sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lymphocyte Apheresis, Lymphocyte Depletion Chemotherapy, and CAR-T Infusion
2.3. Response Criteria
2.4. Establishment of ddPCR for CAR-T Quantification
2.5. Determination of sBCMA Plasma Levels in the Peripheral Blood
3. Results
3.1. Patients Characteristics
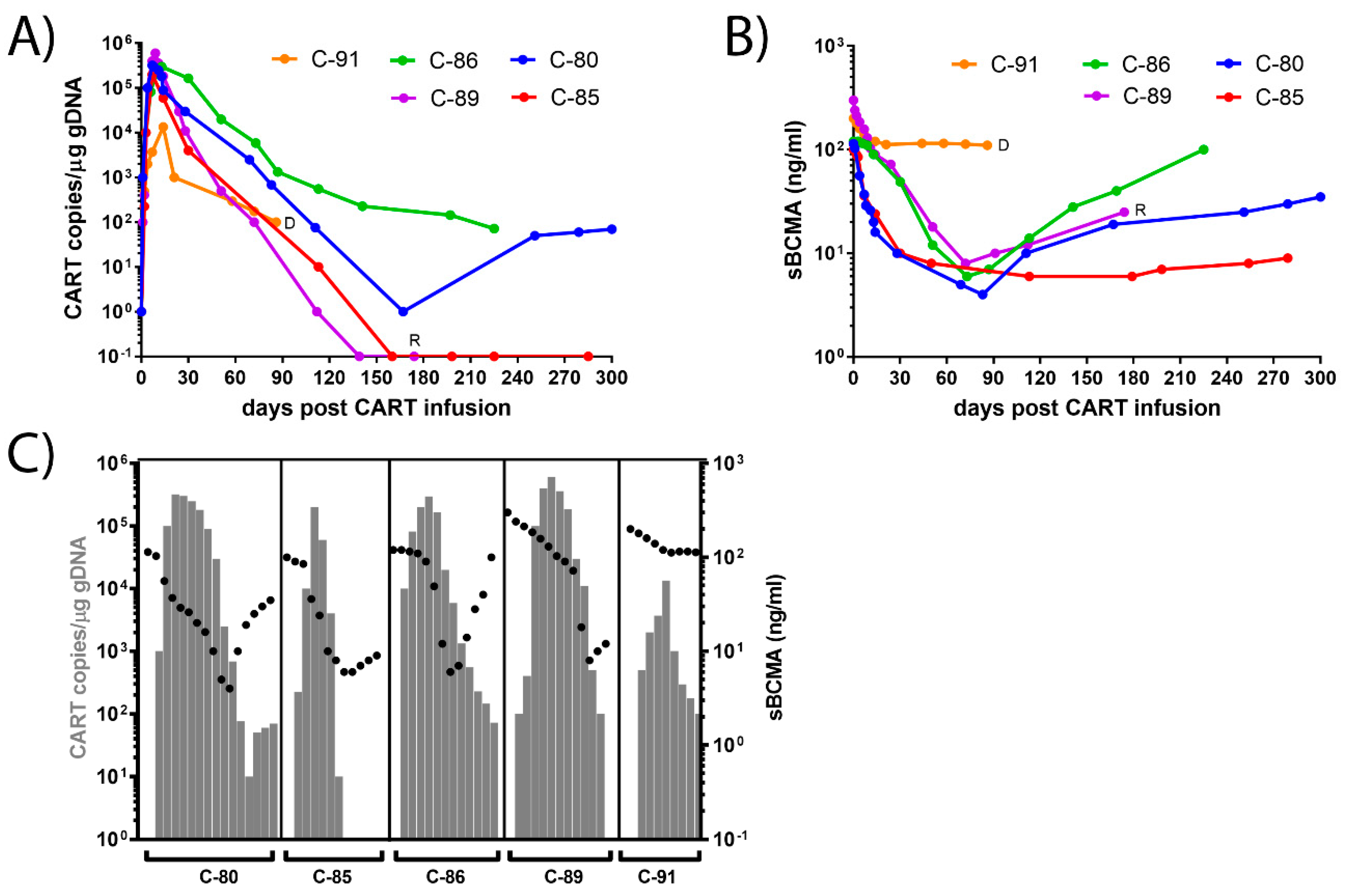
3.2. Dynamics of CAR-T Concentration in the Peripheral Blood
3.3. Dynamics of sBCMA Plasma Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V. Treatment of Multiple Myeloma. Nat. Rev. Clin. Oncol. 2011, 8, 479–491. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued Improvement in Survival in Multiple Myeloma: Changes in Early Mortality and Outcomes in Older Patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, H.; Ashcroft, J.; Szabo, Z.; Garderet, L. Navigating the Treatment Landscape in Multiple Myeloma: Which Combinations to Use and When? Ann. Hematol. 2019, 98, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.B.; Johnson, H. B Cell Maturation Protein Is a Receptor for the Tumor Necrosis Factor Family Member TALL-1. Proc. Natl. Acad. Sci. USA 2000, 97, 9156–9161. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-Cell Maturation Antigen (BCMA) in Multiple Myeloma: Rationale for Targeting and Current Therapeutic Approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Mathys, A.; Bacher, U.; Banz, Y.; Legros, M.; Mansouri Taleghani, B.; Novak, U.; Pabst, T. Outcome of Patients with Mantle Cell Lymphoma after Autologous Stem Cell Transplantation in the Pre-CAR T-Cell Era. Hematol. Oncol. 2021. [Google Scholar] [CrossRef]
- Nydegger, A.; Novak, U.; Kronig, M.-N.; Legros, M.; Zeerleder, S.; Banz, Y.; Bacher, U.; Pabst, T. Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients. Cancers 2021, 13, 6073. [Google Scholar] [CrossRef]
- Meinl, E.; Krumbholz, M. Endogenous Soluble Receptors SBCMA and STACI: Biomarker, Immunoregulator and Hurdle for Therapy in Multiple Myeloma. Curr. Opin. Immunol. 2021, 71, 117–123. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T Cells Expressing an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Multiple Myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Friedman, K.M.; Garrett, T.E.; Evans, J.W.; Horton, H.M.; Latimer, H.J.; Seidel, S.L.; Horvath, C.J.; Morgan, R.A. Effective Targeting of Multiple B-Cell Maturation Antigen-Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum. Gene Ther. 2018, 29, 585–601. [Google Scholar] [CrossRef] [Green Version]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Brechbühl, S.; Bacher, U.; Jeker, B.; Pabst, T. Real-World Outcome in the Pre-CAR-T Era of Myeloma Patients Qualifying for CAR-T Cell Therapy. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021012. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B Cell Maturation Antigen–Specific CAR T Cells Are Clinically Active in Multiple Myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [Green Version]
- Pabst, T.; Joncourt, R.; Shumilov, E.; Heini, A.; Wiedemann, G.; Legros, M.; Seipel, K.; Schild, C.; Jalowiec, K.; Mansouri Taleghani, B.; et al. Analysis of IL-6 Serum Levels and CAR T Cell-Specific Digital PCR in the Context of Cytokine Release Syndrome. Exp. Hematol. 2020, 88, 7–14.e3. [Google Scholar] [CrossRef]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Härmälä, S.K.; Butcher, R.; Roberts, C.H. Copy Number Variation Analysis by Droplet Digital PCR. Methods Mol. Biol. 2017, 1654, 135–149. [Google Scholar] [CrossRef]
- Messmer, A.S.; Que, Y.-A.; Schankin, C.; Banz, Y.; Bacher, U.; Novak, U.; Pabst, T. CAR T-Cell Therapy and Critical Care: A Survival Guide for Medical Emergency Teams. Wien. Klin. Wochenschr. 2021, 133, 1318–1325. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Bladé, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International Uniform Response Criteria for Multiple Myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B Cell Maturation Antigen as a Target for Antibody Drug Conjugate Mediated Cytotoxicity in Multiple Myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E.; et al. Serum B-Cell Maturation Antigen: A Novel Biomarker to Predict Outcomes for Multiple Myeloma Patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ID | C-80 | C-85 | C-86 | C-89 | C-91 | Average (Range) |
|---|---|---|---|---|---|---|
| age at diagnosis (years) | 63 | 58 | 55 | 69 | 45 | 58 (45–69) |
| stage R-ISS | III | II | II | na | II | |
| plasma cell infiltration (%) | 85 | 50 | 80 | 90 | 60 | 73 (50–90) |
| FISH | t(11;14) | t(4;14) | t(11;14)/+1q | na | t(4;14)/+1q | |
| IgA (g/L) | 22 | 50 | 46 | |||
| light chain kappa (mg/L) | 23 | 6 | 223 | |||
| light chain lambda (mg/L) | 13,400 | 382 | 604 | 11 | ||
| lines of prior therapy | 3 | 2 | 5 | 2 | 3 | 3 (2–5) |
| prior ASCT | 1 | 1 | 1 | 0 | 1 | 0.8 (0–1) |
| number of relapses | 3 | 2 | 2 | 2 | 0 | 2 (0–3) |
| time to CART (years) | 7 | 6 | 6 | 1 | 1 | 4.2 (1–7) |
| CAR-T peak (copies/μg gDNA) | 3.2E + 05 | 2.0E + 05 | 3.0E + 05 | 6.0E + 05 | 1.3E + 04 | 2.9E + 05 |
| CAR-T peak day post infusion | 7 | 7 | 13 | 9 | 14 | 10 (7–13) |
| sBCMA pre-infusion (ng/mL) | 115 | 100 | 120 | 280 | 200 | 163 (100–280) |
| sBCMA 4 weeks post infusion | 10 | 10 | 50 | 60 | 120 | 50 (10–120) |
| sBCMA 8 weeks post infusion | 5 | 5 | 12 | 15 | 115 | 33 (5–115) |
| status, 4 weeks post infusion | sCR | sCR | sCR | CR | SD | |
| status, 8 weeks post infusion | sCR | sCR | sCR | CR | PD | |
| status, 6 months post infusion | sCR | sCR | sCR | relapse | deceased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seipel, K.; Porret, N.; Wiedemann, G.; Jeker, B.; Bacher, V.U.; Pabst, T. sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma. Curr. Issues Mol. Biol. 2022, 44, 1463-1471. https://doi.org/10.3390/cimb44040098
Seipel K, Porret N, Wiedemann G, Jeker B, Bacher VU, Pabst T. sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma. Current Issues in Molecular Biology. 2022; 44(4):1463-1471. https://doi.org/10.3390/cimb44040098
Chicago/Turabian StyleSeipel, Katja, Naomi Porret, Gertrud Wiedemann, Barbara Jeker, Vera Ulrike Bacher, and Thomas Pabst. 2022. "sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma" Current Issues in Molecular Biology 44, no. 4: 1463-1471. https://doi.org/10.3390/cimb44040098
APA StyleSeipel, K., Porret, N., Wiedemann, G., Jeker, B., Bacher, V. U., & Pabst, T. (2022). sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma. Current Issues in Molecular Biology, 44(4), 1463-1471. https://doi.org/10.3390/cimb44040098








