, K.; Jin-Nai, A.; Tamura, T. 4-Epicycloeucalenone and 4-Epicyclomusalenone: Two 3-Oxo-28-norcycloartanes from the Fruit Peel of Musa sapientum L. Chem. Pharm. Bull. 1997, 45, 744–746. [Google Scholar] [CrossRef][Green Version]
- Motwani, H.V.; De Rosa, M.; Odell, L.R.; Hallberg, A.; Larhed, M. Aspartic protease inhibitors containing tertiary alcoholtransition-state mimics. Eur. J. Med. Chem. 2015, 90, 462–490. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nishimura, K.; Takeya, K. New Cyclic Peptides from Citrus medica var. sarcodactylis SWINGLE. Chem. Pharm. Bull. 2002, 50, 857–860. [Google Scholar] [CrossRef][Green Version]
- Fauzi, F.M.; Meilanie, S.R.; Zulfikar; Farabi, K.; Herlina, T.; Anshori, J.A.; Mayanti, T. Kokosanolide D: A New Tetranortriterpenoid from Fruit Peels of Lansium domesticum Corr. cv Kokossan. Molbank 2021, 2021, M1232. [Google Scholar] [CrossRef]
- Lewis, B.J.; Herrlinger, K.A.; Craig, T.A.; Mehring-Franklin, C.E.; DeFreitas, Z.; Hinojosa-Laborde, C. Antihypertensive effect of passion fruit peel extract and its major bioactive components following acute supplementation in spontaneously hypertensive rats. J. Nutr. Biochem. 2013, 24, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, Y.G.; Lee, S.H.; Kim, W.S.; Park, K.H.; Moon, J.H. An Ether and Three Ester Derivatives of Phenylpropanoid from Pear (Pyrus pyrifolia Nakai cv. Chuhwangbae) Fruit and Their Radical-Scavenging Activity. Food Sci. Biotechnol. 2014, 23, 253–259. [Google Scholar] [CrossRef]
- Adikaram, N.K.B.; Ewing, D.F.; Karunaratne, M.A.; Wijeratne, M.K. Antifungal compounds from immature Avocado fruit Peel. Phytochemistry 1992, 31, 93–96. [Google Scholar] [CrossRef]
- Aulia, S.H.; Setiawati, Y.; Koendhori, E.B. Antibacterial activity of methanol extract of red dragonfruit peel (Hylocereus polyrhizus) against methicillin-susceptible Staphylococcus aureus (MSSA) ATCC 25923 andmethicillin resistant Staphylococcus aureus (MRSA) in vitro. Indian J. Forensic Med. Toxicol. 2021, 15, 4269–4273. [Google Scholar]
- Rahayu, Y.C.; Sabir, A.; Setyorini, D. Antibacterial activity of red dragon fruit extract (Hylocereuspolyrhizus) on Streptococcus mutans. Int. J. Appl. Pharm. 2019, 11, 60–63. [Google Scholar]
- Hendra, R.; Masdeatresa, L.; Almurdani, M.; Abdulah, R.; Haryani, Y. Antifungal activity of red dragon peel (Hylocereus polyrhizus). In Proceedings of the 2nd International Conference on Chemistry and Material Science, Malanga, Indonesia, 2–3 November 2019; Volume 833. [Google Scholar]
- Febrianti, N.; Purbosari, P.P.; Hertiani, T.; Moeljopawiro, S.; Haryana, S.M. 2020. Antioxidant Potency of Red Dragon Fruit Flesh and Peel Prepared by Different Methods. Curr. Nutr. Food Sci. 2020, 16, 1106–1111. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.J.; Song, X.Z.; Wang, Y.F.; Corlett, R.T.; Xu, Y.K.; Hu, H.B. Chemical composition and the cytotoxic, antimicrobial, and anti-inflammatory activities of the fruit peel essential oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, southwest China. Molecules 2020, 25, 343. [Google Scholar] [CrossRef]
- Musika, S.; Pokratok, N.; Pliankratoke, J.; Khongla, C.; Kupradit, C.; Ranok, A.; Mangkalanan, S. Antioxidant, antityrosinase and antibacterial activities of fruit peel extracts. Int. J. Agricult. Technol. 2021, 17, 1447–1460. [Google Scholar]
- Shuai, L.; Liao, L.Y.; Duan, Z.H.; Song, M.B.; Liang, Y.L.; Xie, Y.H.; Liu, Y.F. Optimization of Extraction Technology of Polysaccharides from Passion Fruit Peel and Its Antioxidant Activity. Sci. Technol. Food Ind. 2020, 41, 156–162. [Google Scholar]
- Gnanasaraswathi, M.; Lakshmipraba, S.; Rajadurai, J.R.P.; Abhinayashree, M.; Fathima, B.M.; Aarthi, L.V.; Kamatchi, S. Potent anti-oxidant behaviour of citrus fruit peels and their bactericidal activity against multi drug resistant organism Pseudomonas aeruginosa. J. Chem. Pharm. Sci. 2014, 2, 139–144. [Google Scholar]
- Gladvin, G.; Santhi, S.K.V. Evaluation of antibacterial and antioxidant property of active ingredient of watermelon peel extract. Res. J. Pharm. Biol. Chem. Sci. 2020, 11, 25–33. [Google Scholar]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.; Ismail, A.; Hamid, A.A.; Azlan, A.; Al-sheraji, S.H. Characterization of fibre-rich powder and antioxidant capacity of Mangifera pajang K. fruit peels. Food Chem. 2011, 126, 283–288. [Google Scholar] [CrossRef]
- Ishfaq, M.; Akhtar, B.; Muhammad, F.; Sharif, A.; Akhtar, M.F.; Hamid, I.; Sohail, K.; Muhammad, H. Antioxidant and Wound Healing Potential of Essential Oil from Citrus reticulata Peel and Its Chemical Characterization. Curr. Pharm. Biotechnol. 2021, 22, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Nawaz, S.; Ahmad, R.; Akram, N.; Iqbal, Z. Evaluation of antibacterial, antifungal and antioxidant activity of essential oil of Citrus reticulata fruit (tangerine fruit peel). Pharmacologyonline 2009, 3, 614–622. [Google Scholar]
- Abudayeh, Z.H.; Al Khalifa, I.I.; Mohammed, S.M.; Ahmad, A.A. Phytochemical content and antioxidant activities of pomelo peel extract. Pharmacogn. Res. 2019, 11, 244–247. [Google Scholar] [CrossRef]
- Lemes, R.S.; Alves, C.C.F.; Estevam, E.B.B.; Santiago, M.B.; Martins, C.H.G.; Dossantos, T.C.L.; Crotti, A.E.M.; Miranda, M.L.D. Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. An. Acad. Bras. Cienc. 2018, 90, 1285–1292. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, R. Phytochemical and antimicrobial activity of different citrus fruit peels. Eur. J. Biomed. Pharm. Sci. 2017, 4, 804–806. [Google Scholar]
- Deng, G.; Craft, J.D.; Steinberg, K.M.; Li, P.L.; Pokharel, S.K.; Setzer, W.N. Influence of different isolation methods on chemical composition and bioactivities of the fruit peel oil of Citrus medica L. var. sarcodactylis (Noot.) Swingle. Medicines 2017, 4, 1. [Google Scholar] [CrossRef]
- Dar, M.S.; Lawrence, R.; Khan, A.N. Evaluation of the chemical constituents and the antibacterial activity of essential oil of Citrus karna fruit peel. Int. J. Pharm. Sci. Res. 2016, 7, 1245–1250. [Google Scholar]
- Aggarwal, V.; Varghese, J.; Joshi, N. Antioxidant potential of fruit peel waste of two species of annonaceae and detection of spathulenol and β-pimaric acid as major bioactive compounds by GC-MS. J. Curr. Pharm. Res. 2018, 9, 2695–2715. [Google Scholar]
- Can-Cauich, C.A.; Sauri-Duch, E.; Betancur-Ancona, D.; Chel-Guerrero, L.; Gonzalez-Aguilar, G.A.; Cuevas-Glory, L.F.; Perez-Pacheco, E.; Moo-Huchin, V.M. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J. Funct. Foods 2017, 37, 501–506. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Al-Zehry, H.H.; Al-Hazmi, N.E. Antibacterial effect of some fruit peels against bacteria causing urinary tract infection. Res. J. Pharm. Biol. Chem. Sci. 2020, 11, 37–42. [Google Scholar]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Sarkar, J.; Garg, A.; Gupta, P. Evaluation of antioxidant activity of Solanum melongena fruit peel extract. Int. J. Pharm. Biol. Sci. 2019, 9, 190–196. [Google Scholar]
- Hasan, M.M.; Tasmin, M.S.; Reza, M.A.; Haque, A. Evaluation of antioxidant and antibacterial activity of Putranjiva roxburghii Wall. fruit peel. Int. J. Biosci. 2019, 15, 396–402. [Google Scholar]
- Abreu, M.M.; De, P.; Nobrega, A.; Sales, P.F.; de Oliveira, F.R.; Nascimento, A.A. Antimicrobial and antidiarrheal activities of methanolic fruit peel extract of Pouteria caimito. Pharmacogn. J. 2019, 11, 944–950. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, L.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chemistry 2020, 305, 125430. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le Thien, T.; Van Camp, J.; Katleen, K.R. 2020. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food Microbiol. 2020, 321, 108539. [Google Scholar] [CrossRef]
- Nguyen, N.M.P.; Le, T.T.; Vissenaekens, H.; Gonzales, G.B.; Van-Camp, J.; Smagghe, G.; Raes, K. In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. Int. J. Food Sci. Technol. 2019, 54, 1169–1178. [Google Scholar] [CrossRef]
- Arumugam, P.; Haritha, M.; Keerthana, R.; Vijayalakshmi, M.; Saraswathi, K. Comparative antioxidant and antimicrobial activities of peel and pulp of fruits of Ficus carica L. World J. Pharm. Res. 2018, 7, 1–20. [Google Scholar]
- Stojanovic, B.T.; Mitic, S.S.; Stojanovic, G.S.; Mitic, M.N.; Kostic, D.A.; Paunovic, D.D.; Arsic, B.B. Phenolic profile and antioxidant activity of pulp and peel from peach and nectarine fruits. Not. Bot. Hort. Agrobot. Cluj-Napoca 2016, 44, 175–182. [Google Scholar] [CrossRef]
- Sihombing, J.R.; Dharma, A.; Chaidir, Z.; Fachrial, E.; Munaf, E. Phytochemical screening and antioxidant activities of 31 fruit peel extract from Sumatera, Indonesia. J. Chem. Pharm. Res. 2015, 7, 190–196. [Google Scholar]
- Monteiro, S.S.; da Silva, R.R.; Martins, S.C.d.; Barin, J.S.; da Rosa, C.S. Phenolic compounds and antioxidant activity of extracts of pequi peel (Caryocar brasiliense Camb.). Int. Food Res. J. 2015, 22, 1985–1992. [Google Scholar]
- Yang, J.; Yu, L.; Wu, M.; Liu, J.; Zhang, Y.; Jiang, K.; Wang, B. Antioxidant activities and total flavonoid content in the peel of Leucaena leucocephala fruits. Shipin Kexue 2015, 36, 187–190. [Google Scholar]
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Antioxidant Activity of Loquat (Eriobotrya japonica Lindl.) Fruit Peel and Pulp Extracts in Stabilization of Soybean Oil During Storage Conditions. Int. J. Food Prop. 2015, 18, 2813–2824. [Google Scholar] [CrossRef]
- Suttirak, W.; Manurakchinakorn, S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014, 51, 3546–3558. [Google Scholar] [CrossRef]
- Nurhuda, H.H.; Maskat, M.Y.; Mamot, S.; Afiq, J.; Aminah, A. Effect of blanching on enzyme and antioxidant activities of rambutan (Nephelium lappaceum) peel. Int. Food Res. J. 2013, 20, 1725–1730. [Google Scholar]
- Wang, Y.F.; Wang, J.; Wu, J.; Xu, P.; Wang, Y.Q.; Gao, J.J.; Hochstetter, D. In vitro antioxidant activity and potential inhibitory action against α-glucosidase of polysaccharides from fruit peel of tea (Camellia sinensis L.). J. Zhejiang Univ. Sci. B 2014, 15, 173–180. [Google Scholar] [CrossRef]
- Sathyan, S.L.P.; Ponnusamy, R.D.; Palanisami, E.; Kingsley, J. In vitro antimicrobial activity of Syzygium cumini fruit peel and identification of anthocyanins. Afr. J. Pharm. Pharmacol. 2013, 7, 1719–1728. [Google Scholar]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A. Fruit Wastes as a Valuable Source of Value-Added Compounds: A Collaborative Perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Chang, Y.; Lacroix, M.; Han, J. Control of microbial growth and lipid oxidation on beef product using an apple peel-based edible coating treatment. LWT 2017, 84, 183–188. [Google Scholar] [CrossRef]
- Al-Anbari, I.H.; Dakhel, A.M.; Adnan, A. The effect of adding local orange peel powder to microbial inhibition and oxidative reaction within edible film component. Plant Arch. 2019, 19, 1006–1012. [Google Scholar]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Alparslan, Y.; Baygar, T. Effect of Chitosan Film Coating Combined with Orange Peel Essential Oil on the Shelf Life of Deepwater Pink Shrimp. Food Bioprocess Technol. 2017, 10, 842–853. [Google Scholar] [CrossRef]
- Rahmawati, D.; Chandra, M.; Santoso, S.; Puteri, M.G. Application of lemon peel essential oil with edible coating agent to prolong shelf life of tofu and strawberry. AIP Conf. Proc. 2017, 1803, 020037. [Google Scholar]
- Radi, M.; Akhavan, H.-R.; Amiri, S.; Akhavan-Darabi, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2018, 42, e13441. [Google Scholar] [CrossRef]
- Alparslan, Y.; Metin, C.; Yapıcı, H.H.; Baygar, T.; Günlü, A.; Baygar, T. Combined effect of orange peel essential oil and gelatin coating on the quality and shelf life of shrimps. J. Food Saf. Food Qual. 2017, 68, 69–78. [Google Scholar]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O. Effect of pineapple waste powder on probiotic growth, antioxidant and antimutagenic activities of yogurt. J. Food Sci. Technol. 2015, 53, 1698–1708. [Google Scholar] [CrossRef]
- Santo, A.P.D.E.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.D.M.; Gioielli, L.; Perego, P.; Converti, A.; de Oliveira, M.N. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vicenssuto, G.M.; De Castro, R.J.S. Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agricult. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Pgi, D.; Jwa, S.; Rmusk, R. Formulation and development of composite fruit peel powder incorporated fat and sugar-free probiotic set yogurt. GSC Biol. Pharm. Sci. 2020, 11, 93–99. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).